Simple Summary
Colorectal cancer (CRC) is a major contributor to cancer-related mortality globally. In this study, we identified hub genes through an integrative approach. Of 989 candidate hub genes identified, 128 genes demonstrated significant prognostic potential. Sixty-seven genes were associated with poor overall survival, while 61 genes were associated with favorable outcomes. This study provides a background for developing more accurate prognostic and diagnostic panels and personalized therapies for CRC.
Abstract
Background: Colorectal cancer (CRC) is a major contributor to cancer-related mortality globally. Despite significant advances in therapeutic strategies, CRC continues to exhibit high recurrence rates. This underscores the urgent need for reliable, non-invasive biomarkers to improve diagnostic precision, early detection, and clinical outcomes. Methods: Gene expression datasets from the GEO database were analyzed to identify differentially expressed genes between CRC and normal tissue samples. Hub genes were identified through an integrative approach combining module membership, gene significance, differential expression, and network centrality. Prognostic significance was assessed via overall survival analysis, and diagnostic utility through ROC curve and AUC. Further integrative analysis included immune cell infiltration, promoter methylation, genetic alterations, and regulatory network construction. Results: An integrated approach identified 989 candidate hub genes. Of these, 128 genes demonstrated significant prognostic potential: 67 were associated with poor overall survival and 61 with favorable outcomes. These genes exhibited patterns of co-expression and positive correlations with immune cell infiltration, particularly B cells, dendritic cells, macrophages, mast cells, and monocytes. Twenty-three hub genes, including MACC1, YEATS4, HMMR, TIGD2, CENPE, GNL3, GMPS, NCAPG, RRM1, DLGAP5, YARS2, CCT8, MET, ZWILCH, KPNA2, KIF15, TRUB1, AURKA, NUDT21, PBK, TOMM20, KIAA1549, and MCM4, showed high diagnostic accuracy in distinguishing CRC from normal tissues. Furthermore, 18 hub genes exhibited statistically significant differential promoter methylation and may serve as promising candidates for epigenetic biomarkers in CRC. Conclusions: Our findings provide a strong foundation for developing more accurate multi-gene prognostic and diagnostic panels and personalized therapies for CRC, with the goal of improving clinical outcomes and reducing the global burden of this disease.
1. Introduction
Colorectal cancer (CRC) is a leading cause of cancer-related mortality in humans and represents a significant global health burden. In the United States, CRC is the second most common cause of cancer deaths, with approximately 1.4 million people affected and 154,270 new cases expected in 2025 [1]. The incidence of CRC continues to increase globally, with Asia alone accounting for nearly 50% of cases reported in 2022 [2]. It is estimated that by 2050, there will be 1.87 million new cases and over 1 million deaths in Asia [2], underscoring the desperate need for improved prevention, detection, and treatment strategies.
The development of CRC is multifactorial, involving complex interactions between genetic and environmental factors [3]. Key contributors include changes in lifestyle, dietary habits, and environmental exposures [4] and chronic inflammatory conditions such as inflammatory bowel disease [5]. Nearly 70–80% of CRC cases are sporadic, and the remaining have an identifiable hereditary cause [6]. Moreover, socioeconomic factors and the human development index have also been linked to CRC incidence and outcomes [7].
Despite significant advances in therapeutics, CRC is still marked by high recurrence rates and limited improvements in long-term survival [8]. Early detection of disease remains the most effective strategy in reducing mortality and is associated with higher survival rates [9]. Current screening methods for CRC include fecal immunochemical test (FIT) [10], colonoscopy [11], and serum biomarkers such as carcinoembryonic antigen (CEA) [12]. However, these methods present certain limitations. FIT is prone to threshold variations [13] and inconsistent patient compliance [14]. Colonoscopy is invasive and often poorly tolerated [15]. Serum biomarkers, such as CEA, lack specificity, and increased levels have been reported in inflammatory diseases [12] and other cancers [16]. Additionally, these approaches are associated with risk of over-diagnosis and over-treatment [12], further emphasizing the need for robust and non-invasive diagnostic tools.
The clinical application of molecular biomarkers for CRC remains limited. There is a critical need to develop reliable, non-invasive biomarkers that can enhance diagnostic accuracy and improve patient outcomes. The identification of key biomarkers associated with CRC not only holds potential for earlier diagnosis but also enhances prognosis and offers novel targets for therapeutic intervention. Recent advances in bioinformatics have significantly accelerated the identification of prognostic biomarkers and novel therapeutic targets in various cancers [17,18,19]. In this study, we aim to identify novel hub genes significantly associated with CRC by analyzing gene expression profiles from the Gene Expression Omnibus (GEO) database. The identified hub genes have the potential to uncover critical molecular signatures associated with CRC pathogenesis and serve as valuable prognostic and diagnostic biomarkers, contributing to improved patient outcomes and treatment responses.
2. Materials and Methods
2.1. Microarray Dataset Collection and Pre-Processing
Gene expression datasets of CRC were retrieved from the GEO database [20]. Inclusion criteria: Only datasets from humans were included in this study. Exclusion criteria: (i) Datasets based on animal models and cell lines, and (ii) datasets without control samples were excluded. A total of four GEO datasets, GSE4107, GSE32323, GSE21510, and GSE24514, met the criteria and were included in the study. Of these, GSE4107, GSE32323, and GSE21510 were generated using GPL570 Affymetrix Human Genome U133 Plus 2.0 Array [HG-U133_Plus_2], while GSE24514 was based on GPL96 Affymetrix Human Genome U133A Array [HG-U133A]. Normalization and background correction of raw data for each dataset were carried out using the “affy” package in R. Gene expression values were obtained from the probe-level data. For genes represented by multiple probes, the average expression value was used as gene expression. Principal component analysis (PCA) was performed to detect outliers and clustering of samples within each dataset. All datasets were analyzed separately.
2.2. Identification of Differentially Expressed Genes
Differentially expressed genes (DEGs) between CRC and normal tissue samples were identified using the limma package in R [21]. The DEGs were screened using the criteria |log2fold change| > 1 and adjusted p-value < 0.05.
2.3. Weighted Gene Co-Expression Network Analysis
Weighted gene co-expression network analysis (WGCNA) was performed using the “WGCNA” (version 1.73) package in R [22]. The analysis was based on the top 5000 most variably expressed genes across all samples. The goodSamplesGenes function of WGCNA was used to filter out genes with zero variance, samples and genes with excessive missing values, and entries below the quality threshold. Pairwise correlations between all gene pairs were computed to construct an adjacency matrix. The soft-thresholding power (β) was determined using the pickSoftThreshold function of WGCNA to approximate a scale-free topology. A β value of ≤6 was selected based on the scale-free topology criterion. The minimum module size was set to 30 genes. Hierarchical clustering was performed using the hclust function in R, which groups genes with similar expression patterns into modules. For each module, module eigengenes (MEs), module membership (MM), and gene significance (GS) were calculated. A topological overlap matrix (TOM) was constructed from the adjacency matrix using TOM similarity values to measure network interconnectedness.
2.4. Construction of Protein–Protein Interaction Network
Genes from the module exhibiting the highest correlation with CRC were used to construct a protein–protein interaction (PPI) network using the STRING database [23]. The igraph package in R was used to calculate normalized centrality measures, including degree, betweenness, and closeness, to evaluate the topological importance of each node (gene) within the network. The ggraph extension of the ggplot2 package was used to visualize the PPI network, highlighting key hub genes and their interactions.
2.5. Hub Gene Identification
A multi-criteria approach was employed to identify a candidate hub gene within the module most strongly correlated with CRC. First, the genes from the selected module were filtered using the following thresholds: MM > 0.8, GS > 0.2, and Log2 fold change (logFC) > 2. This ensured the selection of hub genes with high module connectivity, clinical relevance, and differential expression, respectively. Next, a relevance score was calculated by integrating network topology and expression metrics. For each gene, the following features were normalized: MM, GS, logFC, and three centrality measures (degree, betweenness, and closeness) derived from the PPI network. The network centrality score was computed as the mean of the three normalized centrality measures:
Network centrality = (Degree (normalized) + Betweenness (normalized) + Closeness (normalized)/3
The relevance score was then defined as:
Relevance Score = 0.25 × MM (normalized) + 0.25 × GS (normalized) + 0.25 × logFC (normalized) + 0.25 × Network centrality
Module genes with a relevance score > 0.5 were selected. These genes were then combined with genes passing the original filtering criteria (MM > 0.8, GS > 0.2, logFC > 2) to yield the final list of candidate hub genes.
2.6. Functional Annotation Analysis
Gene Ontology (GO) enrichment analysis for all three major GO categories—biological process (BP), molecular function (MF), and cellular component (CC). and KEGG pathway enrichment analysis was performed for DEGs and candidate hub genes. These analyses were conducted using the enrichGO() and enrichKEGG() functions in the clusterProfiler (version 4.18.1) R package using a p-value < 0.05. For GO enrichment, the genome-wide annotation was based on Entrez gene identifiers, provided by the bioconductor annotation package org.Hs.eg.db.
2.7. Hub Gene Validation
To assess prognostic relevance, overall survival (OS) data correlated with candidate hub gene expression were obtained from Gene Expression Profiling Analysis 2 (GEPIA2) [24]. A p-value < 0.05 was considered statistically significant. Based on survival analysis, hub genes were categorized into two groups: adverse prognostic markers (associated with poor survival) and favorable prognostic markers (associated with better survival outcomes). For diagnostic relevance, the pROC package in R was used to generate receiver operating characteristic (ROC) curves and area under the curve (AUC) for each candidate hub gene. Hub genes with an AUC > 0.75 were considered to have strong detecting ability to distinguish CRC from normal tissues. cBioPortal [25] was used to explore genetic alterations associated with identified hub genes.
2.8. Prognosis-Related Hub Genes and Immune Cell Correlation
The correlation between prognosis-related hub gene expression and immune cell infiltration was evaluated to understand potential immune regulatory roles. Gene set enrichment analysis (GSEA) was performed using the Python (1.1.11) package gseapy. The single-sample GSEA (ssGSEA) method was applied to find the enrichment scores of immune cell types, including T cells, B cells, NK cells, macrophages, dendritic cells, neutrophils, monocytes, regulatory T cells, plasma cells, and mast cells.
2.9. Promoter Methylation Analysis of Prognosis-Related Hub Genes
To investigate epigenetic regulation in CRC, promoter methylation levels of prognosis-related hub genes were analyzed using The Cancer Genome Atlas (TCGA) data from the UALCAN portal [26]. The promoter methylation values (β) from cancerous and normal tissues in colon adenocarcinoma (COAD) and rectal adenocarcinoma (READ) were compared. The genes with differential promoter methylation were screened using the criteria: p-value < 0.05 and |Δβ| ≥ ±0.1.
2.10. Construction of Hub Gene-TF-miRNA Network
Next, a hub gene–transcription factor (TF)–microRNA (miRNA) regulatory network was constructed to investigate the regulatory mechanisms of the identified hub genes. miRNA–hub gene interactions were obtained using the multiMiR (version 1.30.0) R package [27], which integrates data from multiple validated and predicted miRNA-target databases. Transcription factor–hub gene interactions were retrieved from the TRRUST database [28], which contains curated information on human transcription factor-target relationships.
3. Results
3.1. Data Preprocessing and Identification of Differentially Expressed Genes
The raw data were obtained from four GEO datasets: GSE4107 (10 normal and 12 CRC samples), GSE32323 (17 normal and 17 CRC samples), GSE21510 (44 normal and 144 CRC samples), and GSE24514 (15 normal and 34 CRC samples). To ensure data quality and homogeneity, hierarchical clustering was performed to detect outliers and assess grouping consistency (Supplementary Figure S1). The samples identified as outliers or those clustering with the opposite group’s centroid were excluded from further analysis. The final number of samples retained from each dataset after preprocessing is given in Table 1. DEGs were identified by comparing CRC samples with normal samples within each dataset independently, thereby minimizing variability and bias due to inter-dataset differences (Supplementary Figure S2). Genes with an adjusted p-value < 0.05 and log2 fold change (|log2FC|) > 1 were considered differentially expressed. The number of DEGs identified in each dataset was 2141 for GSE4107, 2113 for GSE32323, 2785 for GSE21510, and 895 for GSE24514 (Table 2). Significant differences in gene expression patterns were observed between CRC and normal tissue samples (Supplementary Figure S3). To identify consistently dysregulated genes, we focused on genes that were differentially expressed in ≥3 datasets (Figure 1). This intersection revealed 173 consistently upregulated and 221 consistently downregulated genes in CRC (Supplementary Table S1).

Table 1.
Summary of sample distribution across four GEO datasets used in this study. The table lists the number of normal tissue samples and colorectal cancer (CRC) and included from each dataset.

Table 2.
Summary of differentially expressed genes (DEGs) identified in each GEO dataset. The number of significantly upregulated and downregulated genes is shown for GSE4107, GSE32323, GSE21510, and GSE24514.
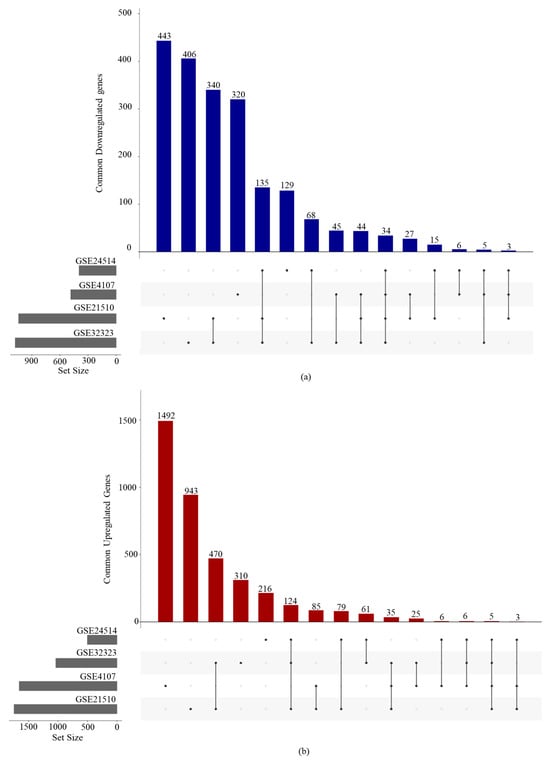
Figure 1.
UpSet plots illustrating the intersection of DEGs across the four GEO datasets: (a) commonly downregulated genes and (b) commonly upregulated genes.
3.2. GO and KEGG Pathway Enrichment Analysis of DEGs
To explore the biological significance of DEGs, GO annotation and KEGG pathway enrichment analyses were performed separately for the upregulated and downregulated DEGs using the clusterProfiler R package. GO enrichment included three major functional categories: biological process (BP), molecular function (MF), and cellular component (CC). Both upregulated and downregulated gene sets showed significant enrichment in several GO terms, indicating their involvement in distinct cellular functions and biological pathways. The top 10 enriched GO terms for each gene set, ranked by adjusted p-value, are presented in Figure 2. It is worth noting that biological processes such as chromosome segregation (GO:0007059), nuclear chromosome segregation (GO:0098813), positive regulation of cell cycle process (GO:0090068), nuclear division (GO:0000280), organelle fission (GO:0048285), sister chromatid segregation (GO:0000819), microtubule cytoskeleton organization involved in mitosis (GO:1902850), mitotic cell cycle phase transition (GO:0044772), regulation of chromosome organization (GO:0033044), and positive regulation of cell cycle (GO:0045787) were enriched in the upregulated genes. Enriched cellular components in the upregulated genes included spindle (GO:0005819), chromosomal region (GO:0098687), spindle pole (GO:0000922), mitotic spindle (GO:0072686), condensed chromosome (GO:0000793), chromosome and centromeric region (GO:0000775), condensed chromosome and centromeric region (GO:0000779), and nuclear chromosome (GO:0000228). KEGG pathway analysis revealed that upregulated DEGs were significantly enriched in pathways associated with the cell cycle (hsa04110), IL-17 signaling pathway (hsa04657), mismatch repair (hsa03430), small cell lung cancer (hsa05222), DNA replication (hsa03030), the NF-κB signaling pathway (hsa04064), the chemokine signaling pathway (hsa04062), and the TNF signaling pathway (hsa04668).
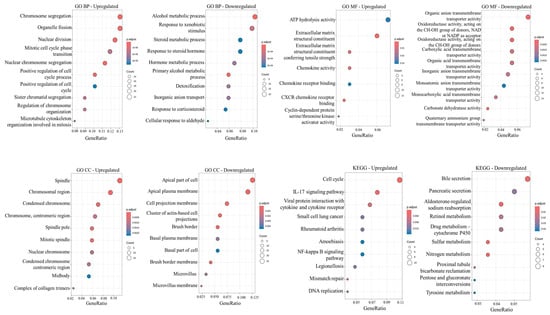
Figure 2.
Functional enrichment analysis of upregulated and downregulated genes identified in ≥3 datasets. Analyses include Gene Ontology (GO) terms for biological process (BP), molecular function (MF), and cellular component (CC) and KEGG pathway enrichment analysis. Dot size represents the number of genes in each GO category or KEGG pathway.
3.3. WGCNA and Identification of Key Co-Expression Modules
The top 5000 most variable genes from each dataset were selected for co-expression network construction. The “WGCNA” package in R was used to classify these variant genes with similar expression patterns into modules. The number of modules obtained for each dataset using a dynamic tree-cutting method was 12 for GSE4107, 24 for GSE32323, 15 for GSE21510, and 20 for GSE24514. Next, the clinically significant module was selected for each dataset. The clinically significant module is the one that showed a maximum correlation coefficient, with a p-value < 0.05. The modules most significantly associated with CRC were turquoise (2683 genes) for GSE4107, turquoise (887 genes) for GSE32323, pink (103 genes) and turquoise (805 genes) for GSE21510, and brown (358 genes) for GSE24514 (Supplementary Figure S4).
3.4. Protein–Protein Interaction (PPI) Network Analysis
Next, the PPI network of genes from the modules that showed the highest correlation with CRC was constructed using the STRINGS database. The three key centrality measures, including degree, betweenness, and closeness, were calculated to evaluate the topological importance of each node (gene) within the network, facilitating the identification of critical hub genes.
3.5. Hub Gene Identification
Hub genes were identified from the key module for each dataset. The first screening filter, |MM| > 0.8 and |GS| > 0.2, led to the identification of 272 genes in GSE4107, 308 genes in GSE32323, 400 genes in GSE21510, and 202 genes in GSE24514. These filtered genes were then intersected with DEGs exhibiting a fold change > 2, yielding 272 genes in GSE4107, 266 genes in GSE32323, 52 genes in GSE21510, and 136 genes in GSE24514. Next, a relevance score was calculated integrating network centrality, module membership (MM), gene significance (GS), and log2fold change. Genes with a relevance score ≥ 0.5 were selected and combined with the previously identified hub genes, resulting in 305 genes for GSE4107, 317 genes for GSE32323, 247 genes for GSE21510, and 141 genes for GSE24514. Finally, hub genes identified from all datasets were merged; duplicate genes were removed to obtain 989 candidate hub genes (Supplementary Table S2). These genes were selected for further analysis.
3.6. Functional Enrichment Analysis of Hub Genes
To further elucidate the biological significance of candidate hub genes, GO annotation and KEGG pathway enrichment analyses were performed (Figure 3). The hub genes showed significant enrichment in several GO terms, indicating their involvement in key cellular processes and regulatory mechanisms. The notable biological processes enriched in hub genes were associated with chromosome organization, segregation and separation (GO:0007059, GO:0033044, GO:0098813, GO:0051983, GO:0000819, GO:0000070, GO:1905818, GO:0051304, GO:0051984, GO:1905820, GO:0033045), nuclear division (GO:0000280), DNA replication (GO:0006260, GO:0006261), transition of cell cycle (GO:0044784, GO:0030071, GO:1902099, GO:1901990), and double-strand break repair (GO:0000727). The notable cellular components enriched in hub genes were related to the chromosomes, including the centromeric region, telomeric region, kinetochore (GO:0098687, GO:0000793, GO:0000775, GO:0000779, GO:0000776, GO:0000781, GO:0000939), and mitotic spindle (GO:0005819, GO:0072686, GO:0051233). Molecular function enrichment was primarily related to DNA replication (GO:0009378, GO:0043138, GO:0003678, GO:0017116, GO:0003697, GO:0061749, GO:1990518, GO:0036121, GO:0003689, GO:0003688). KEGG pathway analysis further supported these findings, highlighting significant enrichment in pathways related to the cell cycle (hsa04110) and DNA replication (hsa03030).
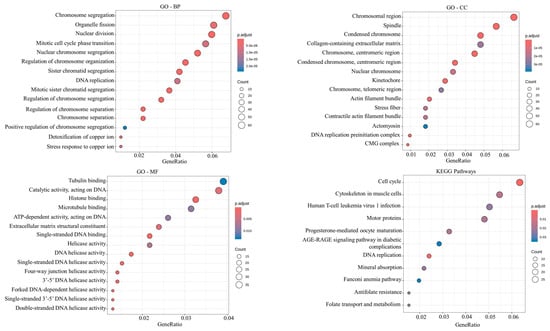
Figure 3.
Functional enrichment analysis of 989 candidate hub genes. Analyses include Gene Ontology (GO) terms for biological process (BP), molecular function (MF), and cellular component (CC) and KEGG pathway enrichment analysis. Dot size represents the number of genes in each GO category or KEGG pathway.
3.7. Validation of Hub Genes
To validate 989 candidate hub genes, their clinical relevance was assessed to evaluate the prognostic value of these genes in CRC. For this, OS analysis of CRC patients was performed using the GEPIA2 platform. A p-value threshold of <0.05 was considered statistically significant. Survival analysis identified 128 hub genes with significant prognostic potential (Table 3). These 128 hub genes were further classified into two categories: 67 genes whose high expression was associated with poor overall survival (Supplementary Figure S5), suggesting their utility as adverse prognostic markers, and 61 genes whose high expression was associated with improved survival outcomes (Supplementary Figure S6), suggesting their function as favorable prognostic markers.

Table 3.
Hub genes associated with prognosis in colorectal cancer. The table presents hub genes categorized by their association with poor or favorable overall survival, as determined through Kaplan–Meier analysis.
3.8. Co-Expression Patterns of Hub Genes
To investigate the relationships among prognostic hub genes, the co-expression patterns of the 67 adverse prognostic markers and 61 favorable prognostic markers were analyzed (Supplementary Figure S7). The results revealed moderate-to-high levels of co-expression within both groups, with stronger co-expression trends observed among the favorable prognostic markers. Many of the favorable prognostic marker hub genes exhibited highly correlated expression patterns, indicating potential co-regulation or involvement in similar biological processes. Contrary to this, the adverse prognostic markers showed a moderate level of co-expression, suggesting diverse functional roles or regulatory mechanisms. The presence of strong co-expression patterns, particularly among favorable prognostic markers, suggests that these hub genes may function together within coordinated regulatory networks. The co-expression patterns also suggest shared contributions to key biological processes that influence the pathophysiology of CRC.
3.9. Genetic Alterations and Mutational Landscape of Hub Genes
To explore the mutational landscape of the prognosis-associated hub genes in CRC, mutation data was retrieved from cBioPortal. Among all genetic alterations, missense mutations were the most prevalent type observed across these genes (Figure 4). Among the adverse prognostic hub genes, the most frequently mutated were AHNAK2 (mutated in 24% of CRC samples), TNS1 (mutated in 17% of CRC samples), PTPRZ1 (mutated in 15% of CRC samples), FLNA (mutated in 13% CRC samples), FILIP1 (mutated in 13% CRC samples), CDH2 (mutated in 13% CRC samples), and THBS2 (mutated in 11% CRC samples). The most frequently mutated hub genes associated with favorable prognosis included SHPRH (mutated in 20% of CRC samples), KIAA1549 (mutated in 16% CRC samples), CENPE (mutated in 15% CRC samples), SHROOM4 (mutated in 12% CRC samples), MCM4 (mutated in 12% CRC samples), MUC2 (mutated in 12% CRC samples), TIMELESS (mutated in 12% CRC samples), CNTN3 (mutated in 11% CRC samples), MET (mutated in 11% CRC samples), NCAPG (mutated in 11% CRC samples), and ZNF443 (mutated in 10% CRC samples).
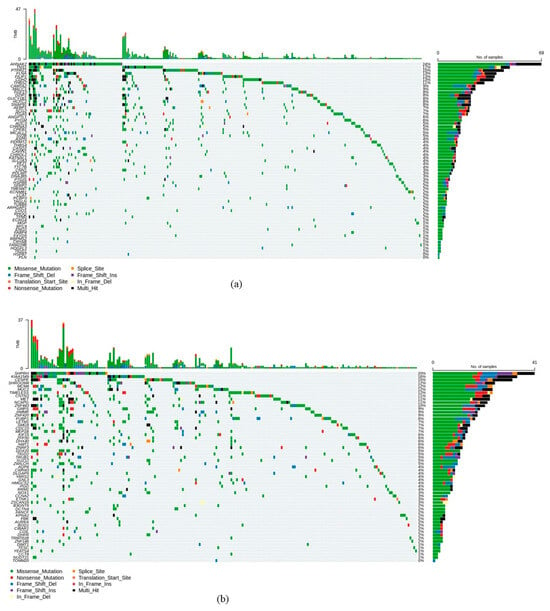
Figure 4.
Genetic alteration profiles of hub genes associated with (a) poor prognosis and (b) favorable prognosis. Oncoplots are based on CRC data in The Cancer Genome Atlas (TCGA).
3.10. Immune Cell Infiltration and Correlation with Hub Gene Expression
Single-sample gene set enrichment analysis (ssGSEA) using curated immune cell marker gene sets was used to evaluate the immune cell infiltration associated with hub gene expression. The majority of hub genes associated with adverse prognosis exhibited positive correlations with immune cell infiltration (Figure 5a). These positive correlations were especially pronounced for B cells, dendritic cells, macrophages, mast cells, and monocytes, highlighting their possible involvement in recruiting or regulating immune populations within the tumor microenvironment. Plasma cells and regulatory T cells show a negative correlation. Similarly, the hub genes associated with a favorable prognosis exhibited a heterogeneous pattern, with some hub genes showing positive correlations and others showing negative correlations with immune cell infiltration (Figure 5b). Again, a negative correlation was observed for plasma cells and regulatory T cells. A heterogeneous pattern suggests that certain hub genes associated with a favorable prognosis may promote immune cell recruitment, while others may promote immune suppression within the tumor microenvironment.
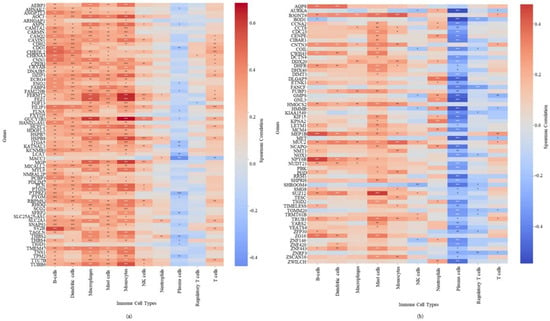
Figure 5.
Correlation between hub gene expression and immune cell infiltration in CRC (a) adverse prognosis hub genes (b) favorable prognosis hub genes. Positive correlations with immune-infiltrating cells are shown in red, negative correlations are shown in blue. * p < 0.05, ** p < 0.01, *** p < 0.001.
3.11. Identification of Potential Diagnostic Biomarkers Among Prognosis-Related Hub Genes
To identify hub genes with diagnostic potential, receiver operating characteristic (ROC) curve analysis was performed. The area under the ROC curve (AUC) was used to evaluate the ability of hub genes to distinguish CRC samples from normal samples. Hub genes with an AUC > 0.75 and a statistically significant difference in expression (p < 0.05) were considered to have a high discriminatory power and thus have strong diagnostic value (Figure 6 and Figure 7). Expression data for hub genes were extracted from all four GEO projects and compared against expression data from TCGA to ensure robustness and consistency. Based on these criteria, 23 hub genes were identified as having significant diagnostic potential. Among these, MACC1 was associated with poor prognosis, while the remaining 22 genes, including YEATS4, HMMR, TIGD2, CENPE, GNL3, GMPS, NCAPG, RRM1, DLGAP5, YARS2, CCT8, MET, ZWILCH, KPNA2, KIF15, TRUB1, AURKA, NUDT21, PBK, TOMM20, KIAA1549, and MCM4, were associated with favorable prognosis.
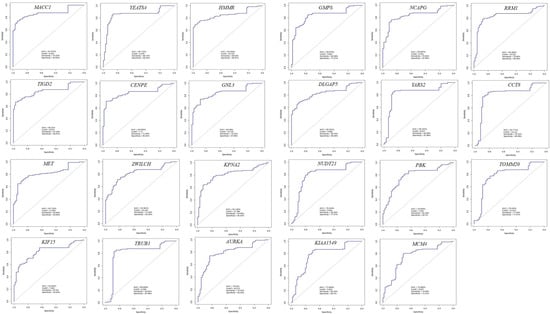
Figure 6.
Receiver operating characteristic (ROC) curves for 23 hub genes demonstrating strong discriminatory potential (AUC > 0.75) in distinguishing CRC from normal tissues.
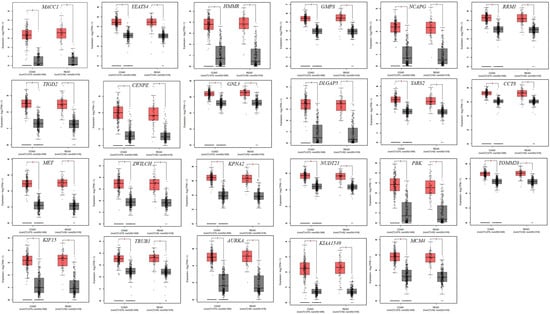
Figure 7.
Expression levels of 23 diagnostically significant hub genes in CRC. CRC samples are shown in red, and normal samples in gray. * Indicates statistical significance (p-value < 0.05).
3.12. Promoter Methylation Analysis of Hub Genes
A total of 18 hub genes (15 genes with worst prognosis and 3 genes with favorable prognosis) exhibited statistically significant differential promoter methylation in CRC (Supplementary Figure S8). Among these, 13 genes were consistently hypermethylated and five genes were consistently hypomethylated in CRC tissues compared to normal controls in both COAD and READ. The hub genes found to be hypermethylated include SFRP2, CDH2, CPEB1, CHRDL2, PTPRZ1, CDO1, SNAP91, HAND2-AS1, LCAT, THBS4, TUBB6, DZIP1, and ANGPTL1. Notably, SNAP91 (ΔβCOAD = 0.419, ΔβREAD = 0.349) and CDH2 (ΔβCOAD = 0.408, ΔβREAD = 0.350) showed the highest levels of promoter hypermethylation suggesting strong transcriptional silencing potential in tumor tissues. The hub genes consistently hypomethylated were FABP4, KCNMB1, NOX1, AQP8, and MEP1B. Interestingly, all three genes with favorable prognosis (NOX1, AQP8, and MEP1B) were found to be hypomethylated.
3.13. Construction of TFs-miRNA–Hub Genes Network
To investigate the transcriptional regulatory landscape of prognosis/diagnosis-related hub genes, we constructed a TFs-miRNA–hub genes network utilizing the multiMiR R package and the TRRUST database. Separate network was constructed for hub genes associated with poor prognosis and those linked to favorable prognosis (Supplementary Figure S9, Supplementary Tables S3 and S4). The TFs-miRNA–hub genes regulatory network highlights the complex interplay between TFs and miRNAs in modulating the expression of hub genes critical to prognosis. Hub genes associated with poor prognosis were regulated by 40 distinct TFs and 1284 unique miRNAs. The most prevalent TFs included: SP1 (regulating CDH2, ITGA5, LCAT, and ASPN), SP3 (regulating CDH2 and LCAT), TFAP2A (regulating CRYAB and SNAP91), SRF (regulating TAGLN and MYL9), HMGA1 (regulating CRYAB and SLC2A3), and NFIC (regulating GUCY1B1 and ITGA5). The most frequent miRNAs regulating these hub genes were hsa-miR-34a-5p (regulating 55 hub genes), hsa-let-7b-5p (50 hub genes), hsa-let-7a-5p (49 hub genes), hsa-let-7g-5p (46 hub genes), hsa-let-7i-5p (45 hub genes), hsa-let-7e-5p (44 hub genes), hsa-let-7d-5p (44 hub genes), hsa-let-7f-5p (43 hub genes), hsa-miR-15a-5p (42 hub genes), hsa-miR-19a-3p (42 hub genes), hsa-miR-423-5p (42 hub genes), hsa-let-7c-5p (42 hub genes), hsa-miR-26b-5p, and hsa-miR-20b-5p (40 hub genes each).
The hub genes associated were favorable prognosis were regulated by 41 distinct TFs and 1364 unique miRNAs. Key TFs identified were E2F1 (regulating AURKA, DHFR and RRM1), SP1 (regulating CCNA2, MET, and MUC2), TP53 (regulating CCNA2, MET, and MUC2), TFDP1 (regulating DHFR and RRM1), YBX1 (regulating KPNA2 and MET), and E2F3 (regulating AURKA and CCNA2). The most frequent miRNAs regulating these hub genes were: hsa-miR-34a-5p (49 hub genes), hsa-miR-16-5p (48 hub genes), hsa-miR-26a-5p (47 hub genes), hsa-miR-103a-3p (46 hub genes), hsa-miR-107 (46 hub genes), hsa-miR-26b-5p (45 hub genes), hsa-miR-15a-5p (44 hub genes), hsa-let-7b-5p (44 hub genes), hsa-let-7i-5p (44 hub genes), hsa-let-7a-5p (44 hub genes), hsa-miR-19a-3p (43 hub genes), hsa-miR-19b-3p (42 hub genes), hsa-let-7c-5p (42 hub genes), hsa-miR-27a-3p (42 hub genes), hsa-miR-17-5p (42 hub genes), hsa-let-7g-5p (41 hub genes), hsa-miR-139-5p (41 hub genes), hsa-let-7e-5p (41 hub genes), hsa-miR-423-5p (41 hub genes), and hsa-miR-196a-5p (41 hub genes).
4. Discussion
CRC is one of the most common cancers and a major contributor to cancer-related deaths worldwide. Although significant progress has been made in diagnosing and treating CRC, the prognosis remains poor. Therefore, it is important to identify key molecular signatures associated with the disease not only for understanding the disease progression but also for developing diagnostic and prognostic markers and novel therapeutic targets. In this study, we analyzed four GEO datasets to identify DEGs between CRC and normal tissues. Upregulated DEGs were significantly enriched in GO processes like chromosome segregation, cell cycle, cell division, extracellular matrix, chemokine activities and cyclin-dependent protein kinase activator activity. KEGG pathway analysis further supported this, identifying enrichment in key cancer-related pathways such as cell cycle, IL17 signaling, NF-κB signaling, mismatch repair and DNA replication. This aligns with the well-established fact that uncontrolled proliferation is a hallmark of cancer. Aberrant activation of cyclin-dependent kinases, particularly CDK1 and CDKN3, has been reported in CRC, and is associated with poor prognosis [29,30,31]. The enrichment of extracellular matrix (ECM) and chemokine activities highlights the importance of tumor-stromal interactions in CRC. ECM remodeling facilitates tumor progression and promotes tumor invasion and metastasis [32]. The dysregulation of genes CHI3L1, COL1A1, COL4A1, COL5A2, COL11A1, and COL12A1, as seen in this study, support epithelial-to-mesenchymal transition (EMT), which drives metastasis [33]. Additionally, upregulated chemokines such as CXCL8, CXCL9 CXCL10, and CXCL11, may induce inflammation and thus promote tumor progression. Similarly, IL-17 and NF-κB signaling mediate chronic inflammation within the tumor microenvironment and promote angiogenesis and immune dysregulation in CRC [34,35]. These findings are in agreement with established CRC biology, which is characterized by uncontrolled cell proliferation, genomic instability, and inflammation-driven signaling.
WGCNA is a robust method for identifying functional gene modules and their relationships to clinical traits and has been used for identification of biomarkers in various cancers [36,37,38]. The modules that show the highest correlation with CRC include those gene clusters that may be co-regulated and functionally relevant in CRC development. The PPI network analysis allowed us to identify genes with topological importance. The use of multi-criteria yielded 989 candidate hub genes; genes that are differentially expressed, highly connected, and tightly associated with CRC. Functional enrichment of these hub genes reiterated their roles in critical tumor-related pathways and processes, such as chromosome organization, segregation, kinetochore, and mitotic spindle. Defects in chromatid cohesion and segregation is associated with tumorigenesis in CRC [39]. Deregulation or mutations of spindle assembly checkpoint genes, such as BUB1 and CDC20 is associated with chromosomal instability and chromosome missegregation in CRC [40]. Therefore, spindle assembly checkpoint is considered a potential therapeutic target in CRC [41]. Similarly, hub gene enrichment in DNA replication and cell—cycle transition underlines dysregulation in S-phase and G2/M checkpoints. In CRC, replication stress is a prevalent driver of chromosomal instability, leading to structural and numerical chromosome abnormalities during mitosis [42,43]. Hub genes involved in DNA double-strand break (DSB) repair mechanisms highlight the pivotal role of DNA repair in CRC pathogenesis. Disruption of DNA repair pathways leads to accumulation of DNA breaks, resulting in genomic instability and tumor progression [44,45]. Hub genes were also enrichment in cellular components such as centromeric region, telomeric region, kinetochore, and mitotic spindle, suggesting that the dysregulation of mitosis and chromosomal architecture plays a central role in CRC progression. These findings are also in line with previous studies linking aberrant cell cycle regulation to tumor growth and metastasis in CRC. Telomere dysfunction is common in CRC and can trigger chromosome breaks that promote genomic rearrangement [39,44]. Additionally, aberrant kinetochore and spindle checkpoint proteins contribute to segregation errors and aneuploidy [43].
An integrative bioinformatics approach integrating differential expression analysis, WGCNA, PPI network construction, and clinical outcome validation, led to the identification of a core set of 128 hub genes implicated in CRC biology. Among these, 67 genes were linked to poor prognosis, suggesting their potential role in tumor aggressiveness, metastasis, or therapy resistance. In contrast, 61 genes were correlated with favorable clinical outcomes, indicating possible tumor-suppressive roles or involvement in protective biological processes. These genes are not only significantly dysregulated in CRC but also strongly associated with patient prognosis. Interestingly, genes associated with favorable prognosis demonstrated strong co-expression, suggesting potential functional synergy in tumor suppression. In contrast, genes linked to poor prognosis exhibited only moderate co-expression, reflecting their involvement in diverse oncogenic pathways, contributing to tumor complexity and heterogeneity. Similar co-expression patterns have been reported in CRC previously [46]. These observations suggest the functional coherence among favorable genes and offer a potential for developing multi-gene therapeutic or prognostic panels.
Mutation analysis of the identified hub genes revealed frequent somatic alterations, further substantiating their relevance and potential roles in CRC biology and clinical outcomes. Missense mutations were most frequent type of alterations, potentially leading to gain-of-function (GOF) or loss-of-function (LOF) effects, depending on the mutation’s location and the gene’s role as an oncogene or tumor suppressor [47]. These functional changes not only affect gene expression but also contribute to tumor progression, or therapeutic response, and offer opportunities for targeted therapies or mutation-based diagnostics. Therefore, characterizing the mutational profiles and functional pathways affected is crucial for identifying cancer-driving variants and their therapeutic applications [48].
Several hub genes identified in this study exhibited a strong correlation with immune cell infiltration, indicating their potential roles in modulating the tumor immune microenvironment. Such correlations highlight the complex immunomodulatory roles of hub genes in influencing immune surveillance. A complex and cell type–specific relationship between hub gene expression and immune cell infiltration in CRC was observed. Notably, hub genes associated with adverse prognosis showed predominantly positive correlations with immune infiltration, particularly involving B cells, dendritic cells, macrophages, mast cells, and monocytes. Tumor-associated B cells and their differentiation into plasma cells play divergent roles in CRC. Elevated B-cell infiltration has been associated with improved prognosis, particularly when organized into tertiary lymphoid structures [49,50]. Macrophages and monocytes are frequently enriched in CRC. M2 macrophages are tumorigenic and immunosuppressive, while M1 macrophages are pro-inflammatory and have anti-tumor properties [51,52]. Tumor-associated macrophages play a key role in CRC metastasis and are often associated with poor prognosis and drug resistance [53]. Their positive correlation with poor-prognosis hub genes aligns with their roles as facilitators of tumor-promoting inflammation. The observed association with mast cells also supports this notion, as mast cell proliferation in CRC is correlated with angiogenesis and lymph node metastasis [54]. Conflicting correlations have been observed between dendritic cell tumor infiltration and prognosis in CRC [55,56,57]. Interestingly, regulatory T cells (Tregs) and plasma cells exhibited negative correlations with hub gene expression. While Tregs usually correlate with poor clinical outcomes in cancer patients and contribute to resistance development to immunotherapy [58], some studies have indicated a protective role in CRC [59]. In contrast, hub genes associated with favorable prognosis displayed heterogeneous correlations with immune cell infiltration, reflecting their functional diversity. This variability reflects the distinct roles these genes play in modulating the tumor-immune microenvironment, with some genes enhancing immune surveillance, while others promote immunoregulatory mechanisms. The tumor microenvironment undergoes selective remodeling of immune cell populations, particularly affecting components of humoral and innate immunity. Importantly, the tumor-suppressive or tumor-promoting effects of immune cells are not universal but are shaped by a complex interplay of gene expression patterns, tumor stage, epigenetic modifications, and tumor mutational burden. A deeper understanding of the immunological implications of hub gene expression may provide valuable insights into tumor-immune dynamics and support the development of personalized immunotherapeutic strategies.
In addition to their prognostic significance, 23 hub genes including MACC1, YEATS4, HMMR, TIGD2, CENPE, GNL3, GMPS, NCAPG, RRM1, DLGAP5, YARS2, CCT8, MET, ZWILCH, KPNA2, KIF15, TRUB1, AURKA, NUDT21, PBK, TOMM20, KIAA1549, and MCM4, demonstrated strong discriminatory potential (AUC > 0.75) in distinguishing CRC from normal tissues. Notably, TIGD2, GMPS, RRM1, and ZWILCH are relatively underexplored in the context of CRC, highlighting them as promising novel candidates for further functional validation and biomarker development. These genes showing strong discriminatory potential have previously been implicated in CRC progression and may serve as potential targets for non-invasive diagnostic development and therapeutic intervention. For instance, MACC1 (metastasis—associated in colon cancer 1), a well-characterized oncogene, acts as a key regulator of HGF-MET signaling and promotes proliferation, migration, invasion, and metastasis of colon cancer cells [60]. It has emerged as a robust prognostic biomarker, not only in CRC but also across multiple cancers [61,62]. A meta-analysis, including fifteen studies encompassing 2161 CRC patients, confirmed that MACC1 overexpression is significantly associated with poor survival [63]. The elevated expression of MACC1 in CRC is often driven by gene amplification [64]. KPNA2 (karyopherin α2) plays a central role in nuclear transport and is frequently upregulated in CRC. Previous studies have linked high KPNA2 expression to lymphatic invasion, advanced tumor-node-metastasis stage, and reduced overall and disease-free survival [65,66]. In contrast, our findings associate KPNA2 expression with improved prognosis. AURKA (aurora kinase A) is typically associated with poor prognosis in CRC patients receiving radiotherapy [67] and progression from adenoma-to-carcinoma and enhanced Wnt and Ras-MAPK signaling [68]. Inhibiting AURKA is considered effective treatment strategy to overcome cetuximab resistance in RAS/RAF wild-type CRC [69]. Nevertheless, our analysis linked AURKA with favorable prognosis, again suggesting possible subtype-specific or patient-specific prognostic divergence. NUDT21 (nudix hydrolase 21), identified in our study as a gene linked to favorable prognosis, has been reported as an oncogene in CRC. HMMR (hyaluronan-mediated motility receptor) appears to have limited prognostic relevance in CRC, as no significant associations with tumor development or survival have been reported [70]. Recently, Chen X et al. [71] found that NUDT21 overexpression promotes CRC cell proliferation and migration by modulating the Hippo signaling pathway, and correlates with poor survival. This discrepancy suggests context-specific roles of NUDT21, possibly dependent on pathway crosstalk or tumor subtype. NOX1 (NADPH oxidase 1) is overexpressed in colon cancer, particularly in patients with activating KRAS mutations [72]. It promotes the proliferation of colon cancer stem cells via mTORC1 and ROS-dependent signaling [73,74], and its inhibition enhances the efficacy of checkpoint inhibitor–based immunotherapy [75], reinforcing its potential as a therapeutic target. TRUB1 (TruB pseudouridine synthase family member 1) has been linked to CRC progression. It is markedly upregulated in CRC and associated with reduced overall survival [76].
Silencing or downregulating YEATS4 (YEATS domain containing 4) suppresses CRC cell proliferation and enhances chemosensitivity [77,78], suggesting a pro-tumorigenic role. DLGAP5 (DLG associated protein 5) overexpression is associated with worse prognosis and increased tumor invasiveness [79]. Interestingly, PBK (PDZ binding kinase) expression is linked to improved survival in our analysis, aligning with previous studies showing that PBK suppresses CRC cell migration and invasion [80,81]. MCM4 (minichromosome maintenance complex component 4), involved in DNA replication and cell cycle control, is closely linked to CRC tumorigenesis and has been proposed as a pan-cancer diagnostic and therapeutic biomarker [82]. KIAA1549 has been implicated in promoting tumor progression and chemoresistance in CRC by enhancing the expression of the DNA repair protein ERCC2, thereby potentially contributing to treatment failure [83]. NCAPG (non-SMC condensin I complex subunit G), which is overexpressed in CRC, promotes tumor invasion and metastasis by regulating EMT and the Wnt/β-catenin pathway [84,85]. Functional studies show that NCAPG knockdown interferes with G2/M–G1 transition and inhibits cell proliferation [86].
YARS2 (mitochondrial tyrosyl-tRNA synthetase) is also upregulated in CRC and has been shown to enhance cell proliferation and migration. Its silencing increases ROS levels, enhancing sensitivity of CRC cells to 5-FU treatment [87]. In contrast, CENPE (centromere-associated protein E), whose high expression is correlated with poor CRC prognosis [88], showed divergent behavior in our study. GNL3 (G protein nucleolar 3) promotes colon cell proliferation, migration, invasion, and EMT through the activation of Wnt/β-catenin signaling pathways [89], emphasizing its role as a therapeutic target. KIF15 (kinesin family member 15) promotes CRC progression by modulating NRAS expression and activating the Rac signaling pathway, whereas its downregulation suppresses cell proliferation and migration while inducing apoptosis [90]. CCT8 (chaperonin containing TCP1 subunit 8), whose expression is elevated in CRC, facilitates tumor progression and EMT by inhibiting nuclear translocation of wild-type p53 [91,92]. TOMM20, a mitochondrial outer membrane protein, is significantly upregulated in CRC, and its expression is associated with enhanced cell proliferation, migration, lymph node involvement, and perineural invasion [93]. c-MET, another critical oncogene, is frequently overexpressed in CRC, particularly in cases with liver metastasis, and correlates with reduced progression-free and overall survival, especially in patients undergoing bevacizumab therapy [94,95].
The promoter methylation analysis highlights the epigenetic landscape of CRC by identifying 18 hub genes that exhibit significant promoter methylation differences between tumor and normal tissues, suggesting a strong regulatory role of DNA methylation in CRC pathogenesis and prognosis. These genes may serve as promising candidates for further exploration as epigenetic biomarkers in CRC. Promoter hypermethylation is a well-established mechanism for gene silencing in cancer [96,97]. This is due to reduced chromatin accessibility and impaired transcription factor binding. In line with this, the majority of hypermethylated hub genes identified in our analysis, including ANGPTL4, SFRP2, CDO1, CPEB1, HAND2-AS1, THBS4, and PTPRZ1, have previously been implicated as tumor suppressors or negative regulators of oncogenic pathways [98,99,100,101,102,103,104]. In contrast, gene hypomethylation is commonly associated with oncogene activation and may contribute to tumor progression [105]. Interestingly, three genes found to be associated with favorable prognosis in our analysis, NOX1, AQP8, and MEP1B, were consistently hypomethylated, suggesting that maintenance of their expression may have anti-tumor effects. Furthermore, KCNQ5 hypomethylation has been reported as a promising epigenetic biomarker for early CRC diagnosis [106]. Collectively, the distinct methylation patterns observed for these hub genes underscore the role of DNA methylation as both a driver and modulator of CRC progression.
The TF–miRNA–hub gene regulatory network was constructed to identify both transcriptional and post-transcriptional regulators of hub genes associated with CRC. This integrated network highlights the complex interplay between TFs and miRNAs in modulating expression of hub genes critical to CRC prognosis and sheds light on key regulatory circuits involved in CRC pathogenesis. Several miRNAs and TFs identified in the network have well-established roles in CRC. Notably, hsa-miR-34a-5p, hsa-let-7d-5p, hsa-miR-19a-3p, hsa-miR-103a-3p, hsa-miR-107, hsa-miR-17-5p, and hsa-miR-27a-3p are frequently up-regulated in CRC, suggesting them to be potential oncogenic regulators [107,108,109,110,111,112,113]. Conversely, several members of the let-7 family, including hsa-let-7a-5p, hsa-let-7b-5p, hsa-let-7e-5p, hsa-let-7f-5p, hsa-let-7c-5p, hsa-let-7g-5p, and hsa-let-7i-5p, and hsa-miR-15a-5p, hsa-miR-423-5p, hsa-miR-26a-5p, and hsa-miR-139-5p are consistently downregulated in CRC, suggesting a tumor suppressive role [114,115,116,117,118,119]. Moreover, reduced expression of hsa-miR-26b-5p has been associated with poor clinical outcomes [120]. Functionally, these miRNAs are known to play diverse roles in CRC biology. For instance, hsa-miR-19b-3p and hsa-miR-196a-5p are known to promote metastasis, while miR-34a-5p and hsa-miR-16-5p are shown to suppress CRC cell proliferation, angiogenesis, and metastasis [121,122,123,124]. TFs identified in the network also play key roles in the initiation and progression of CRC by modulating expression of genes associated with proliferation, apoptosis, EMT, and metastasis. For instance, the transcription factor E2F1 has been shown to induce the expression of SP3 (specificity protein 3) in CRC cells [125]. Notably, the E2F1/SP3/STAT6 axis is integral to IL-4-mediated EMT and enhanced tumor aggressiveness, emphasizing its relevance as a prognostic indicator in CRC. SP1 (specificity protein 1) is markedly overexpressed in CRC and is associated with poor clinical outcomes [126]. TP53, one of the most commonly mutated tumor suppressors in CRC, frequently undergoes LOF alterations that compromise apoptotic signaling [127]. TFAP2A (transcription factor AP-2α) has demonstrated tumor-suppressive functions in CRC, capable of inducing cell cycle arrest and apoptosis through both TP53-dependent and -independent manner [128,129]. E2F3 is significantly overexpressed in CRC and its elevated expression correlates with poor overall survival in colon cancer patients [130]. Another TF, SRF (serum response factor), undergoes alternative splicing in human colon cancer cell lines and contributes to the tumor phenotype [131]. Overexpression of SRF promotes cell motility, invasiveness, and metastatic potential through modulation of the E-cadherin/β-catenin axis [132]. HMGA1 (high mobility group A1), a non-histone chromatin remodeling protein, is frequently upregulated in CRC and facilitates oncogenic metabolic alterations [133]. NFIC (nuclear factor I C) is a pro-tumorigenic regulator, which promotes NLRP3 inflammasome activation via CHPF-mediated MAPK signaling, thereby supporting CRC progression [134]. Further, TFDP1 (transcription factor Dp-1) promotes tumorigenesis and metastasis by positively regulating CKAP2, a protein implicated in microtubule stabilization and cell division [135]. YBX1 (Y-box binding protein 1), a multifunctional TF and RNA-binding protein, is associated with malignant progression, local recurrence, and poor prognosis in CRC tissues [136].
Although this study integrates multi-omics analysis to identify genes with prognostic and diagnostic significance in CRC, it remains an exploratory analysis. The findings from this study must be validated in independent cohorts, and future studies that incorporate patient metadata and functional validation are essential to elucidate the mechanistic roles of these hub genes and their use as clinical biomarkers.
5. Conclusions
By integrating expression analysis, co-expression networks, PPI network, survival analysis, and mutational profiling, we identified 128 prognostically significant hub genes, of which 23 genes show strong diagnostic potential. Collectively, our results provide valuable insight into CRC biology and offer promising candidates to develop prognostic and diagnostic biomarkers and therapeutic targeting. Future research should focus on experimental validation of the identified biomarkers in model systems or larger, independent clinical cohorts and assess their utility in clinical applications such as early detection, risk stratification, and prediction of treatment response. Functional assays and integration with epigenetic and proteomic data would help in determining the contribution of these biomarkers to CRC development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/onco5040050/s1, Supplementary Figure S1: Principal component analysis (PCA) sample clustering and outlier detection in (a) GSE4107 (b) GSE32323 (c) GSE21510, and (d) GSE24514. Outliers and samples falling in opposite group’s centroid are highlighted. Supplementary Figure S2: Volcano plots showing the distribution of differentially expressed genes (DEGs) in (a) GSE4107 (b) GSE32323 (c) GSE21510, and (d) GSE24514. Red dots represent upregulated genes, and green dots represent downregulated genes. Supplementary Figure S3: Heatmaps of top 100 DEGs in (a) GSE4107 (b) GSE32323 (c) GSE21510, and (d) GSE24514. Rows represent genes and columns represent individual samples. Red represents upregulated genes, and blue represents downregulated genes. Supplementary Figure S4: Identification of module eigengenes (MEs) associated with CRC using four GEO datasets. (a, c, e, g) Dendrograms of DEGs clustered by dissimilarity measure (1–TOM) in GSE4107, GSE32323, GSE21510, and GSE24514, respectively. (b, d, f, h) Heatmaps showing module-trait relationships for GSE4107, GSE32323, GSE21510, GSE24514, respectively. Each row denotes a color module, and each column represents a clinical trait (normal vs. cancer). Numbers within cells represent the correlation coefficients, with p-values shown in parentheses. Supplementary Figure S5: Kaplan–Meier survival analysis of 67 hub genes associated with poor overall survival, based on GEPIA2. p < 0.05 considered statistically significant. Supplementary Figure S6: Kaplan–Meier survival analysis of 61 hub genes associated with favorable overall survival, based on GEPIA2. p < 0.05 considered statistically significant. Supplementary Figure S7: Co-expression networks of hub genes associated with (a) poor prognosis and (b) favorable prognosis in CRC. Supplementary Figure S8: Promoter methylation analysis in cancerous vs. normal tissues in colon adenocarcinoma (COAD) and rectal adenocarcinoma (READ) for 18 genes showing significant differential methylation. Genes with differential promoter methylation were identified using the criteria: p-value < 0.05 and |Δβ| ≥ ±0.1. Supplementary Figure S9: Transcriptional regulatory networks of the hub genes, microRNAs (miRNAs), and transcription factors (TFs). Transcriptional regulatory networks are shown for (a) adverse prognosis and (b) favorable prognosis hub genes. Red inner nodes represent the hub genes, green central nodes represent the TFs, and yellow outermost nodes represented regulating miRNA. Interactions are represented by pink edges. Only the top 10 hub genes in each category are shown. Supplementary Table S1. List of common differentially expressed genes (DEGs) identified across the four GEO datasets (GSE4107, GSE32323, GSE21510, and GSE24514). The table includes both upregulated and downregulated genes consistently found in ≥3 datasets. Supplementary Table S2. List of 989 candidate hub genes identified across all four GEO datasets. Supplementary Table S3: Hub genes associated with favorable prognosis and interacting miRNA and transcription factors. Supplementary Table S4: Hub genes associated with worst prognosis and interacting miRNA and transcription factors.
Author Contributions
H.P. and D.L. conceptualized and designed the study; H.P. and D.L. carried out all bioinformatics analysis, D.L. wrote the manuscript; H.P. and D.L. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The GEO datasets used in this study can be accessed through the GEO database: https://www.ncbi.nlm.nih.gov/geo/ (accessed on 6 July 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Li, Y.; Wang, J. Incidence and mortality of colorectal cancer in Asia in 2022 and projections for 2050. J. Gastroenterol. Hepatol. 2025, 40, 1143–1156. [Google Scholar] [CrossRef]
- Pandey, H.; Tang, D.W.T.; Wong, S.H.; Lal, D. Gut microbiota in colorectal cancer: Biological role and therapeutic opportunities. Cancers 2023, 15, 866. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Moreno, V.; Hughes, D.J.; Vodicka, L.; Vodicka, P.; Aglago, E.K.; Gunter, M.J.; Jenab, M. Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol. Asp. Med. 2019, 69, 2–9. [Google Scholar] [CrossRef]
- Pandey, H.; Jain, D.; Tang, D.W.T.; Wong, S.H.; Lal, D. Gut microbiota in pathophysiology, diagnosis, and therapeutics of inflammatory bowel disease. Intest. Res. 2024, 22, 15–43. [Google Scholar] [CrossRef]
- Whiffin, N.; Hosking, F.J.; Farrington, S.M.; Palles, C.; Dobbins, S.E.; Zgaga, L.; Lloyd, A.; Ben Kinnersley, B.; Gorman, M.; Tenesa, A.; et al. Identification of susceptibility loci for colorectal cancer in a genome-wide meta-analysis. Hum. Mol. Genet. 2014, 23, 4729–4737. [Google Scholar] [CrossRef]
- Fidler, M.M.; Soerjomataram, I.; Bray, F. A global view on cancer incidence and national levels of the human development index. Int. J. Cancer 2016, 139, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Mirgayazova, R.; Khadiullina, R.; Mingaleeva, R.; Chasov, V.; Gomzikova, M.; Garanina, E.; Rizvanov, A.; Bulatov, E. Novel Isatin-based activator of p53 transcriptional functions in tumor cells. Mol. Biol. Res. Commun. 2019, 8, 119–128. [Google Scholar]
- Kamel, F.; Eltarhoni, K.; Nisar, P.; Soloviev, M. Colorectal cancer diagnosis: The obstacles we face in determining a non-invasive test and current advances in biomarker detection. Cancers 2022, 14, 1889. [Google Scholar] [CrossRef]
- D’Souza, N.; Georgiou Delisle, T.; Chen, M.; Benton, S.; Abulafi, M.; NICE FIT Steering Group. Faecal immunochemical test is superior to symptoms in predicting pathology in patients with suspected colorectal cancer symptoms referred on a 2WW pathway: A diagnostic accuracy study. Gut 2021, 70, 1130–1138. [Google Scholar] [CrossRef]
- Morgan, J.; Thomas, K.; Lee-Robichaud, H.; Nelson, R.L.; Braungart, S. Transparent cap colonoscopy versus standard colonoscopy to improve caecal intubation. Cochrane Database Syst. Rev. 2012, 12, CD008211. [Google Scholar] [CrossRef]
- Das, V.; Kalita, J.; Pal, M. Predictive and prognostic biomarkers in colorectal cancer: A systematic review of recent advances and challenges. Biomed. Pharmacother. 2017, 87, 8–19. [Google Scholar] [CrossRef]
- Chandrapalan, S.; Arasaradnam, R. Advantages and limitations of faecal immunochemical testing in colorectal cancer. Front. Gastroenterol. 2025, 16, 181–187. [Google Scholar] [CrossRef]
- Gong, C.; Rojas, M.T.M.; Guerrero, M.G.R.; Kladas, M.; Mousakhanian, A.; Sudan, A.; Johnson, A.; Cartmill, K.; Sydney, E.; Kotler, D.P. Fecal immunochemical testing for colorectal cancer prevention in two public hospitals. J. Gastrointest. Cancer 2025, 56, 69. [Google Scholar] [CrossRef] [PubMed]
- Nayor, J.; Saltzman, J.R. Colonoscopy quality: Measuring the patient experience. Endoscopy 2018, 50, 4–5. [Google Scholar] [PubMed]
- Nicholson, B.D.; Shinkins, B.; Pathiraja, I.; Roberts, N.W.; James, T.J.; Mallett, S.; Pereral, R.; Primrose, J.N.; Mant, D. Blood CEA levels for detecting recurrent colorectal cancer. Cochrane Database Syst. Rev. 2015, 2015, CD011134. [Google Scholar] [CrossRef]
- Lu, H.; Li, L.; Sun, D.; Duan, Y.; Yue, K.; Wu, Y.; Wang, X. Identification of novel hub genes associated with lymph node metastasis of head and neck squamous cell carcinoma by completive bioinformatics analysis. Ann. Transl. Med. 2021, 9, 1678. [Google Scholar] [CrossRef]
- Huang, R.; Liu, J.; Li, H.; Zheng, L.; Jin, H.; Zhang, Y.; Ma, W.; Su, J.; Wang, M.; Yang, K. Identification of hub genes and their correlation with immune infiltration cells in hepatocellular carcinoma based on GEO and TCGA databases. Front. Genet. 2021, 12, 647353. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Y.; Shao, S.; Sun, Y.; Lin, Z. Identification of hub genes and biological pathways in hepatocellular carcinoma by integrated bioinformatics analysis. PeerJ 2021, 9, e10594. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets--update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsso, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 2013, 6, pl1. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovov, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef]
- Ru, Y.; Kechris, K.J.; Tabakoff, B.; Hoffman, P.; Radcliffe, R.A.; Bowler, R.; Mahaffey, S.; Rossi, S.; Calin, G.A.; Bemis, L.; et al. The multiMiR R package and database: Integration of microRNA-target interactions along with their disease and drug associations. Nucleic Acids Res. 2014, 42, e133. [Google Scholar] [CrossRef]
- Han, H.; Shim, H.; Shin, D.; Shim, J.E.; Ko, Y.; Shin, J.; Kim, H.; Cho, A.; Kim, E.; Lee, T.; et al. TRRUST: A reference database of human transcriptional regulatory interactions. Sci. Rep. 2015, 5, 11432. [Google Scholar] [CrossRef]
- Ding, X.; Duan, H.; Luo, H. Identification of core gene expression signature and key pathways in colorectal cancer. Front. Genet. 2020, 11, 45. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wang, X.; Yang, Q. CDK1 and CDC20 overexpression in patients with colorectal cancer are associated with poor prognosis: Evidence from integrated bioinformatics analysis. World J. Surg. Oncol. 2020, 18, 50. [Google Scholar] [CrossRef]
- Li, W.H.; Zhang, L.; Wu, Y.H. CDKN3 regulates cisplatin resistance to colorectal cancer through TIPE1. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3614–3623. [Google Scholar]
- Paolillo, M.; Schinelli, S. Extracellular matrix alterations in metastatic processes. Int. J. Mol. Sci. 2019, 20, 4947. [Google Scholar] [CrossRef]
- Salimian, N.; Peymani, M.; Ghaedi, K.; Hashemi, M.; Rahimi, E. Collagen 1A1 (COL1A1) and Collagen11A1(COL11A1) as diagnostic biomarkers in breast, colorectal and gastric cancers. Gene 2024, 892, 147867. [Google Scholar] [CrossRef]
- Wu, D.; Wu, P.; Huang, Q.; Liu, Y.; Ye, J.; Huang, J. Interleukin-17: A promoter in colorectal cancer progression. Clin. Dev. Immunol. 2013, 2013, 436307. [Google Scholar] [CrossRef]
- Bahrami, A.; Khalaji, A.; Najafi, M.B.; Sadati, S.; Raisi, A.; Abolhassani, A.; Eshraghi, R.; Mahabady, M.K.; Rahimian, N.; Mirzaei, H. NF-κB pathway and angiogenesis: Insights into colorectal cancer development and therapeutic targets. Eur. J. Med. Res. 2024, 29, 610. [Google Scholar] [CrossRef]
- Rezaei, Z.; Ranjbaran, J.; Safarpour, H.; Nomiri, S.; Salmani, F.; Chamani, E.; Larki, P.; Brunetti, O.; Silvestris, N.; Tavakoli, T. Identification of early diagnostic biomarkers via WGCNA in gastric cancer. Biomed. Pharmacother. 2022, 145, 112477. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Safarzadeh, A.; Taheri, M.; Jamali, E. Identification of diagnostic biomarkers via weighted correlation network analysis in colorectal cancer using a system biology approach. Sci. Rep. 2023, 13, 13637. [Google Scholar] [CrossRef]
- Lv, J.-H.; Hou, A.-J.; Zhang, S.-H.; Dong, J.-J.; Kuang, H.-X.; Yang, L.; Jiang, H. WGCNA combined with machine learning to find potential biomarkers of liver cancer. Medicine 2023, 102, e36536. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V.; Yamada, H.Y. Genomic instability and colon carcinogenesis: From the perspective of genes. Front. Oncol. 2013, 3, 130. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.S.; Chung, D.C. The chromosomal instability pathway in colon cancer. Gastroenterology 2010, 138, 2059–2072. [Google Scholar] [CrossRef] [PubMed]
- Diogo, V.; Teixeira, J.; Silva, P.M.; Bousbaa, H. Spindle assembly checkpoint as a potential target in colorectal cancer: Current status and future perspectives. Clin. Color. Cancer 2017, 16, 1–8. [Google Scholar] [CrossRef]
- Burrell, R.A.; McClelland, S.E.; Endesfelder, D.; Groth, P.; Weller, M.-C.; Shaikh, N.; Domingo, E.; Kanu, N.; Dewhurst, S.M.; Gronroos, E.; et al. Replication stress links structural and numerical cancer chromosomal instability. Nature 2013, 494, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Coschi, C.H.; Dick, F.A. Chromosome instability and deregulated proliferation: An unavoidable duo. Cell Mol. Life Sci. 2012, 69, 2009–2024. [Google Scholar] [CrossRef] [PubMed]
- Grady, W.M.; Carethers, J.M. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology 2008, 135, 1079–1099. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhu, L.; Liu, C.; Zhou, D.; Zhu, Z.; Xu, N.; Li, W. Current status and prospect of the DNA double-strand break repair pathway in colorectal cancer development and treatment. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167438. [Google Scholar] [CrossRef]
- Xia, W.; Gao, Z.; Jiang, X.; Jiang, L.; Qin, Y.; Zhang, D.; Tian, P.; Wang, W.; Zhang, Q.; Zhang, R.; et al. Alzheimer’s risk factor FERMT2 promotes the progression of colorectal carcinoma via Wnt/β-catenin signaling pathway and contributes to the negative correlation between Alzheimer and cancer. PLoS ONE 2022, 17, e0278774. [Google Scholar] [CrossRef]
- Sinkala, M. Mutational landscape of cancer-driver genes across human cancers. Sci. Rep. 2023, 13, 12742. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Li, X.; Yi, S.; Xu, J. Gain-of-Function mutations: An emerging advantage for cancer biology. Trends Biochem. Sci. 2019, 44, 659–674. [Google Scholar] [CrossRef]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020, 577, 549–555. [Google Scholar] [CrossRef]
- Fridman, W.H.; Galon, J.; Dieu-Nosjean, M.-C.; Cremer, I.; Fisson, S.; Damotte, D.; Pagès, F.; Tartour, E.; Sautès-Fridman, C. Immune infiltration in human cancer: Prognostic significance and disease control. Curr. Top. Microbiol. Immunol. 2011, 344, 1–24. [Google Scholar]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-associated macrophages in tumor immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Zhao, Y.; Chen, J.; Lin, Y.; Qi, X. Tumor-associated macrophages in colorectal cancer metastasis: Molecular insights and translational perspectives. J. Transl. Med. 2024, 22, 62. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Wei, H.; Liu, Y.; Li, N. Mast cells in colorectal cancer tumour progression, angiogenesis, and lymphangiogenesis. Front. Immunol. 2023, 14, 1209056. [Google Scholar] [CrossRef]
- Gulubova, M.V.; Ananiev, J.R.; Vlaykova, T.I.; Yovchev, Y.; Tsoneva, V.; Manolova, I.M. Role of dendritic cells in progression and clinical outcome of colon cancer. Int. J. Color. Dis. 2012, 27, 159–169. [Google Scholar] [CrossRef]
- Dadabayev, A.R.; Sandel, M.H.; Menon, A.G.; Morreau, H.; Melief, C.J.M.; Offringa, R.; van der Burg, S.H.; Rhijn, C.J.-V.; Ensink, N.G.; Tollenaar, R.A.; et al. Dendritic cells in colorectal cancer correlate with other tumor-infiltrating immune cells. Cancer Immunol. Immunother. 2004, 53, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Pryczynicz, A.; Cepowicz, D.; Zaręba, K.; Gryko, M.; Hołody-Zaręba, J.; Kędra, B.; Kemona, A.; Guzińska-Ustymowicz, K. Dysfunctions in the mature dendritic cells are associated with the presence of metastases of colorectal cancer in the surrounding lymph nodes. Gastroenterol. Res. Pract. 2016, 2016, 2405437. [Google Scholar] [CrossRef]
- Saleh, R.; Elkord, E. FoxP3+ T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett. 2020, 490, 174–185. [Google Scholar] [CrossRef]
- Hu, G.; Li, Z.; Wang, S. Tumor-infiltrating FoxP3+ Tregs predict favorable outcome in colorectal cancer patients: A meta-analysis. Oncotarget 2017, 8, 75361–75371. [Google Scholar] [CrossRef]
- Stein, U.; Walther, W.; Arlt, F.; Schwabe, H.; Smith, J.; Fichtner, I.; Birchmeier, W.; Schlag, P.M. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat. Med. 2009, 15, 59–67. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.X.; Wen, J.G.; Zhou, H.H. Metastasis-associated in colon cancer 1: A promising biomarker for the metastasis and prognosis of colorectal cancer. Oncol. Lett. 2017, 14, 3899–3908. [Google Scholar] [CrossRef]
- Schöpe, P.C.; Torke, S.; Kobelt, D.; Kortüm, B.; Treese, C.; Dumbani, M.; Güllü, N.; Walther, W.; Stein, U. MACC1 revisited—An in-depth review of a master of metastasis. Biomark. Res. 2024, 12, 146. [Google Scholar] [CrossRef]
- Zhao, Y.; Dai, C.; Wang, M.; Kang, H.; Lin, S.; Yang, P.; Liu, X.; Liu, K.; Xu, P.; Zheng, Y.; et al. Clinicopathological and prognostic significance of metastasis-associated in colon cancer-1 (MACC1) overexpression in colorectal cancer: A meta-analysis. Oncotarget 2016, 7, 62966–62975. [Google Scholar] [CrossRef] [PubMed]
- Vuaroqueaux, V.; Musch, A.; Kobelt, D.; Risch, T.; Herrmann, P.; Burock, S.; Peille, A.-L.; Yaspo, M.-L.; Fiebig, H.-H.; Stein, U. Elevated MACC1 expression in colorectal cancer is driven by chromosomal instability and is associated with molecular subtype and worse patient survival. Cancers 2022, 14, 1749. [Google Scholar] [CrossRef]
- Takada, T.; Tsutsumi, S.; Takahashi, R.; Ohsone, K.; Tatsuki, H.; Suto, T.; Kato, T.; Fujii, T.; Yokobori, T.; Kuwano, H. KPNA2 over-expression is a potential marker of prognosis and therapeutic sensitivity in colorectal cancer patients. J. Surg. Oncol. 2016, 113, 213–217. [Google Scholar] [CrossRef]
- Yu, L.; Wang, G.; Zhang, Q.; Gao, L.; Huang, R.; Chen, Y.; Tang, Q.; Liu, J.; Liu, C.; Wang, H.; et al. Karyopherin alpha 2 expression is a novel diagnostic and prognostic factor for colorectal cancer. Oncol. Lett. 2017, 13, 1194–1200. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Dong, Y.; Ning, P.; Zhang, Y.; Sun, H.; Li, G. Knockdown of AURKA sensitizes the efficacy of radiation in human colorectal cancer. Life Sci. 2021, 271, 119148. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.; Bosch, L.J.W.; Kemp, S.R.M.-D.; Carvalho, B.; Sillars-Hardebol, A.H.; Dobson, R.J.; de Rinaldis, E.; Meijer, G.A.; Abeln, S.; Heringa, J.; et al. Aurora kinase A (AURKA) interaction with Wnt and Ras-MAPK signalling pathways in colorectal cancer. Sci. Rep. 2018, 8, 7522. [Google Scholar] [CrossRef]
- Rio-Vilariño, A.; Cenigaonandia-Campillo, A.; García-Bautista, A.; Mateos-Gómez, P.A.; Schlaepfer, M.I.; del Puerto-Nevado, L.; Aguilera, O.; García-García, L.; Galeano, C.; de Miguel, I.; et al. Inhibition of the AURKA/YAP1 axis is a promising therapeutic option for overcoming cetuximab resistance in colorectal cancer stem cells. Br. J. Cancer 2024, 130, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-P.; Yin, Y.-X.; Xie, M.-Z.; Liang, X.-Q.; Li, J.-L.; Li, K.-Z.; Hu, B.-L. Systematic analysis of the clinical significance of hyaluronan-mediated motility receptor in colorectal cancer. Front. Mol. Biosci. 2021, 8, 733271. [Google Scholar] [CrossRef]
- Chen, X.; Dai, Z.; Wang, Q.; Chen, W.; Liu, Y.; Wang, Z. NUDT21 functions as a pro-tumorigenic gene in colorectal cancer by upregulating the TAZ protein expression. Biocell 2025, 49, 503–518. [Google Scholar] [CrossRef]
- Laurent, E.; McCoy, J.W.; Macina, R.A.; Liu, W.; Cheng, G.; Robine, S.; Papkoff, J.; Lambeth, J.D. Nox1 is over-expressed in human colon cancers and correlates with activating mutations in K-Ras. Int. J. Cancer 2008, 123, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Ohata, H.; Shiokawa, D.; Obata, Y.; Sato, A.; Sakai, H.; Fukami, M.; Hara, W.; Taniguchi, H.; Ono, M.; Nakagama, H.; et al. NOX1-dependent mTORC1 activation via S100A9 oxidation in cancer stem-like cells leads to colon cancer progression. Cell Rep. 2019, 28, 1282–1295.e8. [Google Scholar] [CrossRef]
- Juhasz, A.; Markel, S.; Gaur, S.; Liu, H.; Lu, J.; Jiang, G.; Wu, X.; Antony, S.; Wu, Y.; Melillo, G.; et al. NADPH oxidase 1 supports proliferation of colon cancer cells by modulating reactive oxygen species-dependent signal transduction. J. Biol. Chem. 2017, 292, 7866–7887. [Google Scholar] [CrossRef]
- Stalin, J.; Garrido-Urbani, S.; Heitz, F.; Szyndralewiez, C.; Jemelin, S.; Coquoz, O.; Ruegg, C.; Imhof, B.A. Inhibition of host NOX1 blocks tumor growth and enhances checkpoint inhibitor-based immunotherapy. Life Sci. Alliance 2019, 2, e201800265. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, Y.; Zhang, T.; Wang, Z.; Gong, J.; Du, Z.; MeI, Y.; Ma, J. TRUB1 is a novel biomarker for promoting malignancy in colorectal cancer via NFκB signaling. Gastroenterol. Rep. 2025, 13, goaf027. [Google Scholar] [CrossRef]
- Tao, K.; Yang, J.; Hu, Y.; Deng, A. Knockdown of YEATS4 inhibits colorectal cancer cell proliferation and induces apoptosis. Am. J. Transl. Res. 2015, 7, 616–623. [Google Scholar] [PubMed]
- Fu, Q.; Cheng, J.; Zhang, J.; Zhang, Y.; Chen, X.; Xie, J.; Luo, S. Downregulation of YEATS4 by miR-218 sensitizes colorectal cancer cells to L-OHP-induced cell apoptosis by inhibiting cytoprotective autophagy. Oncol. Rep. 2016, 36, 3682–3690. [Google Scholar] [CrossRef] [PubMed]
- Branchi, V.; García, S.A.; Radhakrishnan, P.; Győrffy, B.; Hissa, B.; Schneider, M.; Reißfelder, C.; Schölch, S. Prognostic value of DLGAP5 in colorectal cancer. Int. J. Color. Dis. 2019, 34, 1455–1465. [Google Scholar] [CrossRef]
- Lee, D.H.; Jeong, Y.J.; Won, J.Y.; Sim, H.I.; Park, Y.; Jin, H.S. PBK/TOPK is a favorable prognostic biomarker correlated with antitumor immunity in colon cancers. Biomedicines 2022, 10, 299. [Google Scholar] [CrossRef]
- Koshino, A.; Nagano, A.; Ota, A.; Hyodo, T.; Ueki, A.; Komura, M.; Sugimura-Nagata, A.; Ebi, M.; Ogasawara, N.; Kasai, K.; et al. PBK enhances cellular proliferation with histone H3 phosphorylation and suppresses migration and invasion with CDH1 stabilization in colorectal cancer. Front. Pharmacol. 2022, 12, 772926. [Google Scholar] [CrossRef]
- Li, Y.; Gao, W.; Yang, Z.; Hu, Z.; Li, J. Multi-omics pan-cancer analyses identify MCM4 as a promising prognostic and diagnostic biomarker. Sci. Rep. 2024, 14, 6517. [Google Scholar] [CrossRef]
- Ye, F.; Xie, Y.; Lin, M.; Liu, Y.; Fang, Y.; Chen, K.; Zhang, Y.; Ding, Y. KIAA1549 promotes the development and chemoresistance of colorectal cancer by upregulating ERCC2. Mol. Cell Biochem. 2024, 479, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ge, C.; Fang, D.; Wei, W.; Li, L.; Wei, Q.; Yu, H. NCAPG facilitates colorectal cancer cell proliferation, migration, invasion and epithelial-mesenchymal transition by activating the Wnt/β-catenin signaling pathway. Cancer Cell Int. 2022, 22, 119. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Pu, L.; Li, R.; Zhu, R. NCAPG is transcriptionally regulated by CBX3 and activates the Wnt/β-catenin signaling pathway to promote proliferation and the cell cycle and inhibit apoptosis in colorectal cancer. J. Gastrointest. Oncol. 2023, 14, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.P.; Wu, C.C.; Chou, C.L.; Cheng, L.C.; Wang, W.C.; Lin, S.S.; Hung, S.T.; Tian, Y.F.; Fang, C.L.; Lin, K.Y. NCAPG deregulation indicates poor patient survival and contributes to colorectal carcinogenesis. Pathol. Res. Pract. 2023, 241, 154238. [Google Scholar] [CrossRef]
- Fang, Q.; Lin, J.; Gao, L.; Pan, R.; Zheng, X. Targeting mitochondrial tyrosyl-tRNA synthetase YARS2 suppresses colorectal cancer progression. Cancer Biol. Ther. 2022, 23, 1–8. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X. Identification of m6A-related biomarkers associated with prognosis of colorectal cancer. Med. Sci. Monit. 2021, 27, e932370. [Google Scholar] [CrossRef]
- Tang, X.; Zha, L.; Li, H.; Liao, G.; Huang, Z.; Peng, X.; Wang, Z. Upregulation of GNL3 expression promotes colon cancer cell proliferation, migration, invasion and epithelial-mesenchymal transition via the Wnt/β-catenin signaling pathway. Oncol. Rep. 2017, 38, 2023–2032. [Google Scholar] [CrossRef]
- Shi, D.; Wang, J.; Deng, Q.; Kong, X.; Dong, Y.; Yang, Y.; Xu, Y.; Ling, L.; Jiao, Y.; Yu, S. KIF15 knockdown inhibits colorectal cancer proliferation and migration through affecting the ubiquitination modification of NRAS. Am. J. Cancer Res. 2023, 13, 4944–4960. [Google Scholar]
- Liao, Q.; Ren, Y.; Yang, Y.; Zhu, X.; Zhi, Y.; Zhang, Y.; Chen, Y.; Ding, Y.; Zhao, L. CCT8 recovers WTp53-suppressed cell cycle evolution and EMT to promote colorectal cancer progression. Oncogenesis 2021, 10, 84. [Google Scholar] [CrossRef]
- Teng, Y.; Lin, H.; Lin, Z.; Li, X.; Ruan, Y.; Pan, B.; Ge, J.; Zhu, Y.; Lin, D.; Ying, Q.; et al. CCT8 drives colorectal cancer progression via the RPL4-MDM2-p53 axis and immune modulation. BMC Med. Genom. 2025, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, A.R.; Choi, K.; Joung, S.; Yoon, J.B.; Kim, S. TOMM20 as a potential therapeutic target of colorectal cancer. BMB Rep. 2019, 52, 712–717. [Google Scholar] [CrossRef]
- Bradley, C.A.; Dunne, P.D.; Bingham, V.; McQuaid, S.; Khawaja, H.; Craig, S.; James, J.; Moore, W.L.; McArt, D.G.; Lawler, M.; et al. Transcriptional upregulation of c-MET is associated with invasion and tumor budding in colorectal cancer. Oncotarget 2016, 7, 78932–78945. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, J.; Park, S.H.; Park, J.O.; Lim, H.Y.; Kang, W.K.; Park, Y.S.; Kim, S.T. c-MET overexpression in colorectal cancer: A poor prognostic factor for survival. Clin. Color. Cancer 2018, 17, 165–169. [Google Scholar] [CrossRef]
- Esteller, M. Epigenetics in cancer. N. Engl. J. Med. 2008, 358, 1148–1159. [Google Scholar] [CrossRef]
- Jones, P.A.; Baylin, S.B. The epigenomics of cancer. Cell 2007, 128, 683–692. [Google Scholar] [CrossRef]
- Brait, M.; Ling, S.; Nagpal, J.K.; Chang, X.; Park, H.L.; Lee, J.; Okamura, J.; Yamashita, K.; Sidransky, D.; Kim, M.S. Cysteine dioxygenase 1 is a tumor suppressor gene silenced by promoter methylation in multiple human cancers. PLoS ONE 2012, 7, e44951. [Google Scholar] [CrossRef] [PubMed]
- A Greco, S.; Chia, J.; Inglis, K.J.; Cozzi, S.-J.; Ramsnes, I.; Buttenshaw, R.L.; Spring, K.J.; Boyle, G.M.; Worthley, D.L.; A Leggett, B.; et al. Thrombospondin-4 is a putative tumour-suppressor gene in colorectal cancer that exhibits age-related methylation. BMC Cancer 2010, 10, 494. [Google Scholar] [CrossRef]
- Zhang, K.; Zhai, Z.; Yu, S.; Tao, Y. DNA methylation mediated down-regulation of ANGPTL4 promotes colorectal cancer metastasis by activating the ERK pathway. J. Cancer 2021, 12, 5473–5485. [Google Scholar] [CrossRef] [PubMed]
- Boughanem, H.; Pilo, J.; García-Flores, L.A.; Arranz, I.; Ramos-Fernandez, M.; Ortega-Castan, M.; Crujeiras, A.B.; Sandoval, J.; Macias-Gonzalez, M. Identification of epigenetic silencing of the SFRP2 gene in colorectal cancer as a clinical biomarker and molecular significance. J. Transl. Med. 2024, 22, 509. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Park, G.; Lee, J.E.; Park, J.Y.; Kim, T.-H.; Kim, Y.-J.; Lee, S.-H.; Yoo, H.; Kim, J.H.; Park, J.B. CPEB1 modulates differentiation of glioma stem cells via downregulation of HES1 and SIRT1 expression. Oncotarget 2014, 5, 6756–6769. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Hussen, B.M.; Abdullah, S.R.; Dadyar, M.; Taheri, M.; Kiani, A. A review on the role of HAND2-AS1 in cancer. Clin. Exp. Med. 2023, 23, 3179–3188. [Google Scholar] [CrossRef] [PubMed]
- Okochi-Takada, E.; Hattori, N.; Tsukamoto, T.; Miyamoto, K.; Ando, T.; Ito, S.; Yamamura, Y.; Wakabayashi, M.; Nobeyama, Y.; Ushijima, T. ANGPTL4 is a secreted tumor suppressor that inhibits angiogenesis. Oncogene 2014, 33, 2273–2278. [Google Scholar] [CrossRef]
- Ehrlich, M. DNA hypomethylation in cancer cells. Epigenomics 2009, 1, 239–359. [Google Scholar] [CrossRef]
- Xu, Y.; Qu, H.; Liang, R.; Li, M.; Li, M.; Li, X.; Wang, Z. A multi-gene blood-based methylation assay for early diagnosis of colorectal cancer. Transl. Cancer Res. 2024, 13, 6699–6708. [Google Scholar] [CrossRef]
- Hasakova, K.; Reis, R.; Vician, M.; Zeman, M.; Herichova, I. Expression of miR-34a-5p is up-regulated in human colorectal cancer and correlates with survival and clock gene PER2 expression. PLoS ONE 2019, 14, e0224396. [Google Scholar] [CrossRef]
- Chacon-Millan, P.; Lama, S.; Del Gaudio, N.; Gravina, A.G.; Federico, A.; Pellegrino, R.; Luce, A.; Altucci, L.; Facchiano, A.; Caraglia, M.; et al. A combination of microarray-based profiling and biocomputational analysis identified miR331-3p and hsa-let-7d-5p as potential biomarkers of ulcerative colitis progression to colorectal cancer. Int. J. Mol. Sci. 2024, 25, 5699. [Google Scholar] [CrossRef]
- Yu, F.B.; Sheng, J.; Yu, J.M.; Liu, J.H.; Qin, X.X.; Mou, B. MiR-19a-3p regulates the Forkhead box F2-mediated Wnt/β-catenin signaling pathway and affects the biological functions of colorectal cancer cells. World J. Gastroenterol. 2020, 26, 627–644. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, X. MiR-103a-3p contributes to the progression of colorectal cancer by regulating GREM2 expression. Yonsei Med. J. 2022, 63, 520–529. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Liu, J.; Gu, D.; Shi, X. Correlation between microRNA-107 expression level and prognosis in patients with colorectal cancer. Int. J. Clin. Exp. Pathol. 2020, 13, 2342–2347. [Google Scholar]
- Yu, W.; Wang, J.; Li, C.; Xuan, M.; Han, S.; Zhang, Y.; Liu, P.; Zhao, Z. miR-17-5p promotes the invasion and migration of colorectal cancer by regulating HSPB2. J. Cancer 2022, 13, 918–931. [Google Scholar] [CrossRef]
- Su, C.; Huang, D.P.; Liu, J.W.; Liu, W.Y.; Cao, Y.O. miR-27a-3p regulates proliferation and apoptosis of colon cancer cells by potentially targeting BTG1. Oncol. Lett. 2019, 18, 2825–2834. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, R.; Mosakhani, N.; Sarhadi, V.K.; Armengol, G.; Nouraee, N.; Mohammadkhani, A.; Khorrami, S.; Arefian, E.; Paryan, M.; Malekzadeh, R.; et al. Simultaneous underexpression of let-7a-5p and let-7f-5p microRNAs in plasma and stool samples from early stage colorectal carcinoma. Biomark. Cancer 2016, 7, 39–48. [Google Scholar] [CrossRef]
- Niculae, A.M.; Dobre, M.; Herlea, V.; Manuc, T.E.; Trandafir, B.; Milanesi, E.; Hinescu, M.E. Let-7 microRNAs are possibly associated with perineural invasion in colorectal cancer by targeting IGF axis. Life 2022, 12, 1638. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Z.; Wang, Y.; Wang, Y.; Li, W.; Wang, Z.; Zhou, X.; Bao, Y. hsa-miR-15a-5p inhibits colon cell carcinoma via targeting CCND1. Mol. Med. Rep. 2021, 24, 735. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, L.; Zhu, Z.; Gao, W.; Li, D.; Zhou, Z.; Chen, L.; Fu, C.-G. Downregulation of miR-423-5p contributes to the radioresistance in colorectal cancer cells. Front. Oncol. 2021, 10, 582239. [Google Scholar] [CrossRef]
- Ghanbari, R.; Mosakhani, N.; Asadi, J.; Nouraee, N.; Mowla, S.J.; Yazdani, Y.; Mohamadkhani, A.; Poustchi, H.; Knuutila, S.; Malekzadeh, R. Downregulation of Plasma MiR-142-3p and MiR-26a-5p in patients with colorectal carcinoma. Iran. J. Cancer Prev. 2015, 8, e2329. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Yin, Y.; Zhang, J.; Zhang, B.; Bian, Z.; Quan, C.; Zhou, L.; Hu, Y.; Wang, Q.; Ni, S.; et al. MiR-139-5p inhibits migration and invasion of colorectal cancer by downregulating AMFR and NOTCH1. Protein Cell 2014, 5, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Gharib, E.; Rejali, L.; Piroozkhah, M.; Zonoobi, E.; Nasrabadi, P.N.; Arabsorkhi, Z.; Baghdar, K.; Shams, E.; Sadeghi, A.; Kuppen, P.J.K.; et al. IL-2RG as a possible immunotherapeutic target in CRC predicting poor prognosis and regulated by miR-7-5p and miR-26b-5p. J. Transl. Med. 2024, 22, 439. [Google Scholar] [CrossRef]
- Gao, J.; Li, N.; Dong, Y.; Li, S.; Xu, L.; Li, X.; Li, Y.; Li, Z.; Ng, S.S.; Sung, J.J.; et al. miR-34a-5p suppresses colorectal cancer metastasis and predicts recurrence in patients with stage II/III colorectal cancer. Oncogene 2015, 34, 4142–4152. [Google Scholar] [CrossRef]
- Huang, L.; Cai, J.L.; Huang, P.Z.; Kang, L.; Huang, M.J.; Wang, L.; Wang, J.P. miR19b-3p promotes the growth and metastasis of colorectal cancer via directly targeting ITGB8. Am. J. Cancer Res. 2017, 7, 1996–2008. [Google Scholar]
- Xin, H.; Wang, C.; Liu, Z. miR-196a-5p promotes metastasis of colorectal cancer via targeting IκBα. BMC Cancer 2019, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xu, X.; Ke, H.; Pan, X.; Ai, J.; Xie, R.; Lan, G.; Hu, Y.; Wu, Y. microRNA-16-5p suppresses cell proliferation and angiogenesis in colorectal cancer by negatively regulating forkhead box K1 to block the PI3K/Akt/mTOR pathway. Eur. J. Histochem. 2022, 66, 3333. [Google Scholar] [CrossRef]
- Chen, J.; Gong, C.; Mao, H.; Li, Z.; Fang, Z.; Chen, Q.; Lin, M.; Jiang, X.; Hu, Y.; Wang, W.; et al. E2F1/SP3/STAT6 axis is required for IL-4-induced epithelial-mesenchymal transition of colorectal cancer cells. Int. J. Oncol. 2018, 53, 567–578. [Google Scholar] [CrossRef]
- Bajpai, R.; Nagaraju, G.P. Specificity protein 1: Its role in colorectal cancer progression and metastasis. Crit. Rev. Oncol. Hematol. 2017, 113, 1–7. [Google Scholar] [CrossRef]
- Liebl, M.C.; Hofmann, T.G. The role of p53 signaling in colorectal cancer. Cancers 2021, 13, 2125. [Google Scholar] [CrossRef]
- Wajapeyee, N.; Somasundaram, K. Cell cycle arrest and apoptosis induction by activator protein 2alpha (AP-2alpha) and the role of p53 and p21WAF1/CIP1 in AP-2alpha-mediated growth inhibition. J. Biol. Chem. 2003, 278, 52093–52101. [Google Scholar] [CrossRef]
- McPherson, L.A.; Loktev, A.V.; Weigel, R.J. Tumor suppressor activity of AP2alpha mediated through a direct interaction with p53. J. Biol. Chem. 2002, 277, 45028–45033. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Qu, H.; Ren, Y.; Gong, Z.; Ri, H.J.; Zhang, F.; Shao, S.; Chen, X.; Chen, X. Systematic analysis of E2F expression and its relation in colorectal cancer prognosis. Int. J. Gen. Med. 2022, 15, 4849–4870. [Google Scholar] [CrossRef] [PubMed]
- Patten, L.C.; Belaguli, N.S.; Baek, M.J.; Fagan, S.P.; Awad, S.S.; Berger, D.H. Serum response factor is alternatively spliced in human colon cancer. J. Surg. Res. 2004, 121, 92–100. [Google Scholar] [CrossRef]
- Choi, H.N.; Kim, K.R.; Lee, J.H.; Park, H.S.; Jang, K.Y.; Chung, M.J.; Hwang, S.E.; Yu, H.C.; Moon, W.S. Serum response factor enhances liver metastasis of colorectal carcinoma via alteration of the E-cadherin/beta-catenin complex. Oncol. Rep. 2009, 21, 57–63. [Google Scholar] [PubMed]
- Williams, M.D.; Zhang, X.; Belton, A.S.; Xian, L.; Huso, T.; Park, J.-J.; Siems, W.F.; Gang, D.R.; Resar, L.M.S.; Reeves, R.; et al. HMGA1 drives metabolic reprogramming of intestinal epithelium during hyperproliferation, polyposis, and colorectal carcinogenesis. J. Proteome Res. 2015, 14, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, M.; Zhang, Y.; Liu, G.; Xing, Y. Activation of CHPF by transcription factor NFIC promotes NLRP3 activation during the progression of colorectal cancer. Funct. Integr. Genom. 2024, 24, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Cheng, S.; Liu, Y. CKAP2 regulated by TFDP1 promotes metastasis and proliferation of colorectal cancer through affecting the tumor microenvironment. J. Microbiol. Biotechnol. 2024, 34, 2211–2222. [Google Scholar] [CrossRef]
- Yan, X.; Yan, L.; Zhou, J.; Liu, S.; Shan, Z.; Jiang, C.; Tian, Y.; Jin, Z. High expression of Y-box-binding protein 1 is associated with local recurrence and predicts poor outcome in patients with colorectal cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 8715–8723. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).