Abstract
Gallbladder cancer (GBC), an aggressive malignancy of the biliary tract, is characterized by pronounced geographical variation and a poor prognosis, with a five-year survival rate below 20%. Despite its low global incidence, it ranks as the fifth most prevalent gastrointestinal cancer. The aim of this review is to provide a comprehensive understanding of the molecular mechanisms underpinning GBC progression, with a particular focus on the pivotal role of transcription factors (TFs) in its pathogenesis. This review delineates how aberrant regulation of TFs contributes to tumor initiation, progression, and therapeutic resistance, and to discuss the translational potential of targeting these factors for clinical benefit. Tumor suppressor TFs such as p53 and p16 frequently undergo genetic alterations, including mutations, deletions, or epigenetic silencing, leading to impaired cell cycle control, DNA repair, and apoptosis. Conversely, oncogenic TFs including TCF4, MYBL2, NF-kB, AP-1, Snail, c-MYC, SP1, FOXK1, KLF-5, STAT3 and BIRC7 are often upregulated in GBC, promoting unchecked proliferation, epithelial–mesenchymal transition (EMT), metastasis, and therapeutic resistance. This review aims to bridge current molecular insights with emerging therapeutic approaches, with particular emphasis on innovative interventions such as proteolysis-targeting chimeras (PROTACs), RNA-based therapeutics, CRISPR-driven genome editing, and epigenetic modulators, which collectively represent promising strategies for achieving more effective and personalized treatment outcomes in patients with GBC.
1. Introduction
Gallbladder cancer (GBC), a malignancy of the biliary tract, remains a formidable challenge in oncology due to its aggressive nature, late diagnosis, and limited treatment options. It exhibits a striking geographical variation in incidence, underscoring its complex etiology and regional risk factors. Globally, GBC is associated with a dismal prognosis and its association with a high mortality rate, with a median five-year overall survival rate of 5% [1]. The reported number of new cases of GBC worldwide in 2022 was 122,462 with 89,031 death cases, as per Globocan, 2022 [2]. In India, GBC is highly prevalent, accounting for nearly 10% of the global disease burden [3]. There are notable regional variations, with significantly higher incidence rates in the northern and northeastern regions than in the southern states of India. Despite continued research, the underlying molecular mechanisms for GBC pathogenesis in the Indian population remain largely unexplored [4]. The development of GBC is multifactorial, involving a complex interplay of genetic predisposition, chronic inflammation, gallstone disease or cholelithiasis (especially when left untreated), infections (such as Helicobacter or Salmonella species), and lifestyle choices, which all work together to increase the likelihood of GBC tumorigenesis. Central to this pathogenesis is the dysregulation of transcription factors (TFs), key regulators of gene expression. As essential conductors of gene expression, these TFs regulate a broad variety of cellular processes including proliferation, differentiation, DNA repair, immune response and programmed cell death (Figure 1). Disruption in TF activity is a common molecular event across many cancers, including GBC, and contributes to aberrant transcriptional programs of the downstream cellular programs that drive tumorigenesis. An understanding of the altered expression of these upstream regulatory factors may shed light on the core causes of GBC malignancy [5,6].
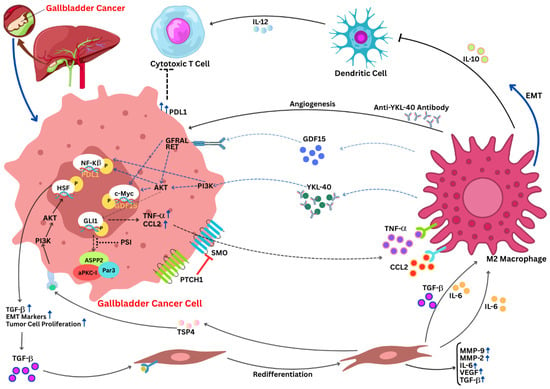
Figure 1.
Oncogenic pathway in gallbladder cancer: an interplay. The tumor microenvironment and signaling factors have an interconnection, and they play in a rhythm to promote tumor progression in GBC. M2 macrophages contribute to immune evasion by secreting chitinase-3-like protein 1 (YKL-40), a chitinase-like glycoprotein that has been linked to increased tumor angiogenesis, immune suppression, and malignancy in a variety of cancers, as well as increased PD-L1 expression on tumor cells. M2 macrophages promote extracellular matrix remodeling and EMT by secreting IL-10 and modulating tumor growth, invasion, and lymph node metastasis via anti-YKL-40 antibodies. Additionally, they upregulate IL-6, TGF-β, and MMPs. Through TGF-β and CCL2 signaling, CAFs aid in tumor cell proliferation and increase EMT markers. Integrin α2 and the PI3K/AKT axis both enhance metastatic behavior. Elevated PD-L1 expression on tumor cells implies immune evasion by suppressing cytotoxic T cell activity, and GDF15-GFRAL interactions may control metabolic reprogramming. The upward blue arrows represent the upregulation; doted blue arrows represent the activation pathways; curved blue arrows represent directional signaling; black arrows represent the flow of molecular process; and the hammer-headed arrows represent inhibition. AKT, protein kinase B; CAF, cancer-associated fibroblast; CCL2, C-C motif chemokine ligand 2; EMT, epithelial–mesenchymal transition; GBC, gallbladder cancer; GDF15, growth differentiation factor 15; GFRAL, glial cell-derived neurotrophic factor receptor alpha-like; IL-6, interleukin-6; IL-10, interleukin-10; IL-12, interleukin-12; MMP, matrix metalloproteinase; PD-L1, programmed death-ligand 1; PI3K, phosphoinositide 3-kinase; PTCH1, patched 1; SMO, smoothened; TGF-β, transforming growth factor-beta; TNF-α, tumor necrosis factor-alpha; YKL-40, chitinase 3-like protein 1 (adapted from Tang et al., 2025 [7]).
This review delves into the dysregulation of TFs and highlights their vital roles in GBC development. It focuses specifically on the TFs that exhibit strong associations with GBC pathogenesis and hold promise as future diagnostic markers or therapeutic targets. Through the analysis of these transcription factors across a wide variety of datasets, ranging from transcriptomic analysis to protein expression and gene copy analysis, it has been observed that GBC emerges not just as the sum of various gene mutations, but as a breakdown of a fine-tuned and essential transcription-control mechanism orchestrated by TFs. Despite the advances, the precise mechanisms linking epidemiology to pathogenesis remain elusive, necessitating integrated studies to develop prevention and early detection strategies. Understanding these risk profiles is foundational to unraveling GBC’s transcriptional dysregulation, as explored in subsequent sections.
2. Transcriptional Factors and Their Roles in GBC
Through their modulation of gene expression patterns, transcription factors significantly contribute to the development and advancement of GBC as depicted in Figure 2 and listed along with their functions in Table 1. These factors impact cell proliferation, metastasis, and survival. Importantly, GBC tissues show a marked increase in Transcription Factor 4 (TCF4) compared to chronic cholecystitis, which is associated with higher tumor grades and advanced stages [8]. TCF4 is a critical component of the Wnt/β-catenin signaling pathway. The overexpression of Forkhead box K1 (FOXK1) in GBC also activates the AKT/mTOR signaling pathway, which in turn promotes tumor growth and metastasis. Increased invasiveness of GBC cells is caused by the interaction of Histone Deacetylase 1 (HDAC1) and Transcription Factor 12 (TCF12), which further promotes epithelial–mesenchymal transition (EMT). Furthermore, Forkhead box O3 (FOXO3) is a tumor suppressor that, when activated, causes GBC cell death and proliferation inhibition, especially through the Janus kinase2/signal transducer and activators of transcription 3 (JAK2/STAT3) pathway. These results demonstrate the possible therapeutic intervention targets and emphasize the intricate regulatory networks involving transcription factors in GBC [9,10].
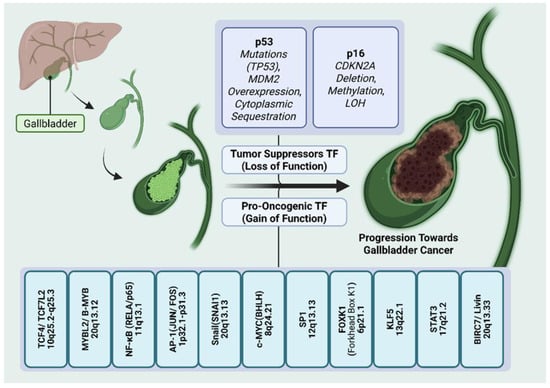
Figure 2.
Role of transcription factors in the progression of gallbladder cancer. Transcriptional disparities in gallbladder cancer involving tumor suppressor and pro-oncogenic transcription factors compounding the process of epithelial–mesenchymal transition and tumor metastasis. Loss of function or mutation in tumor suppressor TFs (top panel), such as p53 and p16, along with MDM2 overexpression and cytoplasmic sequestration of p53 result in cell cycle dysregulation, impaired apoptosis and uncontrolled proliferation. Gain of function or hyperactivation of pro-oncogenic TFs (bottom panel), such as TCF4, MYBL2, NF-KB, AP-1, Snail, c-MYC, SP1, FOXK1, KLF5, STAT3, Livin, etc., drives tumorigenesis by activating various signaling pathways, promoting inflammation, cell proliferation, dedifferentiation, metastasis, angiogenesis, etc.

Table 1.
Transcription factors and their role in GBC development.
3. Loss of Function of Tumor Suppressor Genes
The loss of tumor suppressor genes contributes significantly to the development and progression of gallbladder cancer (GBC). A dysfunctional p53 protein that fails to regulate genomic integrity is the result of mutations and loss of heterozygosity (LOH), which affect more than half of all cases and often occur early in carcinogenesis. Loss of p16 is found in approximately 61% to 75% of gallbladder tumors and is strongly linked to a poor prognosis. This inactivation of the CDKN2A/p16 locus at chromosome 9p21 can occur as a result of point mutations, promoter hypermethylation, loss of heterozygosity (LOH), homozygous deletions, or a mix of these factors. Furthermore, in around 30% of cases, PTEN, a crucial negative regulator of the PI3K/AKT pathway, is downregulated through promoter methylation, LOH, or protein loss, which adds to unregulated proliferative signaling. While SMAD4/DPC4 and pRB are less commonly impacted genes, their inactivation further disturbs cell cycle regulation and tumor differentiation in some tumor types. The aggressive pathobiology of GBC is fueled by the loss of these tumor suppressors, which together dismantle critical checkpoints against cell proliferation and survival [49,50]. The genes associated with loss of tumor function in GBC are given below.
3.1. p53
p53, also known as the tumor protein p53 (TP53), is a potent transcription factor and a well-established tumor suppressor protein. Encoded by the TP53 gene, often dubbed as “Guardian of the genome,” p53 plays a critical role in preserving genomic integrity by orchestrating cellular responses to various stress signals. These stressors include, but are not limited to, DNA damage (from sources like UV radiation or chemotherapeutic agents), hypoxia, and oncogene activation [51]. While p53 protein is exclusively located within the nucleus, executing its transcriptional regulatory functions, it can also be found in the cytoplasm in an inactive state [51,52]. A crucial regulator, Mdm2, is intimately involved in controlling p53′s subcellular localization. It binds to p53, promoting its export from the nucleus to the cytoplasm, where p53 is subsequently targeted for proteasomal degradation [51,52]. Under basal conditions, p53 protein levels are kept low due to rapid degradation primarily mediated by Mdm2. However, upon exposure to cellular stress, such as DNA damage, p53 undergoes post-translational modifications (such as phosphorylation and acetylation) that lead to its stabilization and accumulation in the nucleus [53]. Once activated and accumulated, p53 orchestrates a variety of cellular responses aimed at preserving cellular integrity. p53 can induce cell cycle arrests, often mediated through the transcriptional activation of genes like CDKN1A (which encodes p21), a potent inhibitor of cyclin-dependent kinases (CDKs) [51].
Aberrant p53 activity, whether through mutation or altered expression, is a common finding in GBC. Some evidence also suggests increased prevalence [54]. The implications of p53 deregulation for GBC are multifaceted, encompassing aspects of disease severity, prognosis, and therapeutic response [51]. High levels of p53 protein are a common finding in GBC tumors, reflecting a disruption of normal p53 regulatory mechanisms, which could result due to DNA alterations that can affect the p53 [55,56]. Multiple studies employing immunohistochemistry have reported p53 overexpression in a significant proportion of GBC cases, suggesting an early and frequent event in tumorigenesis [56]. Mutations in the TP53 gene, particularly missense point mutations and loss of heterozygosity (LOH) at the TP53 locus, are prevalent mechanisms leading to impaired p53 function. Mutations in TP53 are frequently observed in GBC, with a systematic review of 3893 GBC samples reporting a weighted average mutation frequency of 38.4%, ranging from 13.2% to 66.7% across studies [57]. Geographical variations in mutation rates and spectra have been noted, highlighting potential roles for environmental or genetic factors [58]. Studies showed varied co-relations from positive and negative findings between patients’ survivability and prognosis, after chemotherapy, radiotherapy, and other treatments [59]. This data can be used with others in the search for a better treatment approach, such as Mdm2 [52], an inhibitor which could have beneficial effects by inducing p53, which is impaired from damage, mutations, etc. [51]. In certain cases, p53 status in GBC has been linked to poor prognosis. Higher p53 expression, reflecting p53 dysfunction, often correlates with more aggressive tumor behavior, higher tumor grade, advanced disease stage, shorter patient survival times, increased rates of metastasis and reduced responsiveness to certain therapies. Also, some other factors such as K-ras mutations can potentially worsen patient state [58]. Takagi and Ohashi suggest differential p53 levels, via tests like IHC and other more accurate tests such as sequencing, to create a better analysis of which biomarkers should be screened, for differential or risk stages, to implement the most effective approach. While the focus is on GBC, it is worth noting that p53′s role is consistent with its function in other cancers, such as of the bladder, lung, and colon, where mutations are also common and linked to poor survival. For instance, combined deletion of p53 and PTEN in bladder epithelium leads to invasive cancer, mirroring findings in GBC [60]. In some cases, even with lower percentages of p53 expression, the risk for metastasis is observed to be greater because of the alteration. In summary, p53 dysregulation is a critical event in GBC development. Its multifaceted roles as a tumor suppressor, a regulator of cellular stress responses, and a potential therapeutic target highlight its importance. A dissection of the precise molecular mechanisms by which p53 is altered and how those alterations contribute to the aggressive nature of GBC is given in Figure 3. Such studies may, one day, inform both prevention strategies and better tailored therapies.
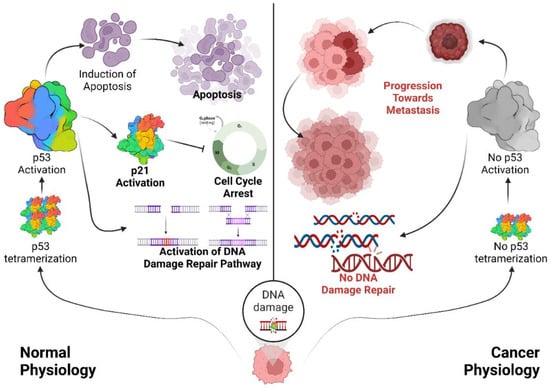
Figure 3.
Role of p53 in regulating the cell cycle to maintain genomic integrity. Under normal conditions, p53 is maintained at low levels. In response to cellular stresses such as DNA damage, p53 accumulates and functions as a tetrameric transcription factor, inducing either cell cycle arrest or apoptosis. DNA damage stimulates p53 tetramerization and activation, which facilitates cell cycle regulation and the maintenance of genomic integrity. Activated p53 binds to specific response elements upstream of target genes such as p21, leading to cell cycle arrest and activation of the DNA repair machinery, primarily via ATR/ATM kinase pathways. When DNA damage is irreparable, p53 triggers the expression of pro-apoptotic genes such as Bax and Bak to initiate apoptosis. In contrast, under carcinogenic conditions, mutations in various domains of p53 impede its stabilization and tetramer formation, resulting in p53 inactivation. Some p53 mutants with a functional tetramerization domain can form mixed tetramers with wild-type p53, exerting dominant-negative effects (DNE) that further inactivate p53, ultimately contributing to genomic instability and metastasis. Black arrows represent the flow of molecular process; and the hammer-headed arrows represent inhibition.
3.2. p16INK4a
p16INK4a, encoded by the CDKN2A gene on chromosome 9p21, stands as a critical gatekeeper-like suppressive regulator of cell cycle progression, and its deregulation is a frequent hallmark of gallbladder cancer (GBC) [61,62,63,64,65,66]. As a potent cyclin-dependent kinase inhibitor (CDKI), p16INK4a normally exerts its tumor-suppressive function by binding to CDK4 and CDK6, preventing their association with D-type cyclins and thereby inhibiting phosphorylation of the retinoblastoma protein (RB) [67,68], a key event required for the G1-S transition of cell cycle. By maintaining RB in its hyperphosphorylated, active state, p16INK4a sequesters E2F transcription factors, preventing the expression of genes essential for DNA replication and cell cycle progression [68,69]. Thus, functional p16INK4a serves as a crucial brake on uncontrolled cellular proliferation [70]. Dysfunctional p16 fails to inhibit Cyclin/CDK activity, allowing unchecked entry into the S phase of DNA synthesis. This leads to Rb pathway disruption, triggering continuous activation of downstream signals that drive uncontrolled cell proliferation [5].
In GBC, this brake is frequently released, contributing significantly to tumorigenesis. In contrast to other cancers where RB mutations are prevalent, GBC often exhibits alterations that target p16INK4a itself [71]. The most common mechanisms leading to p16INK4a inactivation in GBC are homozygous deletion of the CDKN2A gene, and epigenetic silencing via promoter hypermethylation [69,72,73]. Loss of heterozygosity (LOH) at 9p21-22 also provided further evidence from a study in 2007, which found homozygous deletions in 26.0% (13/50) of cases, LOH in 56.9% (29/51), and promoter hypermethylation in 72.5% (37/51), with loss of p16 protein expression in 62.7% (32/51) of cases [74]. Although some studies find a significant correlation with loss and/or alterations of p16 with other factors [69], mutations in p16 have been shown to be only secondary events in GBC tumorigenesis [5]. While several molecular methods show similar results, PCR analysis proved more practical when evaluating bile in an endoscopic retrograde study. The consequence of p16INK4a loss is profound. Without functional p16INK4a, CDK4/6 are free to complex with D-type cyclins, leading to RB hyperphosphorylation, E2F release, and ultimately, uncontrolled cell cycle progression [68].
The relationship between p16INK4a alterations and clinicopathological features in GBC remains under debate. Studies report a prevalence in the range of promoter hypermethylation [5,75]. Also, studies have provided conflicting evidence for such an interaction; while some studies suggest that loss of p16INK4a expression correlates with poor prognosis, advanced tumor stage, and increased invasiveness [69,76], others have found no significant association with clinicopathological parameters [5]. These discrepancies may arise from differences in study populations, sample sizes, methodologies (e.g., immunohistochemistry versus methylation-specific PCR), and the inclusion of different histological subtypes of GBC. It is also present in “patients with PSC”, showing similarity with other frank biliary tract cancers [75]. It is believed that hypermethylation occurs before homozygous dilatation [74,77,78,79]. Importantly, the in vitro and preclinical data strongly support p16INK4a’s role as a tumor suppressor in the biliary tract. Furthermore, the development of selective CDK4/6 inhibitors (e.g., palbociclib, abemaciclib, LEE011) has provided a powerful therapeutic approach that, in essence, restores p16INK4a-like function by blocking the downstream consequences of its loss [68,80,81,82,83,84,85]. Clinical trials with these agents, particularly in combination with other targeted therapies or hormone therapy, are yielding encouraging results, highlighting the importance of the p16INK4a/CDK4/6/RB pathway in GBC and other cancers. However, the response to these agents is likely influenced by other gene alterations like methylation and its effectiveness is further proven in combination therapies [5]. Further assessments with larger patient populations can bring valuable inputs to our understanding of its involvement.
4. Gain of Function of Pro-Oncogenic Transcription Factors
The growth, invasion, and metastasis of tumors are driven by various pro-oncogenic transcription factors, which are frequently upregulated in gallbladder cancer. Approximately 83% of tumors have overexpressed STAT3, a critical effector of the JAK/STAT pathway; increased levels of STAT3 are associated with advanced tumor stage, poor differentiation, early recurrence, and enhanced VEFG-driven angiogenesis and epithelial–mesenchymal transition (EMT) [8]. The GPRC5A-JAK2-STAT3-TNS4 axis enhances metastatic potential, and inflammatory cytokine loops like IL-11/STAT3 and IL-6/JAK-STAT3 via fibroblasts maintain this activation. Furthermore, there is an upregulation of FOXK1, a forkhead-box transcription factor, in GBC [86]. This upregulation promotes cell cycle progression, EMT, and AKT/mTOR signaling. It is closely linked to poorly differentiated cells, liver metastasis, advanced TNM stage, and decreased survival. Furthermore, TCF4, an important transcription factor in the Wnt/β-catenin pathway, is found to be overexpressed in more than 80% of GBC samples. Its levels are even higher in late-stage and high-grade tumors, suggesting that it is involved in the growth and advancement of tumors. These abnormalities in STAT3, FOXK1, and TCF4 function, when combined, form a transcriptional network that promotes aggressive behavior and may be therapeutic targets for gallbladder cancer [87,88].
4.1. TCF4
Transcription factor 4, alternatively termed as transcription factor 7-like 2 (TCF7L2), is encoded by the TCF 4 gene, spanning 442 Kb (approx.) across chromosomal region 18q21.2 [89,90]. As a pivotal mediator of the Wnt/β-catenin signaling cascade, TCF4 has dual functionality as a transcriptional repressor or activator, depending on its interaction with Groucho/TLE family members, while β-catenin association enables transcriptional activation [91]. In the absence of Wnt signaling, phosphorylation by GSK3β directs β-catenin to undergo ubiquitin-mediated degradation in the β-catenin destruction complex (DC). Activity of GSK3β is inhibited by Wnt signaling. Following this, β-catenin builds up in the nucleus, where it attaches to TCF family members and changes these WNT effectors from transcriptional repressors to activators [92]. Since TCF4 is widely expressed, it binds to the consensus sequence CANNTG (“Ephrussi box or E-box”) either as homo- or heterodimers [93] and is classified under the class I HLH (helix–loop–helix) group often referred to as E-proteins [94]. It has been reported in regulation of hematopoiesis [95], myogenesis [96], neurogenesis [97], melanogenesis [98], and osteogenesis [99], as well as the differentiation of endothelial [100], mammary gland [101], placental [102], and Sertoli cells [103].
TCF4-driven dysregulation of Wnt/β-catenin signaling is implicated in cancer stem cell maintenance in cancers like colorectal cancer [92], lung cancer [104], hepatocellular carcinoma [105] and GBC [10]. Neogi et al. used RT-qPCR and immunohistochemistry (IHC) to demonstrate that the mRNA levels of TCF4 and β catenin in GBC tissues were considerably (p < 0.05) higher than chronic cholecystitis [10]. Furthermore, TCF4 overexpression was associated with an increased chance of advanced disease state, as seen by the fact that TCF4 expression was higher in high tumor grades than low grades and higher in stages 2 and 3 than stage 1. These outcomes suggested that specific inhibitors of the β catenin/TCF4 interaction or downregulation of Wnt/β catenin signaling may be effective treatments for GBC.
4.2. MYBL-2 (B-MYB)
A member of the myb transcription factor family, MYBL2 (B-myb, v-Myb avian myeloblastosis viral oncogene homolog-like 2) is found on chromosome 20q13.12 and is frequently expressed in embryonic stem cells (ESC). Structurally this protein consists of multiple domains (DNA-binding domain and C-terminal domain) allowing it to regulate multiple crucial cellular pathways like cell proliferation, differentiation, DNA damage repair and apoptosis including various phases of the cell cycle. Dysregulation of the MYBL-2 gene is reported in several cancers (breast, ovarian, liver, colorectal, blood, neural and bladder) [106], often arising from deregulation of DREAM complex assembly or aberrant regulation at the post-transcriptional level via microRNAs [4,5,6,7,8,9,107,108]. Notably, the p53-p21-DREAM pathway suppresses MYBL2 expression, particularly in response to DNA damage (cellular stress). MYBL2 is also disproportionately elevated in a number of p53-mutant cancers [109,110]. Moreover, the oncoprotein HPV16 E7 can induce MYBL2 expression by deregulating the assembly of the DREAM complex [111].
Surprisingly, downregulation of MYBL2 is often linked with certain types of hematological malignancies [112,113], whereas in solid tumors, expression is elevated [106]. Using RT-qPCR and immunohistochemistry (IHC), Liang et al. reported that MYBl2 is increased in GBC compared to cholecystitis tissue and promotes DNA replication and GBC cell proliferation [114]. Using Cell Counting Kit-8 (CCK-8) and colony formation assays, they demonstrated that MYBL2 overexpression promotes cell growth, but RNA interference-based MYBL2 knockdown markedly inhibits cell proliferation. Additionally, MYBL2 promotes passage through the S and G2/M phases by controlling the GBC cell cycle. Its function in cell division was suggested by the fact that overexpression promoted cell cycle progression, whereas knockdown trials caused G1-phase arrest. Using xenograft tumor models in mice, the oncogenic potential of MYBL2 was confirmed in vivo. MYBL2-overexpressing cells induced tumors that were bigger than controls, but knockdown cells produced tumors that were noticeably smaller [114]. This suggests a prospective alternative for future clinical treatments meant to act as both a prognostic marker and as a possible therapeutic target in GBC.
4.3. NF-kappa B
NF-kB is a family of related protein complexes that function as hetero- and homodimers. It can produce up to 15 NF-kB complexes by selecting from a pool of five monomers (p50, p52, Rel A (p65), Rel B, and c-Rel) [115]. Hundreds of genes incorporate NF-kB within their enhancers or promoters due to variations of the palindromic κB site, which follows the consensus sequence 5′-GGGRNWYYCC-3′ (where N represents any base, R denotes purines, W signifies adenine or thymine, and Y corresponds to pyrimidines) (https://www.bu.edu/nf-kb/gene-resources/target-genes/, accessed on 13 April 2025). NF-kB is widely distributed and inhibited by IκBs in the cytoplasm. A variety of cancers like breast cancer [116], melanoma [117], lung cancer [118], colon cancer [119], multiple myeloma [120], pancreatic cancer [121], esophageal adenocarcinoma [122], leukemia and lymphoma [123] are associated with overexpression of NF-kB, which is typically measured by the presence of nuclear RelA. Bao et al. reported that human immortalized normal biliary epithelial cells (H69) had higher levels of lncRNA MEG3 expression than GBC cell lines (EH-GB1, GBC-SD, and OCUG-1; p < 0.05) [124]. When compared to the negative control (NC) group, the overexpression of lncRNA MEG3 dramatically decreased the proliferation rate and colony formation capacity of GBC-SD cells, as determined by the CCK-8 and colony formation assays. Furthermore, the lncRNA MEG3 overexpression sample exhibited higher levels of apoptosis than the NC group. This implies that MEG3 had the capacity to inhibit tumors [124]. Additionally, they discovered that overexpression of lncRNA MEG3 significantly increased the amount of NF-kB protein in the nucleus, suggesting that lncRNA MEG3 overexpression triggers the NF-kB signal. Moreover, they also suggest that the tumor suppressor ability of MEG3 is facilitated by NF-kB expression by using NF-kB inhibitor SN50 on lncRNA MEG3 transfected GBC cells.
4.4. AP-1
The AP-1 (activating protein-l) family of transcription factors consists of homodimers and heterodimers of Jun (v-Jun, c-Jun, JunB, JunD), Fos (v-Fos, c-Fos, FosB, Fral, Fra2) or activating transcription factor (ATF2, ATF3/LRF1, B-ATF) bZIP (basic region leucine zipper) proteins [125]. Dimerization between members of the AP-1 family occurs through a structure known as a leucine zipper, which can dimerize with another α-helix via the formation of a coiled-coil structure with contacts between hydrophobic leucine zipper domains. Jun-Jun and Jun-Fos dimers preferentially bind to the phorbol 12-O-tetradecanoate-13-acetate (TPA)-responsive element (TRE). On the other hand, Jun-ATF dimers or ATF homodimers prefer to bind to the cAmp-responsive element (CRE) [126]. MAF (c-MAF, MAFA, -B, -F, -G, -K, and Nrl) multigene families bind either to the MARE I or MARE II motifs that are the extension of TRE and CRE motifs respectively [26,127]. It is found that AP-1 plays a very significant role in T-cell activation and the differentiation of naïve T-cells to T helper cells by using the MAPK (Mitogen-activated protein kinase) pathway and also controls the expression of the cytokines TNF-α, IL-1, interleukin 2 (IL-2), IFNγ, and GM-CSF as well as matrix-degrading matrix metalloproteases (MMPs) such as collagenase 1 and stromelysin [128]. AP-1 is necessary for skin homeostasis and differentiation. It modulates the activity of the human distal regulatory enhancer [129].
Cancer is undoubtedly the most documented pathology involving AP-1 dysregulation, which leads to cell transformation and tumor progression [26,27]. It acts as a tumor suppressor or oncogene and is a crucial effector of the upstream oncogenic events [130]. c-JUN-deficient cells were found to undergo premature senescence as a result of spontaneous DNA damage, suggesting that c-JUN plays a role in stimulating DNA [131]. C-JUN acts as a proliferating agent by inhibiting p53 and stimulating cyclin D1. A reduction in levels of p53 leads to the dampening of the cyclin-dependent kinase inhibitor (CDKI) p21, leading to the transition from the G1 to the S phase [132,133]. JUN-B suppresses the proliferation by the activation of CDKI p16INK4a and repression of cyclin D1 and leads to inhibition of progression into the S phase. In various cancers the AP-1 component level is upregulated, for example in breast cancer, endometrial carcinoma, colorectal cancer, acute myeloid leukemia, Hodgkin’s, and anaplastic large cell lymphoma [134].
Fos-like antigen-1 (FOSL1) is a member of the AP-1 complex and it exerts oncogenic effects like proliferation, invasion, and epithelial–mesenchymal transition (EMT) [135]. It was confirmed that the miR-195-5p/FOSL1 regulatory axis is closely related to GBC by using bioinformatics and qRT-PCR analysis. FOSL1 is the direct target of miR-195-5p and the overexpression of miR-195-5p leads to the inhibition of the proliferation, migration, and invasion ability of GBC. It was found that the GBC cells were blocked in the Go/G1 phase by directly targeting FOSL1 and regulating the Wnt/β-catenin signaling pathway [136]. Further research is required to know the interaction mechanism between FOSL1 and the Wnt/β-catenin signaling pathway. Studies on malignant melanoma and ovarian cancer found that the decoy oligodeoxynucleotides act as the therapeutic drug against c-JUN. This leads to the decrease in proliferation ability of the cancer as well as an increase in the apoptosis inside the cancerous cell by inhibition of downstream targets like cyclin D1, p53, BCL3 and matrix metalloproteinase 9. Given these findings, similar strategies may be applicable for GBC treatment, though further research is required to validate their efficacy [137,138].
4.5. Snail
Snail is a zinc-finger transcription factor that which is highly conserved in Drosophila and higher vertebrates. The highly conserved C2H2-type zinc-finger domain helps in the binding to the E-box motif-containing CANNTG-specific nucleotide sequence. Snail is the transcription repressor that plays a very important role in various cell differentiation processes, mainly in the epithelial–mesenchymal transition (EMT). Studies have shown that the Snail promoter is directly regulated by different growth factors like TGF-beta, FGF-2, and EGF along with different signaling molecules like integrin-linked kinase (ILK), H-ras, v-Akt and NF-KappaB/p65 [139,140,141]. Research studies indicate that the human Snail (hSnail) modulates epithelial-specific cytokeratin leading to the EMT. It is one of the major structural proteins in the epithelial cells forming a cytoplasmic network of intermediate filaments [139,141].
In cancer, EMT dysregulation can be triggered by various molecules, promoting carcinoma cell invasion and metastasis [142]. Findings from colorectal cancer studies revealed that the human Snail leads to morphologic changes and plays a crucial role in promoting cancer invasion [142]. In ovarian cancer, high expression of the Snail transcription factor is seen and provides resistance to apoptosis [143]. In cases of GBC, the Snail transcription factor is a tumor-promoting factor. The expression of Snail is higher in gallbladder adenocarcinoma as compared to peritumoral tissues and cholecystitis [11]. Through the screening process, a novel lncRNA, HOXA-AS2, is identified to be highly expressed in GBC and promotes GBC cells’ migration by sponging miR-6867-5p which targets YAP (overexpressed in GBC) [144,145,146]. lncRNA HOXA-AS2 in GBC cells upregulates YAP1, which plays a role in EMT and potentially impacts the Snail transcription factor [147]; however, further research is required for better understanding of the mechanism.
4.6. c-MYC
c-MYC is a protooncogene present in the cell, playing a role in cellular growth, differentiation and apoptosis [148]. It is a DNA-binding protein containing the helix–loop–helix and leucine zipper protein dimerization domain. The basic region in the C-terminal acts as the major DNA contact surface [149]. The complete binding site of the c-MYC is GACCACGTGGTC [149]. Max is another bHLHZip protein that forms a heterodimer with c-MYC and they have a common DNA target (Ebox) site [150,151]. Studies have shown that the MYC-Max transcription factor complex regulates the target genes mediating the multiple effects of MYC [152,153,154]. This heterodimer promotes Cyclin D1, D2, E1, A2, and Cyclin-Dependent Kinase 4 for cell cycle progression and represses transcription of cell cycle checkpoint genes like GADD45 and GADD153 as well as cyclin-dependent kinase inhibitor p15 and p21 [155,156,157,158].
In cancer, c-MYC is frequently dysregulated and exhibits overexpression in the majority of human malignancies [159]. Recent studies have revealed that small nucleolar RNAs (SNORNs), a class of non-coding RNA about 60–300 nucleotides long located in the nucleolus, play a crucial role in cancer. For instance, SNORA42 is significantly overexpressed in non-small-cell lung cancer (NSCLC) and colorectal cancer [160,161]. qRT-PCR analysis has revealed that SNORA21 is significantly downregulated in GBC. Studies indicate that SNORA21 overexpression induces G1-G0-phase cell cycle arrest, thereby inhibiting proliferation, migration, and invasion of GBC cells. This effect is further supported by a reduction in cyclin D1 and c-MYC levels, along with an increase in p21 expression. Given these findings, SNORA21 holds potential as a therapeutic target for GBC, though further research is required [162].
4.7. Sp1
Sp1 is a transcription factor that is necessary for the transcription of several “housekeeping genes” involved in metabolism, cell growth and proliferation and cell death [163]. The majority of Sp1′s transcriptional activity is supported by its two primary transactivation domains, A and B, which work in tandem with the DNA-binding domain Sp1 to function as an activator or as a part of a repressor complex [164]. Sp1 expression is closely associated with patient survival in most cancers, with elevated levels often indicating a poor prognosis. Numerous studies have investigated the potential of targeting Sp1 to eliminate cancer cells or enhance their sensitivity to treatment [165,166,167,168].
Sp1 regulates a broad range of target genes, including proto-oncogenes such as c-MYC and tumor suppressors like TP53, which are critical for controlling cell proliferation and oncogenesis. Additionally, Sp1 modulates the expression of both pro- and anti-angiogenic factors involved in invasion and metastasis, as well as pro- and anti-apoptotic genes that influence genomic stability. It also governs key regulators of the cell cycle, such as cyclins, thereby impacting cell cycle progression and arrest [169]. Sp1 is also a key transcriptional regulator of the hTERT gene, which encodes the catalytic subunit of telomerase. Activation of hTERT promotes telomere maintenance, enabling replicative immortality—a hallmark of cancer. Sp1 binding sites within the hTERT promoter are essential for its transcriptional activation, and overexpression of Sp1 has been linked to increased hTERT expression in various cancers [170]. In addition, Sp1 collaborates with other transcription factors and epigenetic modifiers to regulate hTERT expression through chromatin remodeling and histone modifications, further contributing to tumorigenesis [171]. Multiple Sp1 binding sites are also present in the gene that codes for the RNA component of telomerase, human telomerase RNA component (hTERC). Additionally, Sp1 controls acetylation and other histone modifications to control the expression of hTERT [172]. Sp1 is also known to regulate the long non-coding RNA, LINC00152, by directly binding to its promoter region and upregulates its expression in GBC cells, NOZ. Elevated LINC00152, in turn, activates the PI3K/AKT signaling pathway, thereby promoting tumor growth, invasion, and metastasis. This Sp1–LINC00152–PI3K/AKT regulatory axis underscores a potential therapeutic target in GBC. Additionally, arsenic trioxide (As2O3) has been reported to inhibit GBC cell proliferation by downregulating Cyclin D1 expression in an Sp1-dependent manner, although the exact mechanism of Sp1 modulation in this context remains to be properly explored [173].
4.8. FOXK1
FOXK1 is a member of the Human Forkhead-box (FOX) gene family [174]. The characteristic of FOX proteins is their conserved forkhead DNA-binding domain (DBD). In order to modify gene expression, this domain permits certain interactions with DNA sequences [175]. Human genetic illnesses, cancer, and/or aging are caused by genetic changes or dysregulation of FOX genes [176]. In stem cell populations and cancer, FOXK1 plays a crucial role in regulating cell division, proliferation, and quiescence [177]. In gastric cancer, increased FOXK1 expression was shown to suppress autophagy via the PI3K/AKT/mTOR pathway [178]. Similarly, FOXK1 is found upregulated in several cancers and is thought to participate in tumorigenesis [179,180]. FOXK1 is reported to be overexpressed in GBC cells. The overexpression of FOXK1 increases the ability of GBC cells to migrate and invade by initiating EMT and promoting cell proliferation through cell cycle regulation [87]. Furthermore, the activation of the Akt/mTOR axis may be partially linked to the tumor-promoting impact of FOXK1. Mechanistic studies confirmed that FOXK1 knockdown could result in G1/S cell cycle arrest by downregulating CDK4, CDK6, cyclin D1, and cyclin E1 [87]. Additionally, FOXK1 may control the expression of proteins linked to the epithelial–mesenchymal transition (EMT), including vimentin, N-cadherin, and E-cadherin. FOXK1 stimulates GBC cells to proliferate in vitro, while the inactivation of FOXK1 induces G1/S arrest [87]. In GBC patients, FOXK1 may therefore be a viable prognosis biomarker and therapeutic target.
4.9. KLF5
Kruppel-like factor 5 (KLF5) is a zinc-finger transcription factor encoded on chromosome 13q21 and consists of 457 amino acid residues [181]. It contains three C2H2-type zinc-finger motifs at its C-terminus which facilitate the binding to GC-rich promoter regions [182]. KLF5 plays a crucial role in the regulation of multiple genes, such as Cyclin D1, p27, Nanog and Slug [181], and is implicated in numerous cellular processes such as proliferation, apoptosis, stemness, autophagy, cell cycle progression, metabolism and cellular homeostasis. Functionally, KLF5 exhibits context-dependent behavior, acting as either a tumor suppressor or an oncogene across different cancer types [183]. Its dysregulation is associated with various pathological conditions, including cancer and cardiovascular diseases. Post-translational modifications such as phosphorylation and acetylation enhance KLF5 transcriptional activity [184,185]. KLF5 is expressed in multiple tissues and plays diverse context-dependent roles. In breast cancer cells, enhanced KLF5 expression increases the expression of many genes, such as Cyclin A, chromatin licensing and DNA replication factor 1 (cdc-10 dependent transcript 1; CDT1), and E2F transcription factor 3 (E2F3), progesterone and progesterone receptor (PR) thereby promote the proliferation of cells [186]. Additionally, KLF5 maintains the stemness in mammary cells by promoting the transcription of the Slug gene, a known regulator of stemness and epithelial–mesenchymal transition [187].
KLF5 is implicated in multiple signaling pathways and has been increasingly recognized for its oncogenic potential. In non-small-cell lung cancer (NSCLC), KLF5 is thought to promote tumorigenesis by activating the PI3K/AKT signaling pathway [188]. According to various studies, KLF5 triggers NF-kB signaling. For instance, in thyroid cancer cells, KLF5 enhances the nuclear translocation of p65 and promotes phosphorylation of IKKβ and IκBα [189]. Also, KLF5 has been demonstrated to increase Sox4 expression, which in turn promotes lung cancer cell proliferation and tumor progression [190]. According to recent research, KLF5 promotes tumor growth in gallbladder cancer. GBC progression is suppressed when KLF5 is silenced, likely through the downregulation of PDGFA, which affects cell proliferation, metastasis, angiogenesis, and apoptosis of GBC cells [191]. Given its central role in modulating key oncogenic transcriptional networks, targeting KLF5 may offer therapeutic potential not only in GBC but across a wide spectrum of cancers.
4.10. STAT3
Signal Transducer and Activator of Transcription 3 (STAT3) is a transcription factor and signal transducer protein which can be activated by a variety of cytokines, growth factors and intracellular kinases, particularly through the JAK/STAT signaling pathway. It is an 89 KDa (770-amino-acid) latent cytoplasmic protein characterized by several distinct structural protein domains, including N-terminal, coiled-coil, DNA binding, linker, SH2 (Src homology) and transcriptional-activation domains [192]. STAT3 expression and activity are tightly regulated through alternative splicing, post-translational modification and subcellular localization. One key splice variant of the mammalian STAT3 with deletion of a 50-nucleotide sequence near the C-terminus codes for an 80 kDa STAT3 isomer (STAT3β) that functions as a negative regulator of canonical STAT3α isoform [192]. Unlike STAT3α, which primarily promotes oncogenic transcriptional programs, STAT3β is associated with pro-apoptotic functions and exerts context-dependent roles in tumor suppression, thus playing a role distinct from STAT3α. Despite the different mechanism, STAT3 activation through phosphorylation or acetylation can facilitate tumorigenesis synergistically. STAT3 shuttles between the cytoplasm, nucleus, mitochondria and other possible organelles, and exerts its diverse functions in transcriptional regulation, cellular respiration, proliferation and apoptosis. A variety of animal models reveal that STAT3 is essential for embryo development, pluripotency of stem cells, embryo implantation and decidualization. Among the seven members of the STAT protein family, STAT3 is arguably the most important for tumor progression [193]. In cancer, STAT3 dysregulation leads to its prolonged activation, resulting in cell proliferation, invasion, angiogenesis, decreased apoptosis, and immune evasion [194].
STAT3 is highly expressed in various cancer types, such as endometrial cancer [195], glioma of the brain [196], breast cancer [197], lung cancer [198], and gall blader cancer [199]. Higher expression of STAT3 was correlated with clinical stage and positively associated with early postoperative recurrence of the GBC malignancy. This overexpression is closely linked to the formation of vasculogenic mimicry (VM), a process where tumor cells form vessel-like structures independent of endothelial cells. VM is a tumor vascular model that differs from angiogenesis that has been detected in melanoma, inflammatory breast cancer, liver cancer and other malignant tumor types with highly invasive characteristics [200]. In GBC, VM was observed in over 92% of tumors with high STAT3 expression, and statistical analysis confirmed a significant positive correlation between STAT3 levels and VM presence. Furthermore, both high STAT3 expression and VM formation were strongly associated with postoperative recurrence [201]. Specifically, patients with high STAT3 expression had a mean time to recurrence (TTR) of 17.35 months, compared to 35.65 months in those with low STAT3 expression. Similarly, VM-positive tumors recurred at a mean of 22.38 months, versus 36.00 months in VM-negative cases [201]. These findings suggest that STAT3 not only contributes to GBC cell proliferation and invasion but also promotes alternative vascularization through VM, underscoring its potential as a therapeutic target for antitumor angiogenesis therapy and metastasis inhibition in gallbladder carcinoma.
4.11. BIRC7
Baculoviral IAP repeat-containing 7 (BIRC7), commonly referred to as Livin, encodes a member of the inhibitor of apoptosis protein (IAP) family. The BIRC7 gene is localized in the cytoplasm and is situated on the long arm of chromosome 20, specifically at the cytogenetic band 20q13.33. The Livin gene, spanning approximately 4.6 kilobases, is located on chromosome 20 at cytogenetic band q13. This gene consists of six introns and seven exons. The Livin protein comprises 298 amino acids with a molecular weight of approximately 32.8 kDa. It is characterized by the presence of a single baculovirus IAP repeat (BIR) domain and a RING-type zinc-finger domain. The BIR domain plays a critical role in mediating interactions with caspases and inhibiting apoptosis. The BIR domain, approximately 70 amino acids in length, is characterized by zinc-binding residues. Its core structure is enriched with conserved cysteine and histidine residues, which play a pivotal role in anti-apoptotic activity. Structurally, the BIR domain exhibits a globular architecture formed by four α-helices and a three-stranded anti-parallel β-sheet, along with specific residues contributing to its hydrophobic core. In contrast, the RING finger domain may enhance anti-apoptotic activity in certain contexts but is insufficient to inhibit apoptosis independently. The RING finger motif, found at the carboxyl terminus, is defined by seven cysteine residues and one histidine residue that coordinates two zinc atoms. This motif facilitates interactions with the cellular ubiquitination machinery, thereby regulating the degradation of key cellular proteins [202].
The Livin gene encodes two splice variants, Livin-α and Livin-β. These variants are almost identical except for a 54-base-pair truncation at the 5′ end of exon 6 in Livin-β. The open reading frames for Livin-α and Livin-β encode proteins consisting of 298 and 280 amino acids, respectively. Both variants share the N-terminal BIR domain and the C-terminal RING domain. However, Livin-β lacks 18 amino acids in the region linking the BIR and RING domains, which may influence its structural and functional properties [202]. Livin plays a pivotal role in the inhibition of apoptosis. Studies have demonstrated that Livin directly interacts with caspase-3 and caspase-7 in vitro, as well as with caspase-9 in vivo, through its Baculovirus IAP Repeat (BIR) domain [203]. Its anti-apoptotic activity is regulated by Smac/DIABLO, a mitochondrial protein that antagonizes IAPs. The anti-apoptotic function of Livin is primarily attributed to its singular BIR domain. This domain enables Livin to bind to and inhibit caspase-9, effectively preventing the activation of apoptotic pathways. Mutations in the BIR domain significantly impair Livin’s ability to inhibit caspase-9, thereby diminishing its overall anti-apoptotic activity. Furthermore, Smac/DIABLO interacts physically with Livin and neutralizes its ability to inhibit apoptosis, underscoring the regulatory dynamics between Livin and pro-apoptotic factors [203]. In addition to its role in apoptosis inhibition, evidence indicates that Livin is also implicated in the regulation of cell proliferation. It was reported that in human malignant melanoma LiBr cells, silencing of Livin significantly induces apoptosis, arrests the cell cycle at the G0/G1 phase, and inhibits cellular proliferation [204]. These findings suggest that Livin expression is closely linked to cell cycle regulation and may play a critical role in maintaining cell proliferation dynamics [205].
The BIRC7 protein (Livin) has been implicated in the pathogenesis of gallbladder cancer (GBC), contributing to tumor progression through its anti-apoptotic functions. By inhibiting caspases via its BIR domain, BIRC7 effectively suppresses apoptosis, allowing malignant cells to evade programmed cell death and sustain unchecked growth. Furthermore, the RING-type zinc-finger domain may augment the anti-apoptotic effects, promoting cancer cell survival and resistance to therapy. In GBC, overexpression of BIRC7 has been associated with poor prognosis, increased tumor aggressiveness, and resistance to chemotherapeutic agents [206]. This highlights its potential as a biomarker for disease progression and a therapeutic target. Targeting BIRC7 through specific inhibitors could restore apoptotic pathways, thereby enhancing the efficacy of conventional treatments, and may improve patient outcomes.
5. Clinical Implications of Transcription Factors Targeting Signaling Pathways in Gallbladder Cancer
There are substantial therapeutic implications of transcription factors in GBC as diagnostic and prognostic biomarkers and as possible therapeutic targets (Table 1 and Table 2). Several small-molecule inhibitors targeting key molecular and/or signaling pathways are currently under clinical investigation for suppressing tumorigenesis in GBC as therapeutic developments (Figure 4). Studies have demonstrated that dysregulation of specific TFs correlates with tumor aggressiveness, progression, and patient outcomes. The Wnt/β-catenin pathway’s essential effector, TCF4, has been found to be associated with higher tumor grade and stage, suggesting that it could be used as a biomarker to track disease progression and a possible target for personalized molecular therapies. Overexpression of certain tumor factors is associated with a worse prognosis for patients; for example, FOXK1 has been found to activate AKT/mTOR signaling, which in turn promotes proliferation and metastasis in GBC. The roles of tumor-suppressing TFs such as SMAD4 and p53 are highlighted by dysregulation of these proteins. Mutations in SMAD4 have been associated with disrupted TGF-β signaling in around 6% of GBC cases, while mutations in p53 are prevalent in over 40%. Detection of mutant p53 protein could help with early diagnosis or risk stratification [207].
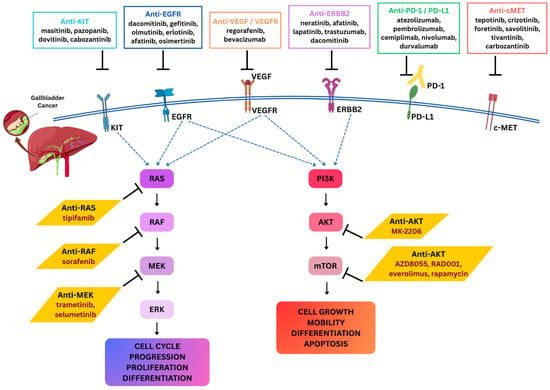
Figure 4.
Targeted therapeutic strategies and key signaling pathways in gallbladder cancer. Schematic illustration of major receptor tyrosine kinases (KIT, EGFR, VEGFR, ERBB2, PD-1/PD-L1, and c-MET) and their downstream signaling cascades implicated in GBC progression. Activation of these receptors drives the RAS signaling and AKT signaling regulating cell cycle progression, proliferation, differentiation, cell growth, mobility, differentiation, and apoptosis. Various inhibitors belonging to anti-KIT, anti-EGFR, anti-VEGF/VEGFR, anti-PD-1/PD-L1, and anti-c-MET directly target these tyrosine kinase receptors. Additionally, small-molecule inhibitors against intracellular signaling components like anti-RAS, anti-RAF, anti-MEK, and anti-AKT/mTOR directly target the downstream targets of these signaling pathways. Together, these therapeutic approaches highlight potential avenues for targeted and immune-based interventions in GBC. Doted blue arrows represent the activation pathways; black arrows represent the flow of molecular process; and the hammer-headed arrows represent inhibition.
These transcription factors are not only becoming potential therapeutic vulnerabilities, but they are also used as biomarkers. Master regulators orchestrating super-enhancer-driven programs, like SOX9, TCF7L2, and other components of colorectal cancer, display “transcriptional addiction” in GBC cells. Key oncogenic TF networks can be suppressed and tumor growth can be impaired through transcription inhibition, as demonstrated in preclinical models, especially with CDK7 inhibitors. Additionally, a reasonable anti-cancer strategy involves targeting downstream effectors of TF pathways, such as blocking AKT/mTOR signaling in tumors overexpressing FOXK1. Potentially better early detection, risk assessment, and treatment results for GBC patients might result from additional research into transcription-focused therapies and the ongoing integration of TF profiling into clinical practice [208,209]. Some of the relevant clinical trials are listed in Table 2.

Table 2.
Clinical drug trials and molecular targets of GBC along with their clinical phases [210].
Table 2.
Clinical drug trials and molecular targets of GBC along with their clinical phases [210].
| Drug Investigated | Molecular Target | Clinical Phase |
|---|---|---|
| Sorafenib | Multitargeted TKI | 2/3 |
| Afatinib | EGFR, HER2 | 2 |
| Apatinib | EGFR, HER2 | 2 |
| Trametinib | MEK | 2 |
| Guadecitabine | DNMT | 1 |
| Pembrolizumab | PD-1 (Immune checkpoint inhibitor) | 2 |
| Nivolumab + Ipilimumab | PD-1 + CTLA-4 (Immunotherapy combo) | 2 |
| Zanidatamab (Ziihera) | HER2 (bispecific antibody) | 2 |
| Neratinib | Pan-HER (HER2, EGFR) | 2 |
| Pertuzumab + Trastuzumab | HER2 (dual antibody therapy) | 2 |
| Trastuzumab deruxtecan | HER2 (antibody-drug conjugate) | 2 |
| Crelosidenib (LY3410738) | IDH1 mutation | 1 |
| Ponatinib | FGFR1–4 (pan-FGFR inhibitor) | 2 |
| Erdafitinib (JNJ-42756493) | FGFR1–4 | 2 |
| Atezolizumab + CDX-1127 (Varlilumab) ± Cobimetinib | PD-L1 + CD27 (immunotherapy) ± MEK inhibitor | 2 |
| Sintilimab + Bevacizumab + Gemcitabine + Nab-paclitaxel | PD-1 + VEGF + chemotherapy | 2 |
Abbreviations: TKI, Tyrosine kinase inhibitor; EGFR, Epidermal growth factor receptor; HER2, Human epidermal growth factor receptor 2; MEK, Mitogen-activated protein kinase; DNMT, DNA methyltransferase; PD-1, Programmed cell death protein 1; CTLA-4, Cytotoxic T-lymphocyte-associated protein 4, FGFR, Fibroblast growth factor receptor; IDH1, Isocitrate dehydrogenase 1; PD-L1, Programmed cell death ligand 1; CD27, Cluster of Differentiation 27; VEGF, Vascular endothelial growth factor.
6. Emerging Trends and Future Directions
Although various drugs targeting the dysregulated TFs in GBC are under clinical trial, effective treatment of GBC remains a significant challenge. This is largely due to the tumor heterogeneity, non-specificity, immunogenic response and chemo-resistivity arising from diverse genetic and epigenetic alterations, as well as the dynamic rewiring of TF networks and cell cycle pathways within cancer cells. Therefore, there is a need for more precise, effective and advanced therapy to deal with this lethal malignancy.
6.1. Proteolysis-Targeting Chimeras (PROTACs)
Over the past two decades, proteolysis-targeting chimeras (PROTACs) have emerged as a novel class of therapeutic molecules that exploit the ubiquitin–proteasome system (UPS) to selectively degrade target proteins. Structurally, PROTACs are bifunctional molecules composed of two ligands: one that binds the protein of interest and another that recruits an E3 ubiquitin ligase, thereby facilitating ubiquitination and subsequent proteasomal degradation [211,212]. Recent studies have demonstrated the feasibility of PROTACs in targeting transcription factors (TFs) across multiple cancer types. Notably, two small-molecule PROTACs—ARV-110, an androgen receptor (AR) degrader, and ARV-471, an estrogen receptor (ER) degrader—have advanced into clinical trials [213]. ARV-110 has shown therapeutic efficacy in prostate cancers harboring both wild-type and mutant AR, while ARV-471 has produced promising clinical responses in breast cancer patients with wild-type or mutant ER [214]. PROTAC techniques could be transferred to the treatment of GBC by leveraging their unique ability to target and degrade oncogenic proteins, specifically, transcription factors and signaling molecules that are often considered “undruggable” by conventional therapies. Identifying key oncogenic transcription factors (e.g., MYBL2, NF-kB, TCF4) that are consistently dysregulated in GBC and contribute to tumor progression, EMT, and therapy resistance could provide a more specific and precise therapy to manage the oncogenesis and progression of this cancer.
6.2. RNA-Based Therapeutics
RNA-based therapies represent an emerging class of treatments that harness or modulate RNA molecules to achieve therapeutic effects. They often exploit non-coding RNAs, including microRNAs (miRNAs), circular RNAs (circRNAs), and long non-coding RNAs (lncRNAs) [215]. A recent study demonstrated that miR-145-5p, frequently downregulated in GBC, restores tumor-suppressive signaling by activating Signal Transducer and Activator of Transcription 1 (STAT1) through direct targeting of the oncogenic regulator Protein Tyrosine Phosphatase Receptor Type F (PTPRF) [216]. Similarly, an lncRNA ZEB2-NAT binds to TF Zeb2 and masks the splicing site of internal ribosome entry sites (IRES), resulting in the enhanced expression of Zeb2, which activates EMT in colon cancer cells [217]. RNA-based therapeutics present a promising approach towards regulation of TFs and the better management of GBC.
6.3. CRISPR-Based Gene Editing
Clustered regularly interspaced palindromic repeats (CRISPR) is a genome-editing platform composed of a guide RNA that directs the system to a specific target sequence and the Cas endonuclease, which introduces a double-stranded DNA break to enable precise genetic modifications in the target sequence [218]. By employing the CRISPR/Cas system, the expression of oncogenic or tumor-suppressive TFs can be edited in order to manage the cancer progression [219]. In addition to this, recently the new concept of artificial transcription factors (ATFs) emerged for cancer drug repurposing and increased chemosensitivity. ATFs are small molecules with the ability to modulate the transcriptional ability of a gene. Among the various types of ATFs, CRISPR/dCas9 (deactivated Cas9) is the most advanced one. CRISPR/dCas9-based ATFs have an effector domain that interacts with the DNA-binding domain to either activate or repress the transcription of target gene [220]. It was observed that lung adenocarcinoma (LAD) and lung cancer, with overexpressed lncRNA KCNQ1OT1, were resistant to paclitaxel. When the drug was used in combination with CRISPR/dCas9-based ATFs, the chemosensitivity of the LAD cells significantly increased [221]. Developing these target-specific CRISPR-based gene editing strategies to directly modulate the expression of dysregulated TFs in GBC could offer a highly specific and effective therapeutic avenue, addressing a critical un-met need in the management of this malignancy.
7. Conclusions
GBC remains a highly aggressive malignancy with poor prognosis, largely due to the dysregulation of transcription factors that govern key cellular processes. This review highlights the critical role of both tumor-suppressor and oncogenic transcription factors in GBC pathogenesis, emphasizing how their altered expression drives uncontrolled proliferation, metastasis, and therapeutic resistance. While this review underscores the critical role of both oncogenic and tumor-suppressor transcription factors in GBC pathogenesis, the translation of innovative molecular therapies like PROTACs, RNA-based therapeutics, and CRISPR-based gene editing into clinical practice remains at an early but promising stage. Key milestones for translating these molecular therapies into clinical practice for GBC include comprehensive target validation in preclinical models, development of high-affinity ligands and effective delivery systems tailored to gallbladder tissue, rigorous safety and off-target profiling, early-phase clinical trials to establish safety and efficacy, integration of precision oncology tools for patient stratification, and exploration of combination therapies with existing treatments to overcome resistance. These focused efforts are essential to bridge molecular findings and enable effective targeted interventions that improve patient outcomes in this aggressive cancer.
Emerging technologies such as single-cell transcriptomics, artificial intelligence-driven drug discovery, and multi-omics integration will further enhance our understanding of transcription factor networks in GBC. Future research should also focus on identifying reliable biomarkers for early detection. Moving forward, a multidisciplinary approach incorporating genomics, immunotherapy, and personalized medicine will be key to translating these molecular insights into effective clinical interventions. While significant challenges remain, ongoing research and novel therapeutic strategies offer hope for improved diagnosis, treatment, and survival outcomes in GBC patients.
Author Contributions
All authors contributed to the manuscript as follows: The study was conceived and designed by S.K.S. (Samarendra Kumar Singh). The drafting of the manuscript was carried out by S.S., K.Y., A.S., D.A., T.S., S.K. and S.K.S. (Sonika Kumari Sharma). S.S., S.K. and S.K.S. (Sonika Kumari Sharma) were also responsible for designing the figures. Critical review and final editing of the manuscript was performed by S.K., S.K.S. (Samarendra Kumar Singh) and S.K.S. (Sonika Kumari Sharma). All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no specific funding for this study.
Acknowledgments
We would like to acknowledge the Director and Dean of the Institute of Science, Banaras Hindu University, and the Coordinator of the School of Biotechnology, Banaras Hindu University, for providing space and facilities. We are also grateful for the support and facilities provided through the DST-FIST program.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Goetze, T.O. Gallbladder carcinoma: Prognostic factors and therapeutic options. World J. Gastroenterol. 2015, 21, 12211–12217. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Dutta, U.; Bush, N.; Kalsi, D.; Popli, P.; Kapoor, V.K. Epidemiology of gallbladder cancer in India. Chin. Clin. Oncol. 2019, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sarangi, Y.; Gupta, A.; Sharma, A. Gallbladder cancer: Progress in the Indian subcontinent. World J. Clin. Oncol. 2024, 15, 695–716. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, H.; Shigihara, T.; Ikeda, T.; Takase, M.; Suyama, M. Two distinct pathways of p16 gene inactivation in gallbladder cancer. World J. Gastroenterol. 2007, 13, 6396–6403. [Google Scholar] [CrossRef] [PubMed]
- Wee, A.; Teh, M.; Raju, G.C. Clinical importance of p53 protein in gall bladder carcinoma and its precursor lesions. J. Clin. Pathol. 1994, 47, 453–456. [Google Scholar] [CrossRef]
- Tang, Q.; Guan, Y.; Ma, Y.; Li, Q.; Geng, Z. Molecular Mechanisms of Lymph Node Metastasis in Gallbladder Cancer: Insights into the Tumor Microenvironment. Biomedicines 2025, 13, 1372. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Kumar, V.; Gupta, S. Molecular Oncology of Gall Bladder Cancer. India. J. Surg. Oncol. 2021, 12, 57–64. [Google Scholar] [CrossRef]
- Zou, L.; Yang, Y.; Zhou, B.; Li, W.; Liu, K.; Li, G.; Miao, H.; Song, X.; Yang, J.; Geng, Y.; et al. tRF-3013b inhibits gallbladder cancer proliferation by targeting TPRG1L. Cell. Mol. Biol. Lett. 2022, 27, 99. [Google Scholar] [CrossRef]
- Neogi, K.; Tewari, M.; Singh, A.K.; Sharma, K.; Tej, G.N.V.C.; Verma, S.S.; Gupta, S.C.; Nayak, P.K. Transcription factor 4 expression and correlation with tumor progression in gallbladder cancer. J. Cancer Res. Ther. 2022, 18, 668–676. [Google Scholar] [CrossRef]
- Xiong, L.; Wen, Y.; Miao, X.; Yang, Z. Expressions of cell junction regulatory proteins and their association with clinicopathologic parameters in benign and malignant gallbladder lesions. Am. J. Med. Sci. 2011, 342, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Puisieux, A.; Brabletz, T.; Caramel, J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell. Biol. 2014, 16, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tilló, E.; Liu, Y.; De Barrios, O.; Siles, L.; Fanlo, L.; Cuatrecasas, M.; Darling, D.S.; Dean, D.C.; Castells, A.; Postigo, A. EMT-activating transcription factors in cancer: Beyond EMT and tumor invasiveness. Cell. Mol. Life Sci. 2012, 69, 3429–3456. [Google Scholar] [CrossRef] [PubMed]
- De Craene, B.; Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 2013, 13, 97–110. [Google Scholar] [CrossRef]
- Sasaki, T.; Kuniyasu, H.; Luo, Y.; Kato, D.; Shinya, S.; Fujii, K.; Ohmori, H.; Yamashita, Y. Significance of epithelial growth factor in the epithelial-mesenchymal transition of human gallbladder cancer cells. Cancer Sci. 2012, 103, 1165–1171. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Shu, Y.J.; Weng, H.; Ye, Y.Y.; Hu, Y.P.; Bao, R.F.; Cao, Y.; Wang, X.A.; Zhang, F.; Xiang, S.S.; Li, H.F.; et al. SPOCK1 as a potential cancer prognostic marker promotes the proliferation and metastasis of gallbladder cancer cells by activating the PI3K/AKT pathway. Mol. Cancer 2015, 14, 12. [Google Scholar] [CrossRef]
- Kawamoto, M.; Onishi, H.; Ozono, K.; Yamasaki, A.; Imaizumi, A.; Nakamura, M. TrkB/BDNF signaling promotes EMT mediated invasiveness and is a potential therapeutic target for gallbladder cancer. Ann. Oncol. 2016, 27, vi236. [Google Scholar] [CrossRef][Green Version]
- Lee, D.G.; Lee, S.H.; Kim, J.S.; Park, J.; Cho, Y.L.; Kim, K.S.; Jo, D.Y.; Song, I.C.; Kim, N.; Yun, H.J.; et al. Loss of NDRG2 promotes epithelial-mesenchymal transition of gallbladder carcinoma cells through MMP-19-mediated Slug expression. J. Hepatol. 2015, 63, 1429–1439. [Google Scholar] [CrossRef]
- Adachi, Y.; Takeuchi, T.; Nagayama, T.; Ohtsuki, Y.; Furihata, M. Zeb1-mediated T-cadherin repression increases the invasive potential of gallbladder cancer. FEBS Lett. 2009, 583, 430–436. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, Y.; Ma, L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle 2015, 14, 481–487. [Google Scholar] [CrossRef]
- Qin, Y.; Gong, W.; Zhang, M.; Wang, J.; Tang, Z.; Quan, Z. Forkhead box L1 is frequently downregulated in gallbladder cancer and inhibits cell growth through apoptosis induction by mitochondrial dysfunction. PLoS ONE 2014, 9, e102084. [Google Scholar] [CrossRef]
- Zhang, M.; Gong, W.; Zhang, Y.; Yang, Y.; Zhou, D.; Weng, M.; Qin, Y.; Jiang, A.; Ma, F.; Quan, Z. Expression of interleukin-6 is associated with epithelial-mesenchymal transition and survival rates in gallbladder cancer. Mol. Med. Rep. 2015, 11, 3539–3546. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Dai, Y.; Wang, J.; Suo, T.; Pan, H.; Liu, H.; Shen, S.; Liu, H. CIZ1 promoted the growth and migration of gallbladder cancer cells. Tumor Biol. 2015, 36, 3539–3546. [Google Scholar] [CrossRef] [PubMed]
- Eferl, R.; Wagner, E.F. AP-1: A double-edged sword in tumorigenesis. Nat. Rev. Cancer 2003, 3, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Shaulian, E. AP-1—The Jun proteins: Oncogenes or tumor suppressors in disguise? Cell. Signal. 2010, 22, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Lian, S.; Shao, Y.; Liu, H.; He, J.; Lu, W.; Zhang, Y.; Jiang, Y.; Zhu, J. PDK1 induces JunB, EMT, cell migration and invasion in human gallbladder cancer. Oncotarget 2015, 6, 29076–29086. [Google Scholar] [CrossRef]
- Shi, Y.J.; Matson, C.; Lan, F.; Iwase, S.; Baba, T.; Shi, Y. Regulation of LSD1 histone demethylase activity by its associated factors. Mol. Cell. 2005, 19, 857–864. [Google Scholar] [CrossRef]
- Lian, S.; Shao, Y.; Liu, H.; He, J.; Lu, W.; Zhang, Y.; Jiang, Y.; Zhu, J. Lysine-specific demethylase 1 promotes tumorigenesis and predicts prognosis in gallbladder cancer. Oncotarget 2015, 6, 33065–33076. [Google Scholar] [CrossRef][Green Version]
- Shi, Y.; Lan, F.; Matson, C.; Mulligan, P.; Whetstine, J.R.; Cole, P.A.; Casero, R.A.; Shi, Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 2004, 119, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, Y.; Li, J.; Dong, C.; Ye, X.; Chi, Y.I.; Evers, B.M.; Zhou, B.P. The SNAG domain of snail1 functions as a molecular hook for recruiting lysine-specific demethylase 1. EMBO J. 2010, 29, 1803–1816. [Google Scholar] [CrossRef] [PubMed]
- Den Hollander, P.; Kumar, R. Cip-Interacting Zinc Finger Protein 1 Ciz1. In Encyclopedia of Cancer; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Lin, B.; Hong, H.J.; Jiang, X.J.; Li, C.Z.; Zhu, S.Y.; Tang, N.H.; Wang, X.Q.; She, F.F.; Chen, Y.L. WNT inhibitory factor 1 promoter hypermethylation is an early event during gallbladder cancer tumorigenesis that predicts poor survival. Gene 2017, 622, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Hong, H.; Jiang, X.; Li, C.; Zhu, S.; Tang, N.; Wang, X.; She, F.; Chen, Y. C-Jun suppresses the expression of WNT inhibitory factor 1 through transcriptional regulation and interaction with DNA methyltransferase 1 in gallbladder cancer. Mol. Med. Rep. 2018, 17, 8180–8188. [Google Scholar] [CrossRef]
- Qiang, J.; Zhao, C.; Shi, L.Q.; Sun, S.R.; Wang, H.K.; Liu, S.L.; Yang, Z.Y.; Dong, P.; Xiang, S.S.; Wang, J.D.; et al. BRD9 promotes the progression of gallbladder cancer via CST1 upregulation and interaction with FOXP1 through the PI3K/AKT pathway and represents a therapeutic target. Gene Ther. 2024, 31, 594–606. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, K.L.; Gupta, A.; Yadav, A.; Kumar, A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J. Gastroenterol. 2017, 23, 3978–3998. [Google Scholar] [CrossRef]
- Wistuba, I.I.; Albores-Saavedra, J. Genetic abnormalities involved in the pathogenesis of gallbladder carcinoma. J. Hepatobiliary Pancreat. Surg. 1999, 6, 237–244. [Google Scholar] [CrossRef]
- Itoi, T.; Watanabe, H.; Ajioka, Y.; Oohashi, Y.; Takei, K.; Nishikura, K.; Nakamura, Y.; Horii, A.; Saito, T. APC, K-ras codon 12 mutations and p53 gene expression in carcinoma and adenoma of the gall-bladder suggest two genetic pathways in gall-bladder carcinogenesis. Pathol. Int. 1996, 46, 333–340. [Google Scholar] [CrossRef]
- Kuroki, T.; Tajima, Y.; Matsuo, K.; Kanematsu, T. Genetic alterations in gallbladder carcinoma. Surg. Today 2005, 35, 101–105. [Google Scholar] [CrossRef]
- Maurya, S.K.; Tewari, M.; Mishra, R.R.; Shukla, H.S. Genetic abberations in gallbladder cancer. Surg. Oncol. 2012, 21, 37–43. [Google Scholar] [CrossRef]
- Lam, J.Y.C.; Choo, S.P.; Teh, B.T. HER2 as a therapeutic target in gallbladder cancer-aye or nay? Transl. Cancer Res. 2016, 5, 7–10. [Google Scholar] [CrossRef]
- Sicklick, J.K.; Fanta, P.T.; Shimabukuro, K.; Kurzrock, R. Genomics of gallbladder cancer: The case for biomarker-driven clinical trial design. Cancer Metastasis Rev. 2016, 35, 263–275. [Google Scholar] [CrossRef]
- Andrén-Sandberg, Å. Molecular biology of Gallbladder cancer: Potential clinical implications. N. Am. J. Med. Sci. 2012, 4, 435–441. [Google Scholar] [CrossRef]
- Nigam, P.; Misra, U.; Negi, T.S.; Mittal, B.; Choudhuri, G. Alterations of p53 gene in gallbladder cancer patients of North India. Trop. Gastroenterol. 2010, 31, 96–100. [Google Scholar] [CrossRef]
- Barreto, S.G.; Dutt, A.; Chaudhary, A. A genetic model for gallbladder carcinogenesis and its dissemination. Ann. Oncol. 2014, 25, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Mishra, P.K.; Saluja, S.S.; Talikoti, M.A.; Kirtani, P.; Najmi, A.K. Prognostic significance of HER-2 and p53 expression in gallbladder carcinoma in North Indian Patients. Oncology 2016, 91, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Patel, B.; Kumar, M.; Shukla, M.; Pandey, M. Cyclin d1, retinoblastoma and p16 protein expression in carcinoma of the gallbladder. Asian Pac. J. Cancer Prev. 2013, 14, 2711–2715. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.T.; Wang, J.H.; Yu, Y.; Liu, C.; Li, B.; Cheng, Q.B.; Jiang, X.Q. Tumor suppressor LKB1 inhibits the progression of gallbladder carcinoma and predicts the prognosis of patients with this malignancy. Int. J. Oncol. 2018, 53, 1215–1226. [Google Scholar] [CrossRef]
- Zong, H.; Yin, B.; Zhou, H.; Cai, D.; Ma, B.; Xiang, Y. Loss of angiotensin-converting enzyme 2 promotes growth of gallbladder cancer. Tumor Biol. 2015, 36, 5171–5177. [Google Scholar] [CrossRef]
- Levine, A.J. p53, the Cellular Gatekeeper Review for Growth and Division. Cell 1997, 88, 323–331. [Google Scholar] [CrossRef]
- Vogelstein, B.; Kinzler, K.W. p53 Function and Dysfunction. Cell 1992, 70, 523–526. [Google Scholar] [CrossRef]
- Shieh, S.-Y.; Ikeda, M.; Taya, Y.; Prives, C. DNA Damage-Induced Phosphorylation of p53 Alleviates Inhibition by MDM2 phosphorylated at sites within its N-terminal and C-termi-nal regions, and several protein kinases have been shown to phosphorylate p53 in vitro. One such kinase. Cell 1997, 91, 325–334. [Google Scholar] [CrossRef]
- Saetta, A.A. K-ras, p53 Mutations, and Microsatellite Instability (MSI) in Gallbladder Cancer. J. Surg. Oncol. 2006, 93, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Takagawa, M.; Muguruma, N.; Oguri, K.; Imoto, Y.; Okamoto, K.; Ii, K.; Ito, S. Prediction of prognosis in gallbladder carcinoma by mucin and p53 immunohistochemistry. Dig. Dis. Sci. 2005, 50, 1410–1413. [Google Scholar] [CrossRef] [PubMed]
- Oohashi, Y.; Watanabe, H.; Ajioka, Y.; Hatakeyama, K. p53 lmrnunostaining distinguishes malignant from benign lesions of the gall-bladder. Pathol. Int. 1995, 45, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, H.; de Bitter, T.J.J.; de Boer, M.T.; van der Post, R.S.; Nijkamp, M.W.; de Reuver, P.R.; Fehrmann, R.S.N.; Hoogwater, F.J.H. Gallbladder Cancer: Current Insights in Genetic Alterations and Their Possible Therapeutic Implications. Cancers 2021, 13, 5257. [Google Scholar] [CrossRef]
- Tewari, M. Contribution of silent gallstones in gallbladder cancer. J. Surg. Oncol. 2006, 93, 629–632. [Google Scholar] [CrossRef]
- Ishida, H.; Irie, K.; Itoh, T.; Furukawa, T.; Tokunaga, O. The prognostic significance of p53 and bcl-2 expression in lung adenocarcinoma and its correlation with Ki-67 growth fraction. Cancer 1997, 80, 1034–1045. [Google Scholar] [CrossRef]
- Puzio-Kuter, A.M.; Castillo-Martin, M.; Kinkade, C.W.; Wang, X.; Shen, T.H.; Matos, T.; Shen, M.M.; Cordon-Cardo, C.; Abate-Shen, C. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes. Dev. 2009, 23, 675. [Google Scholar] [CrossRef]
- Kim, Y.-T.; Kim, J.; Jang, Y.H.; Lee, W.J.; Ryu, J.K.; Park, Y.-K.; Kim, S.W.; Kim, W.H.; Yoon, Y.B.; Kim, C.Y. Genetic alterations in gallbladder adenoma, dysplasia and carcinoma. Cancer Lett. 2001, 169, 59–68. [Google Scholar] [CrossRef]
- Ma, H.B.; Hu, H.T.; Di, Z.L.; Wang, Z.R.; Shi, J.S.; Wang, X.J.; Li, Y. Association of cycl1in D1, p16 and retinoblastoma protein expressions with prognosis and metastasis of gallbladder carcinoma. World J. Gastroenterol. 2005, 11, 744–747. [Google Scholar] [CrossRef]
- Shi, Y.-Z.; Hui, A.-M.; Li, X.; Takayama, T.; Makuuchi, M. Overexpression of Retinoblastoma Protein Predicts Decreased Survival and Correlates with Loss of p16 INK4 Protein in Gallbladder Carcinomas. Clin. Cancer Res. 2000, 6, 4096–4100. [Google Scholar]
- Quan, Z.W.; Wu, K.; Wang, J.; Shi, W.; Zhang, Z.; Merrell, R.C. Association of p53; p16, and Vascular Endothelial Growth Factor Protein Expressions with the Prognosis and Metastasis of Gallbladder Cancer. J. Am. Coll. Surg. 2001, 193, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Wistuba, I.I.; Sugio, K.; Hung, J.; Kishimoto, Y.; Virmani, A.K.; Roa, I.; Albores-Saavedra, J.; Gazdar, A.F. Allele-specific Mutations Involved in the Pathogenesis of Endemic Gallbladder Carcinoma in Chile. Cancer Res. 1995, 55, 2511–2515. [Google Scholar] [PubMed]
- Witkiewicz, A.K.; Knudsen, K.E.; Dicker, A.P.; Knudsen, E.S. The meaning of p16ink4a expression in tumors: Functional significance, clinical associations and future developments. Cell Cycle 2011, 10, 2497–2503. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.A. The Retinoblastoma Protein and Cell Cycle Control. Cell 1995, 81, 323–330. [Google Scholar] [CrossRef]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef]
- Feng, Z.; Chen, J.; Wei, H.; Gao, P.; Shi, J.; Zhang, J.; Zhao, F. The risk factor of gallbladder cancer: Hyperplasia of mucous epithelium caused by gallstones associates with p16/CyclinD1/CDK4 pathway. Exp. Mol. Pathol. 2011, 91, 569–577. [Google Scholar] [CrossRef]
- Yoshida, S.; Todoroki, T.; Ichikawa, Y.; Hanai, S.; Suzuki, H.; Hori, M.; Fukao, K.; Miwa, M.; Uchida, K. Mutations of p16Ink4/CDKN2 and p15Ink4B/MTS2 genes in biliary tract cancers. Cancer Res. 1995, 55, 2756–2760. [Google Scholar]
- Tannapfel, A.; Benicke, M.; Katalinic, A.; Uhlmann, D.; Köckerling, F.; Hauss, J.; Wittekind, C. Frequency of p16 INK4A alterations and k-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut 2000, 47, 721–727. [Google Scholar] [CrossRef]
- Caca, K.; Feisthammel, J.; Klee, K.; Tannapfel, A.; Witzigmann, H.; Wittekind, C.; Mössner, J.; Berr, F. Inactivation of the INK4a/ARF locus and p53 in sporadic extrahepatic bile duct cancers and bile tract cancer cell lines. Int. J. Cancer 2002, 97, 481–488. [Google Scholar] [CrossRef]
- Ku, J.L.; Yoon, K.A.; Kim, I.J.; Kim, W.H.; Jang, J.Y.; Suh, K.S.; Kim, S.W.; Park, Y.H.; Hwang, J.H.; Yoon, Y.B.; et al. Establishment and characterisation of six human biliary tract cancer cell lines. Br. J. Cancer 2002, 87, 187–193. [Google Scholar] [CrossRef]
- Ueki, T.; Hsing, A.W.; Gao, Y.-T.; Wang, B.-S.; Shen, M.-C.; Cheng, J.; Deng, J.; Fraumeni, J.F.; Rashid, A. Alterations of p16 and Prognosis in Biliary Tract Cancers from a Population-Based Study in China. Clin. Cancer Res. 2004, 10, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Klump, B.; Hsieh, C.-J.; Dette, S.; Holzmann, K.; Kielich, R.; Jung, M.; Sinn, U.; Ortner, M.; Porschen, R.; Gregor, M. Promoter Methylation of INK4a/ARF As Detected in Bile-Significance for the Differential Diagnosis in Biliary Disease. Clin. Cancer Res. 2003, 9, 1773–1778. [Google Scholar] [PubMed]
- Kim, K.; Kim, D.H.; Chae, S.W.; Shin, J.H.; Kim, H.J.; Do, S.I.; Lee, H.J.; Koo, J.H.; Pyo, J.S.; Sohn, J.H. Expression of cell cycle-related proteins, p16, p53 and p63 as important prognostic markers in gallbladder Adenocarcinoma. Pathol. Oncol. Res. 2014, 20, 409–415. [Google Scholar] [CrossRef] [PubMed]
- House, M.G.; Wistuba, I.I.; Argani, P.; Guo, M.Z.; Schulick, R.D.; Hruban, R.H.; Herman, J.G.; Maitra, A. Progression of gene hypermethylation in gallstone disease leading to gallbladder cancer. Ann. Surg. Oncol. 2003, 10, 882–889. [Google Scholar] [CrossRef]
- Tozawa, T.; Tamura, G.; Honda, T.; Nawata, S.-I.; Kimura, W.; Makino, N.; Kawata, S.; Sugai, T.; Suto, T.; Motoyama, T. Promoter hypermethylation of DAP-kinase is associated with poor survival in primary biliary tract carcinoma patients. Cancer Sci. 2004, 95, 736–740. [Google Scholar] [CrossRef]
- Takahashi, T.; Shivapurkar, N.; Riquelme, E.; Shigematsu, H.; Reddy, J.; Suzuki, M.; Miyajima, K.; Zhou, X.; Bekele, B.N.; Gazdar, A.F.; et al. Aberrant Promoter Hypermethylation of Multiple Genes in Gallbladder Carcinoma and Chronic Cholecystitis. Clin. Cancer Res. 2004, 10 Pt 1, 6126–6133. [Google Scholar] [CrossRef]
- Rader, J.; Russell, M.R.; Hart, L.S.; Nakazawa, M.S.; Belcastro, L.T.; Martinez, D.; Li, Y.; Carpenter, E.L.; Attiyeh, E.F.; Diskin, S.J.; et al. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin. Cancer Res. 2013, 19, 6173–6182. [Google Scholar] [CrossRef]
- Dickson, M.A. Molecular pathways: CDK4 inhibitors for cancer therapy. Clin. Cancer Res. 2014, 20, 3379–3383. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Sicinska, E.; Czaplinski, J.T.; Remillard, S.P.; Moss, S.; Wang, Y.; Brain, C.; Loo, A.; Snyder, E.L.; Demetri, G.D.; et al. Antiproliferative effects of CDK4/6 inhibition in CDK4-amplified human liposarcoma in vitro and in vivo. Mol. Cancer Ther. 2014, 13, 2184–2193. [Google Scholar] [CrossRef]
- Vora, S.R.; Juric, D.; Kim, N.; Mino-Kenudson, M.; Huynh, T.; Costa, C.; Lockerman, E.L.; Pollack, S.F.; Liu, M.; Li, X.; et al. CDK 4/6 Inhibitors Sensitize PIK3CA Mutant Breast Cancer to PI3K Inhibitors. Cancer Cell 2014, 26, 136–149. [Google Scholar] [CrossRef]
- Gelbert, L.M.; Cai, S.; Lin, X.; Sanchez-Martinez, C.; Del Prado, M.; Lallena, M.J.; Torres, R.; Ajamie, R.T.; Wishart, G.N.; Flack, R.S.; et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: In-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Investig. New Drugs 2014, 32, 825–837. [Google Scholar] [CrossRef]
- Tate, S.C.; Cai, S.; Ajamie, R.T.; Burke, T.; Beckmann, R.P.; Chan, E.M.; De Dios, A.; Wishart, G.N.; Gelbert, L.M.; Cronier, D.M. Semi-mechanistic pharmacokinetic/pharmacodynamic modeling of the antitumor activity of LY2835219, a new cyclin-dependent kinase 4/6 inhibitor, in mice bearing human tumor xenografts. Clin. Cancer Res. 2014, 20, 3763–3774. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Chen, S.; Li, G.; Pu, P.; Yang, Y.; Wu, W.; Geng, Y.; Liu, Y. GPRC5A promotes gallbladder cancer metastasis by upregulating TNS4 via the JAK2–STAT3 pathway. Cancer Lett. 2024, 598, 217067. [Google Scholar] [CrossRef]
- Wencong, M.; Jinghan, W.; Yong, Y.; Jianyang, A.; Bin, L.; Qingbao, C.; Chen, L.; Xiaoqing, J. FOXK1 Promotes Proliferation and Metastasis of Gallbladder Cancer by Activating AKT/mTOR Signaling Pathway. Front. Oncol. 2020, 10, 545. [Google Scholar] [CrossRef]
- FENG, Y.; BAI, Z.; SONG, J.; ZHANG, Z. FOXK1 plays an oncogenic role in the progression of hilar cholangiocarcinoma. Mol. Med. Rep. 2021, 23, 91. [Google Scholar] [CrossRef]
- Sepp, M.; Kannike, K.; Eesmaa, A.; Urb, M.; Timmusk, T. Functional diversity of human basic helix-loop-helix transcription factor TCF4 isoforms generated by alternative 5′ exon usage and splicing. PLoS ONE 2011, 6, e22138. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.R.; Szeto, R.A.; Carvalho, V.M.A.; Muotri, A.R.; Papes, F. Transcription factor 4 and its association with psychiatric disorders. Transl. Psychiatry 2021, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, M.D.; Polleux, F. Neuronal and glial cell biology. Curr. Opin. Neurobiol. 2010, 20, 529–530. [Google Scholar] [CrossRef] [PubMed]
- Van De Wetering, M.; Sancho, E.; Verweij, C.; De Lau, W.; Oving, I.; Hurlstone, A.; Van Der Horn, K.; Batlle, E.; Coudreuse, D.; Haramis, A.-P.; et al. The beta-Catenin/TCF-4 Complex Imposes a Crypt Progenitor Phenotype on Colorectal Cancer Cells renewal occurs in these crypts through a coordinated series of events involving proliferation, differentiation, and migration toward the intestinal lumen. Pluripotent cation compartment divide approximately every 12 h. Cell 2002, 111, 241–250. [Google Scholar]
- Sepp, M.; Pruunsild, P.; Timmusk, T. Pitt-Hopkins syndrome-associated mutations in TCF4 lead to variable impairment of the transcription factor function ranging from hypomorphic to dominant-negative effects. Hum. Mol. Genet. 2012, 21, 2873–2888. [Google Scholar] [CrossRef]
- Forrest, M.; Chapman, R.M.; Doyle, A.M.; Tinsley, C.L.; Waite, A.; Blake, D.J. Functional analysis of TCF4 missense mutations that cause Pitt-Hopkins syndrome. Hum. Mutat. 2012, 33, 1676–1686. [Google Scholar] [CrossRef]
- De Pooter, R.F.; Kee, B.L. E proteins and the regulation of early lymphocyte development. Immunol. Rev. 2010, 238, 93–109. [Google Scholar] [CrossRef]
- Kawai-Kowase, K.; Kumar, M.S.; Hoofnagle, M.H.; Yoshida, T.; Owens, G.K. PIAS1 Activates the Expression of Smooth Muscle Cell Differentiation Marker Genes by Interacting with Serum Response Factor and Class I Basic Helix-Loop-Helix Proteins. Mol. Cell Biol. 2005, 25, 8009–8023. [Google Scholar] [CrossRef]
- Fischer, B.; Azim, K.; Hurtado-Chong, A.; Ramelli, S.; Fernández, M.; Raineteau, O. E-proteins orchestrate the progression of neural stem cell differentiation in the postnatal forebrain. Neural Dev. 2014, 9, 23. [Google Scholar] [CrossRef]
- Furumura, M.; Potterf, S.B.; Toyofuku, K.; Matsunaga, J.; Muller, J.; Hearing, V.J. Involvement of ITF2 in the Transcriptional Regulation of Melanogenic Genes. J. Biol. Chem. 2001, 276, 28147–28154. [Google Scholar] [CrossRef] [PubMed]
- Beck, G.R.; Zerler, B.; Moran, E. Gene Array Analysis of Osteoblast Differentiation. Cell Growth Differ. 2001, 12, 61–83. [Google Scholar] [PubMed]
- Tanaka, A.; Itoh, F.; Nishiyama, K.; Takezawa, T.; Kurihara, H.; Itoh, S.; Kato, M. Inhibition of endothelial cell activation by bHLH protein E2-2 and its impairment of angiogenesis. Blood 2010, 115, 4138–4147. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, S.; Lin, C.Q.; Murata, K.; Itahana, Y.; Singh, J.; Krtolica, A.; Campisi, J.; Desprez, P.Y. Id-1, ITF-2, and Id-2 Comprise a Network of Helix-Loop-Helix Proteins That Regulate Mammary Epithelial Cell Proliferation, Differentiation, and Apoptosis. J. Biol. Chem. 2001, 276, 39213–39219. [Google Scholar] [CrossRef]
- Meinhardt, G.; Husslein, P.; Knöfler, M. Tissue-specific and ubiquitous basic helix-loop-helix transcription factors in human placental trophoblasts. Placenta 2005, 26, 527–539. [Google Scholar] [CrossRef]
- Muir, T.; Sadler-Riggleman, I.; Stevens, J.D.; Skinner, M.K. Role of the basic helix-loop-helix protein ITF2 in the hormonal regulation of sertoli cell differentiation. Mol. Reprod. Dev. 2006, 73, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Y.; Guo, S.; Chen, H. T cell factor-4 functions as a co-activator to promote NF-kB-dependent MMP-15 expression in lung carcinoma cells. Sci. Rep. 2016, 6, 24025. [Google Scholar] [CrossRef]
- Jun, C.L.; Zhao, D.H.; Hong, J.J.; Guo, S.Y.; Yang, R.L.; Yuan, J.; Wen, C.J.; Zhou, K.Y. Aberrant expression and function of TCF4 in the proliferation of hepatocellular carcinoma cell line BEL-7402. Cell Res. 2004, 14, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Musa, J.; Aynaud, M.M.; Mirabeau, O.; Delattre, O.; Grünewald, T.G.P. MYBL2 (B-Myb): A central regulator of cell proliferation, cell survival and differentiation involved in tumorigenesis. Cell Death Dis. 2017, 8, e2895. [Google Scholar] [CrossRef]
- Martinez, I.; DiMaio, D. B-Myb, cancer, senescence, and MicroRNAs. Cancer Res. 2011, 71, 5370–5373. [Google Scholar] [CrossRef]
- Yu, Z.; Kim, J.; He, L.; Creighton, C.J.; Gunaratne, P.H.; Hawkins, S.M.; Matzuk, M.M. Functional analysis of miR-34c as a putative tumor suppressor in high-grade serous ovarian cancer. Biol. Reprod. 2014, 91, 113. [Google Scholar] [CrossRef]
- Fischer, M.; Quaas, M.; Steiner, L.; Engeland, K. The p53-p21-DREAM-CDE/CHR pathway regulates G2/M cell cycle genes. Nucleic Acids Res. 2016, 44, 164–174. [Google Scholar] [CrossRef]
- Fischer, M.; Quaas, M.; Nickel, A. Engeland, Indirect p53-dependent transcriptional repression of Survivin, CDC25C, and PLK1 genes requires the cyclin-dependent kinase inhibitor p21/CDKN1A and CDE/CHR promoter sites binding the DREAM complex. Oncotarget 2015, 6, 41402–41417. [Google Scholar] [CrossRef]
- Lam, E.W.; Morris, J.D.; Davies, R.; Crook, T.; Watson, R.J.; Vousden, K.H. HPV16 E7 oncoprotein deregulates B-myb expression: Correlation with targeting of p107/E2F complexes. EMBO J. 1994, 13, 871–878. [Google Scholar] [CrossRef]
- Heinrichs, S.; Conover, L.F.; Bueso-Ramos, C.E.; Kilpivaara, O.; Stevenson, K.; Neuberg, D.; Loh, M.L.; Wu, W.S.; Rodig, S.J.; Garcia-Manero, G.; et al. MYBL2 is a sub-haploinsufficient tumor suppressor gene in myeloid malignancy. eLife 2013, 2013, e00825. [Google Scholar] [CrossRef]
- Clarke, M.; Dumon, S.; Ward, C.; Jäger, R.; Freeman, S.; Dawood, B.; Sheriff, L.; Lorvellec, M.; Kralovics, R.; Frampton, J.; et al. MYBL2 haploinsufficiency increases susceptibility to age-related haematopoietic neoplasia. Leukemia 2013, 27, 661–670. [Google Scholar] [CrossRef][Green Version]
- Liang, H.B.; Cao, Y.; Ma, Q.; Shu, Y.J.; Wang, Z.; Zhang, F.; Ye, Y.Y.; Li, H.F.; Xiang, S.S.; Song, X.L.; et al. MYBL2 is a potential prognostic marker that promotes cell proliferation in gallbladder cancer. Cell. Physiol. Biochem. 2017, 41, 2117–2131. [Google Scholar] [CrossRef]
- Smale, S.T. Dimer-specific regulatory mechanisms within the NF-jB family of transcription factors. Immunol. Rev. 2012, 246, 193–204. [Google Scholar] [CrossRef]
- Chua, H.L.; Bhat-Nakshatri, P.; Clare, S.E.; Morimiya, A.; Badve, S.; Nakshatri, H. NF-kB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2. Oncogene 2007, 26, 711–724. [Google Scholar] [CrossRef]
- Yang, J.; Pan, W.H.; Clawson, G.A.; Richmond, A. Systemic targeting inhibitor of κB kinase inhibits melanoma tumor growth. Cancer Res. 2007, 67, 3127–3134. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Z.; Bai, L.; Lin, Y. NF-kappaB in lung cancer, a carcinogenesis mediator and a prevention and therapy target. Front. Biosci. 2011, 16, 1172–1185. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Mullany, L.E.; Sakoda, L.; Samowitz, W.S.; Wolff, R.K.; Stevens, J.R.; Herrick, J.S. The NF-kB signalling pathway in colorectal cancer: Associations between dysregulated gene and miRNA expression. J. Cancer Res. Clin. Oncol. 2018, 144, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, C.M.; Davis, R.E.; Demchenko, Y.; Bellamy, W.; Gabrea, A.; Zhan, F.; Lenz, G.; Hanamura, I.; Wright, G.; Xiao, W.; et al. Frequent Engagement of the Classical and Alternative NF-kB Pathways by Diverse Genetic Abnormalities in Multiple Myeloma. Cancer Cell 2007, 12, 115–130. [Google Scholar] [CrossRef]
- Weichert, W.; Boehm, M.; Gekeler, V.; Bahra, M.; Langrehr, J.; Neuhaus, P.; Denkert, C.; Imre, G.; Weller, C.; Hofmann, H.P.; et al. High expression of RelA/p65 is associated with activation of nuclear factor-κB-dependent signaling in pancreatic cancer and marks a patient population with poor prognosis. Br. J. Cancer 2007, 97, 523–530. [Google Scholar] [CrossRef]
- Izzo, J.G.; Malhotra, U.; Wu, T.T.; Luthra, R.; Correa, A.M.; Swisher, S.G.; Hofstetter, W.; Chao, K.S.C.; Hung, M.C.; Ajani, J.A. Clinical biology of esophageal adenocarcinoma after surgery is influenced by nuclear factor-κB expression. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1200–1205. [Google Scholar] [CrossRef][Green Version]
- Rae, C.; Langa, S.; Tucker, S.J.; Macewan, D.J. Elevated NF-B responses and FLIP levels in leukemic but not normal lymphocytes: Reduction by salicylate allows TNF-induced apoptosis. Proc. Natl. Acad. Sci. USA 2007, 104, 12790–12795. [Google Scholar] [CrossRef]
- Bao, D.; Yuan, R.X.; Zhang, Y. Effects of lncRNA MEG3 on proliferation and apoptosis of gallbladder cancer cells through regulating NF-kB signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6632–6638. [Google Scholar] [CrossRef]
- Angel, P.; Karin, M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta 1991, 1072, 129–157. [Google Scholar] [CrossRef]
- Hai, T.; Currant, T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity (gene regulation/oncogenes/leucine zipper/protein dimerization/transcription). Proc. Natl. Acad. Sci. USA 1911, 88, 3720–3724. [Google Scholar] [CrossRef] [PubMed]
- Chinenov, Y.; Kerppola, T.K. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 2001, 20, 2438–2452. [Google Scholar] [CrossRef] [PubMed]
- Atsaves, V.; Leventaki, V.; Rassidakis, G.Z.; Claret, F.X. AP-1 transcription factors as regulators of immune responses in cancer. Cancers 2019, 11, 1037. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.Y.; Albea, D.M.; Goodwin, Z.A.; Quiggle, A.M.; Baker, B.P.; Guggisberg, A.M.; Geahlen, J.H.; Kroner, G.M.; De Guzman Strong, C. Regulation of the dynamic chromatin architecture of the epidermal differentiation complex is mediated by a c-Jun/AP-1-modulated enhancer. J. Investig. Dermatol. 2014, 134, 2371–2380. [Google Scholar] [CrossRef]
- Bejjani, F.; Evanno, E.; Zibara, K.; Piechaczyk, M.; Jariel-Encontre, I. The AP-1 transcriptional complex: Local switch or remote command? Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 11–23. [Google Scholar] [CrossRef]
- MacLaren, A.; Black, E.J.; Clark, W.; Gillespie, D.A.F. c-Jun-Deficient Cells Undergo Premature Senescence as a Result of Spontaneous DNA Damage Accumulation. Mol. Cell Biol. 2004, 24, 9006–9018. [Google Scholar] [CrossRef]
- Johnston, I.M.; Spence, H.J.; Winnie, J.N.; Mcgarry, L.; Vass, J.K.; Meagher, L.; Stapleton, G.; Ozanne, B.W. Regulation of a multigenic invasion programme by the transcription factor, AP-1: Re-expression of a down-regulated gene, TSC-36, inhibits invasion. Oncogene 2000, 19, 5348–5358. [Google Scholar] [CrossRef]
- Schreiber, M.; Kolbus, A.; Piu, F.; Szabowski, A.; Hle-Steinlein, U.M.; Tian, J.; Karin, M.; Angel, P.; Wagner, E.F. Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev. 1999, 13, 607–619. [Google Scholar] [CrossRef]
- Wu, Z.; Nicoll, M.; Ingham, R.J. AP-1 family transcription factors: A diverse family of proteins that regulate varied cellular activities in classical hodgkin lymphoma and ALK+ ALCL. Exp. Hematol. Oncol. 2021, 10, 4. [Google Scholar] [CrossRef]
- Talotta, F.; Casalino, L.; Verde, P. The nuclear oncoprotein Fra-1: A transcription factor knocking on therapeutic applications’ door. Oncogene 2020, 39, 4491–4506. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, Z.; Yu, J.; Wu, J.; Zhuo, X.; Chen, Q.; Liang, Y.; Li, G.; Wan, Y. MiR-195-5p suppresses the proliferation, migration, and invasion of gallbladder cancer cells by targeting FOSL1 and regulating the Wnt/β-catenin pathway. Ann. Transl. Med. 2022, 10, 893. [Google Scholar] [CrossRef] [PubMed]
- Fialka, I.; Schwarz, H.; Reichmann, E.; Oft, M.; Busslinger, M.; Beug, H. The Estrogen-dependent c-JunER Protein Causes a Reversible Loss of Mammary Epithelial Cell Polarity Involving a Destabilization of Adherens Junctions. J. Cell Biol. 1996, 132, 1115–1132. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.F.; Morais, A.S.; Jasiulionis, M.G. Biomarkers as key contributors in treating malignant melanoma metastases. Dermatol. Res. Pract. 2012, 2012, 156068. [Google Scholar] [CrossRef]
- Lu, Z.; Ghosh, S.; Wang, Z.; Hunter, T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of-catenin, and enhanced tumor cell invasion. Cancer Cell 2003, 4, 499–515. [Google Scholar] [CrossRef]
- Peinado, H.; Quintanilla, M.; Cano, A. Transforming growth factor β-1 induces Snail transcription factor in epithelial cell lines. Mechanisms for epithelial mesenchymal transitions. J. Biol. Chem. 2003, 278, 21113–21123. [Google Scholar] [CrossRef]
- Peiró, S.; Escrivà, M.; Puig, I.; Barberà, M.J.; Dave, N.; Herranz, N.; Larriba, M.J.; Takkunen, M.; Francí, C.; Muñoz, A.; et al. Snail1 transcriptional repressor binds to its own promoter and controls its expression. Nucleic Acids Res. 2006, 34, 2077–2084. [Google Scholar] [CrossRef]
- De Craene, B.; Van Roy, F.; Berx, G. Unraveling signalling cascades for the Snail family of transcription factors. Cell. Signal. 2005, 17, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Kielbik, M.; Szulc-Kielbik, I.; Klink, M. Snail transcription factors—Characteristics, regulation and molecular targets relevant in vital cellular activities of ovarian cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119705. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.A.; Wang, R.; Miao, J.; Oliva, E.; Shen, X.; Wheeler, T.; Hilsenbeck, S.G.; Orsulic, S.; Goode, S. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 2010, 70, 8517–8525. [Google Scholar] [CrossRef] [PubMed]
- Steinhardt, A.A.; Gayyed, M.F.; Klein, A.P.; Dong, J.; Maitra, A.; Pan, D.; Montgomery, E.A.; Anders, R.A. Expression of Yes-associated protein in common solid tumors. Hum. Pathol. 2008, 39, 1582–1589. [Google Scholar] [CrossRef]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes. Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef]
- Xiao, T.; Yan, A.; Tan, L.; Zhu, H.; Gao, W. LncRNA HOXA-AS2 is a prognostic and clinicopathological predictor in patients with cancer: A meta-analysis. Oncol. Lett. 2024, 27, 226. [Google Scholar] [CrossRef]
- Bister, K.; Jansen, H.W. Oncogenes in retroviruses and cells: Biochemistry and molecular genetics. Adv. Cancer Res. 1986, 47, 99–188. [Google Scholar]
- Halazonetis, T.D.; Kandil, A.N. Determination of the c-MYC DNA-binding site (oncogene/transcription factor TFEB/helixloop-helix motif). Proc. Natl. Acad. Sci. USA 1991, 88, 6162–6166. [Google Scholar] [CrossRef]
- Blackwood, E.M.; Kretzner, L.; Eisenman, R.N. Myc and Max function as a nucleoprotein complex. Curr. Opin. Genet. Dev. 1992, 2, 227–235. [Google Scholar] [CrossRef]
- Ayer, D.E.; Kretzner, L.; Eisenman, R.N. IMad: A Heterodimeric Partner for Max That Antagunizes Myc Transcriptional Activity. Cell 1993, 72, 211–222. [Google Scholar] [CrossRef]
- Dang, C.V. c-Myc Target Genes Involved in Cell Growth. Mol. Cell. Biol. 1999, 19, 1–11. [Google Scholar] [CrossRef]
- Iritani, B.M.; Eisenman, R.N. c-Myc enhances protein synthesis and cell size during B lymphocyte development. Proc. Natl. Acad. Sci. USA 1999, 96, 13180–13185. [Google Scholar] [CrossRef] [PubMed]
- Coller, H.A.; Grandori, C.; Tamayo, P.; Colbert, T.; Lander, E.S.; Eisenman, R.N.; Golub, T.R. Expression analysis with oligonucleotide microarrays reveals that MYC regulates genes involved in growth, cell cycle, signaling, and adhesion. Proc. Natl. Acad. Sci. USA 1999, 97, 3260–3265. [Google Scholar] [CrossRef] [PubMed]
- Pelengaris, S.; Khan, M. The many faces of c-MYC. Arch. Biochem. Biophys. 2003, 416, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Grandori, C.; Cowley, S.M.; James, L.P.; Eisenman, R.N. The myc/max/mad network and the transcriptional control of cell behavior. Annu. Rev. Cell Dev. Biol. 2000, 16, 653–699. [Google Scholar] [CrossRef]
- Hurlin, P.J.; Dezfouli, S. Functions of Myc:Max in the Control of Cell Proliferation and Tumorigenesis. Int. Rev. Cytol. 2004, 238, 183–226. [Google Scholar]
- Nasi, S.; Ciarapica, R.; Jucker, R.; Rosati, J.; Soucek, L. Making decisions through Myc. FEBS Lett. 2001, 490, 153–162. [Google Scholar] [CrossRef]
- Sorolla, A.; Wang, E.; Golden, E.; Duffy, C.; Henriques, S.T.; Redfern, A.D.; Blancafort, P. Precision medicine by designer interference peptides: Applications in oncology and molecular therapeutics. Oncogene 2020, 39, 1167–1184. [Google Scholar] [CrossRef]
- Mei, Y.P.; Liao, J.P.; Shen, J.; Yu, L.; Liu, B.L.; Liu, L.; Li, R.Y.; Ji, L.; Dorsey, S.G.; Jiang, Z.R.; et al. Small nucleolar RNA 42 acts as an oncogene in lung tumorigenesis. Oncogene 2012, 31, 2794–2804. [Google Scholar] [CrossRef]
- Okugawa, Y.; Toiyama, Y.; Toden, S.; Mitoma, H.; Nagasaka, T.; Tanaka, K.; Inoue, Y.; Kusunoki, M.; Boland, C.R.; Goel, A. Clinical significance of SNORA42 as an oncogene and a prognostic biomarker in colorectal cancer. Gut 2017, 66, 107–117. [Google Scholar] [CrossRef]
- Qin, Y.; Zhou, Y.; Ge, A.; Chang, L.; Shi, H.; Fu, Y.; Luo, Q. Overexpression of SNORA21 suppresses tumorgenesis of gallbladder cancer in vitro and in vivo. Biomed. Pharmacother. 2019, 118, 109266. [Google Scholar] [CrossRef]
- Black, A.R.; Black, J.D.; Azizkhan-Clifford, J. Sp1 and Krü ppel-Like Factor Family of Transcription Factors in Cell Growth Regulation and Cancer. J. Cell. Physiol. 2001, 188, 143–160. [Google Scholar] [CrossRef]
- Suske, G. The Sp-family of transcription factors. Gene 1999, 238, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.K.; Eun, H.K.; Seok, S.P.; Jun, H.L.; Taeg, K.K.; Kyeong, S.C. Quercetin sensitizes human hepatoma cells to TRAIL-induced apoptosis via Sp1-mediated DR5 up-regulation and proteasome-mediated c-FLIPS down-regulation. J. Cell Biochem. 2008, 105, 1386–1398. [Google Scholar] [CrossRef]
- Chae, J.I.; Cho, J.H.; Lee, K.A.; Choi, N.J.; Seo, K.S.; Kim, S.B.; Lee, S.H.; Shim, J.H. Role of transcription factor Sp1 in the quercetin-mediated inhibitory effect on human malignant pleural mesothelioma. Int. J. Mol. Med. 2012, 30, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Gong, A.; Young, C.Y.F. Involvement of transcription factor Sp1 in quercetin-mediated inhibitory effect on the androgen receptor in human prostate cancer cells. Carcinogenesis 2005, 26, 793–801. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, J.; Wei, D.; Wang, L.; Yuan, P.; Le, X.; Li, Q.; Yao, J.; Xie, K. Molecular basis of the synergistic antiangiogenic activity of bevacizumab and mithramycin A. Cancer Res. 2007, 67, 4878–4885. [Google Scholar] [CrossRef]
- Li, L.; Davie, J.R. The role of Sp1 and Sp3 in normal and cancer cell biology. Ann. Anat. 2010, 192, 275–283. [Google Scholar] [CrossRef]
- Takakura, M.; Kyo, S.; Kanaya, T.; Hirano, H.; Takeda, J.; Yutsudo, M.; Inoue, M. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999, 59, 551–557. [Google Scholar]
- Won, J.; Yim, J.; Kim, T.K. Sp1 and Sp3 recruit histone deacetylase to repress transcription of human telomerase reverse transcriptase (hTERT) promoter in normal human somatic cells. J. Biol. Chem. 2002, 277, 38230–38238. [Google Scholar] [CrossRef]
- Beishline, K.; Azizkhan-Clifford, J. Sp1 and the “hallmarks of cancer”. FEBS J. 2015, 282, 224–258. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.; Lu, W.; Ton, S.; Liu, H.; Sou, T.; Shen, Z.; Qin, X. Arsenic trioxide-mediated growth inhibition in gallbladder carcinoma cells via down-regulation of Cyclin D1 transcription mediated by Sp1 transcription factor. Biochem. Biophys. Res. Commun. 2007, 360, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Human FOX gene family. Int. J. Oncol. 2004, 25, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Obsil, T.; Obsilova, V. Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene 2008, 27, 2263–2275. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Graves, D.T. FOXO transcription factors: Their clinical significance and regulation. Biomed. Res. Int. 2014, 2014, 925350. [Google Scholar] [CrossRef]
- Herman, L.; Todeschini, A.L.; Veitia, R.A. Forkhead Transcription Factors in Health and Disease. Trends Genet. 2021, 37, 460–475. [Google Scholar] [CrossRef]
- Garry, D.J.; Maeng, G.; Garry, M.G. Foxk1 regulates cancer progression. Ann. Transl. Med. 2020, 8, 1041. [Google Scholar] [CrossRef]
- Cao, H.; Chu, X.; Wang, Z.; Guo, C.; Shao, S.; Xiao, J.; Zheng, J.; Zhang, D. High FOXK1 expression correlates with poor outcomes in hepatocellular carcinoma and regulates stemness of hepatocellular carcinoma cells. Life Sci. 2019, 228, 128–134. [Google Scholar] [CrossRef]
- Li, L.; Gong, M.; Zhao, Y.; Zhao, X.; Li, Q. FOXK1 facilitates cell proliferation through regulating the expression of p21, and promotes metastasis in ovarian cancer. Oncotarget 2017, 8, 70441–70451. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, C. The roles and regulation of the KLF5 transcription factor in cancers. Cancer Sci. 2021, 112, 2097–2117. [Google Scholar] [CrossRef]
- Shao, M.; Ge, G.-Z.; Liu, W.-J.; Xiao, J.; Xia, H.-J.; Fan, Y.; Zhao, F.; He, B.-L.; Chen, C. Characterization and phylogenetic analysis of Krüppel-like transcription factor (KLF) gene family in tree shrews (Tupaia belangeri chinensis). Oncotarget 2017, 8, 16325–16339. [Google Scholar] [CrossRef]
- Diakiw, S.M.; D’Andrea, R.J.; Brown, A.L. The double life of KLF5: Opposing roles in regulation of gene-expression, cellular function, and transformation. IUBMB Life 2013, 65, 999–1011. [Google Scholar] [CrossRef]
- Zhang, Z.; Teng, C.T. Phosphorylation of Kruppel-like factor 5 (KLF5/IKLF) at the CBP interaction region enhances its transactivation function. Nucleic Acids Res. 2003, 31, 2196–2208. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhao, K.W.; Dong, X.Y.; Sun, X.; Dong, J.T. Acetylation of KLF5 alters the assembly of p15 transcription factors in transforming growth factor-β-mediated induction in epithelial cells. J. Biol. Chem. 2009, 284, 18184–18193. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhou, Z.; Zhao, D.; Chen, C. The induction of KLF5 transcription factor by progesterone contributes to progesterone-induced breast cancer cell proliferation and dedifferentiation. Mol. Endocrinol. 2011, 25, 1137–1144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, R.; Shi, P.; Zhou, Z.; Zhang, H.; Li, W.; Zhang, H.; Chen, C. Krüpple-like factor 5 is essential for mammary gland development and tumorigenesis. J. Pathol. 2018, 246, 497–507. [Google Scholar] [CrossRef]
- Gong, T.; Cui, L.; Wang, H.; Wang, H.; Han, N. Knockdown of KLF5 suppresses hypoxia-induced resistance to cisplatin in NSCLC cells by regulating HIF-1α-dependent glycolysis through inactivation of the PI3K/Akt/mTOR pathway. J. Transl. Med. 2018, 16, 164. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Liu, F.; Ma, X.; Wu, L.; Guo, F.; Zhao, S.; Huang, F.; Qin, G. KLF5 promotes the tumorigenesis and metastatic potential of thyroid cancer cells through the NF-κB signaling pathway. Oncol. Rep. 2018, 40, 2608–2618. [Google Scholar] [CrossRef]
- Li, Q.; Dong, Z.; Zhou, F.; Cai, X.; Gao, Y.; Wang, L.W. Krüppel-like factor 5 promotes lung tumorigenesis through upregulation of Sox4. Cell. Physiol. Biochem. 2014, 33, 1–10. [Google Scholar] [CrossRef]
- Lu, X.; Hu, K.; Zhang, D.; Yin, X.; Nie, J.; Zhao, K. KLF5 silencing restrains proliferation; invasion, migration and angiogenesis of gallbladder carcinoma cells by transcriptional regulation of PDGFA. J. Cancer Res. Clin. Oncol. 2025, 151, 11. [Google Scholar] [CrossRef]
- Egwuagu, C.E. STAT3 in CD4+ T helper cell differentiation and inflammatory diseases. Cytokine 2009, 47, 149–156. [Google Scholar] [CrossRef]
- Fisher, M.L.; Balinth, S.; Mills, A.A. ΔNp63α in cancer: Importance and therapeutic opportunities. Trends Cell Biol. 2023, 33, 280–292. [Google Scholar] [CrossRef] [PubMed]
- El-Tanani, M.; Al Khatib, A.O.; Aladwan, S.M.; Abuelhana, A.; McCarron, P.A.; Tambuwala, M.M.; STAT, I.O. metastasis and therapeutic interventions. Cell Signal 2022, 92, 110275. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Wang, H.-H.; Tian, F.-J.; He, X.-Y.; Qiu, M.-T.; Wang, J.-Y.; Zhang, H.-J.; Wang, L.-H.; Wan, X.-P. A TrkB-STAT3-miR-204-5p regulatory circuitry controls proliferation and invasion of endometrial carcinoma cells. Mol. Cancer 2013, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Kruczyk, M.; Przanowski, P.; Dabrowski, M.; Swiatek-Machado, K.; Mieczkowski, J.; Wallerman, O.; Ronowicz, A.; Piotrowski, A.; Wadelius, C.; Kaminska, B.; et al. Integration of genome-wide of Stat3 binding and epigenetic modification mapping with transcriptome reveals novel Stat3 target genes in glioma cells. Biochim. Biophys. Acta Gene Regul. Mech. 2014, 1839, 1341–1350. [Google Scholar] [CrossRef]
- Cheng, G.Z.; Zhang, W.Z.; Sun, M.; Wang, Q.; Coppola, D.; Mansour, M.; Xu, L.M.; Costanzo, C.; Cheng, J.Q.; Wang, L.H. Twist is transcriptionally induced by activation of STAT3 and mediates STAT3 oncogenic function. J. Biol. Chem. 2008, 283, 14665–14673. [Google Scholar] [CrossRef]
- Cao, C.; Zhao, G.W.; Yu, W.; Xie, X.M.; Wang, W.T.; Yang, R.F.; Lv, X.; Liu, D.P. Activation of STAT3 stimulates AHSP expression in K562 cells. Sci. China Life Sci. 2014, 57, 488–494. [Google Scholar] [CrossRef]
- Enyu, L.; Na, W.; Chuanzong, Z.; Ben, W.; Xiaojuan, W.; Yan, W.; Zequn, L.; Jianguo, H.; Jiayong, W.; Benjia, L.; et al. The clinical significance and underlying correlation of pStat-3 and integrin αvβ6 expression in gallbladder cancer. Oncotarget 2017, 8, 19467–19477. [Google Scholar] [CrossRef][Green Version]
- Andonegui-Elguera, M.A.; Alfaro-Mora, Y.; Cáceres-Gutiérrez, R.; Caro-Sánchez, C.H.S.; Herrera, L.A.; Díaz-Chávez, J. An Overview of Vasculogenic Mimicry in Breast Cancer. Front. Oncol. 2020, 10, 220. [Google Scholar] [CrossRef]
- Zhou, H.; Yuan, Y.; Qian, H. Expression of STAT3 and vasculogenic mimicry in gallbladder carcinoma promotes invasion and metastasis. Exp. Ther. Med. 2021, 22, 738. [Google Scholar] [CrossRef]
- Liu, B.; Han, M.; Wen, J.K.; Wang, L. Livin/ML-IAP as a new target for cancer treatment. Cancer Lett. 2007, 250, 168–176. [Google Scholar] [CrossRef]
- Kasof, G.M.; Gomes, B.C. Livin, a Novel Inhibitor of Apoptosis Protein Family Member. J. Biol. Chem. 2001, 276, 3238–3246. [Google Scholar] [CrossRef]
- Wang, H.; Tan, S.S.; Wang, X.Y.; Liu, D.H.; Yu, C.S.; Bai, Z.L.; He, D.L.; Zhao, J. Silencing livin gene by siRNA leads to apoptosis induction, cell cycle arrest, and proliferation inhibition in malignant melanoma LiBr cells. Acta Pharmacol. Sin. 2007, 28, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Song, X.; Zhang, J.; Ye, L.; Wang, S.; Che, X.; Wang, J.; Zhang, Z.; Wang, L. Suppression of livin gene expression by siRNA leads to growth inhibition and apoptosis induction in human bladder cancer T24 cells. Biosci. Biotechnol. Biochem. 2010, 74, 1039–1044. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roy, N.; Kshattry, M.; Mandal, S.; Jolly, M.K.; Bhattacharyya, D.K.; Barah, P. An integrative systems biology approach identifies molecular signatures associated with gallbladder cancer pathogenesis. J. Clin. Med. 2021, 10, 3520. [Google Scholar] [CrossRef] [PubMed]
- Lozano, E.; Sanchon-Sanchez, P.; Morente-Carrasco, A.; Chinchilla-Tábora, L.M.; Mauriz, J.L.; Fernández-Palanca, P.; Marin, J.J.G.; Macias, R.I.R. Impact of Aberrant β-Catenin Pathway on Cholangiocarcinoma Heterogeneity. Cells 2023, 12, 1141. [Google Scholar] [CrossRef]
- Azizi, A.A.; Lamarca, A.; McNamara, M.G.; Valle, J.W. Chemotherapy for advanced gallbladder cancer (GBC): A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 163, 103328. [Google Scholar] [CrossRef]
- Song, X.; Hu, Y.; Li, Y.; Shao, R.; Liu, F.; Liu, Y. Overview of current targeted therapy in gallbladder cancer. Signal Transduct. Target. Ther. 2020, 5, 230. [Google Scholar] [CrossRef]
- Canale, M.; Monti, M.; Rapposelli, I.G.; Ulivi, P.; Sullo, F.G.; Bartolini, G.; Tiberi, E.; Frassineti, G.L. Molecular targets and emerging therapies for advanced gallbladder cancer. Cancers 2021, 13, 5671. [Google Scholar] [CrossRef]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, M.; Yang, Y.; Du, C.; Zhou, H.; Liu, C.; Chen, Y.; Fan, L.; Ma, H.; Gong, Y.; et al. An overview of PROTACs: A promising drug discovery paradigm. Mol. Biomed. 2022, 3, 46. [Google Scholar] [CrossRef]
- Li, Y.; Song, J.; Zhou, P.; Zhou, J.; Xie, S. Targeting Undruggable Transcription Factors with PROTACs: Advances and Perspectives. J. Med. Chem. 2022, 65, 10183–10194. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.M.; Dong, J.; Xu, Z.Y.; Cheng, X.D.; Zhang, W.D.; Qin, J.J. PROTAC: An Effective Targeted Protein Degradation Strategy for Cancer Therapy. Front. Pharmacol. 2021, 12, 692574. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Sharma, S.K.; Mishra, N.; Soni, A.; Kumari, M.; Singh, S.K. Targeting cell cycle regulators: A new paradigm in cancer therapeutics. Biocell 2024, 48, 1639–1666. [Google Scholar] [CrossRef]
- Goeppert, B.; Truckenmueller, F.; Ori, A.; Fritz, V.; Albrecht, T.; Fraas, A.; Scherer, D.; Silos, R.G.; Sticht, C.; Gretz, N.; et al. Profiling of gallbladder carcinoma reveals distinct miRNA profiles and activation of STAT1 by the tumor suppressive miRNA-145-5p. Sci. Rep. 2019, 9, 4796. [Google Scholar] [CrossRef] [PubMed]
- Beltran, M.; Puig, I.; Peña, C.; García, J.M.; Álvarez, A.B.; Peña, R.; Bonilla, F.; De Herreros, A.G. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial-mesenchymal transition. Genes Dev. 2008, 22, 756–769. [Google Scholar] [CrossRef]
- Redman, M.; King, A.; Watson, C.; King, D. What is CRISPR/Cas9? Arch. Dis. Child. Educ. Pract. 2016, 101, 213–215. [Google Scholar] [CrossRef]
- Riedel, M.; Cai, H.; Stoltze, I.C.; Vendelbo, M.H.; Wagner, E.F.; Bakiri, L.; Thomsen, M.K. Targeting AP-1 transcription factors by CRISPR in the prostate. Oncotarget 2021, 12, 1956–1961. [Google Scholar] [CrossRef]
- Martinez-Escobar, A.; Luna-Callejas, B.; Ramón-Gallegos, E. CRISPR-dCas9-Based Artificial Transcription Factors to Improve Efficacy of Cancer Treatment with Drug Repurposing: Proposal for Future Research. Front. Oncol. 2021, 10, 604948. [Google Scholar] [CrossRef]
- Ren, K.; Xu, R.; Huang, J.; Zhao, J.; Shi, W. Knockdown of long non-coding RNA KCNQ1OT1 depressed chemoresistance to paclitaxel in lung adenocarcinoma. Cancer Chemother. Pharmacol. 2017, 80, 243–250. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).