Revisiting the Role of PD-L1 Overexpression in Prognosis and Clinicopathological Features in Patients with Oral Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. CPS Measured in Biopsy and Surgical Piece
3.2. Clinicopathological Correlation
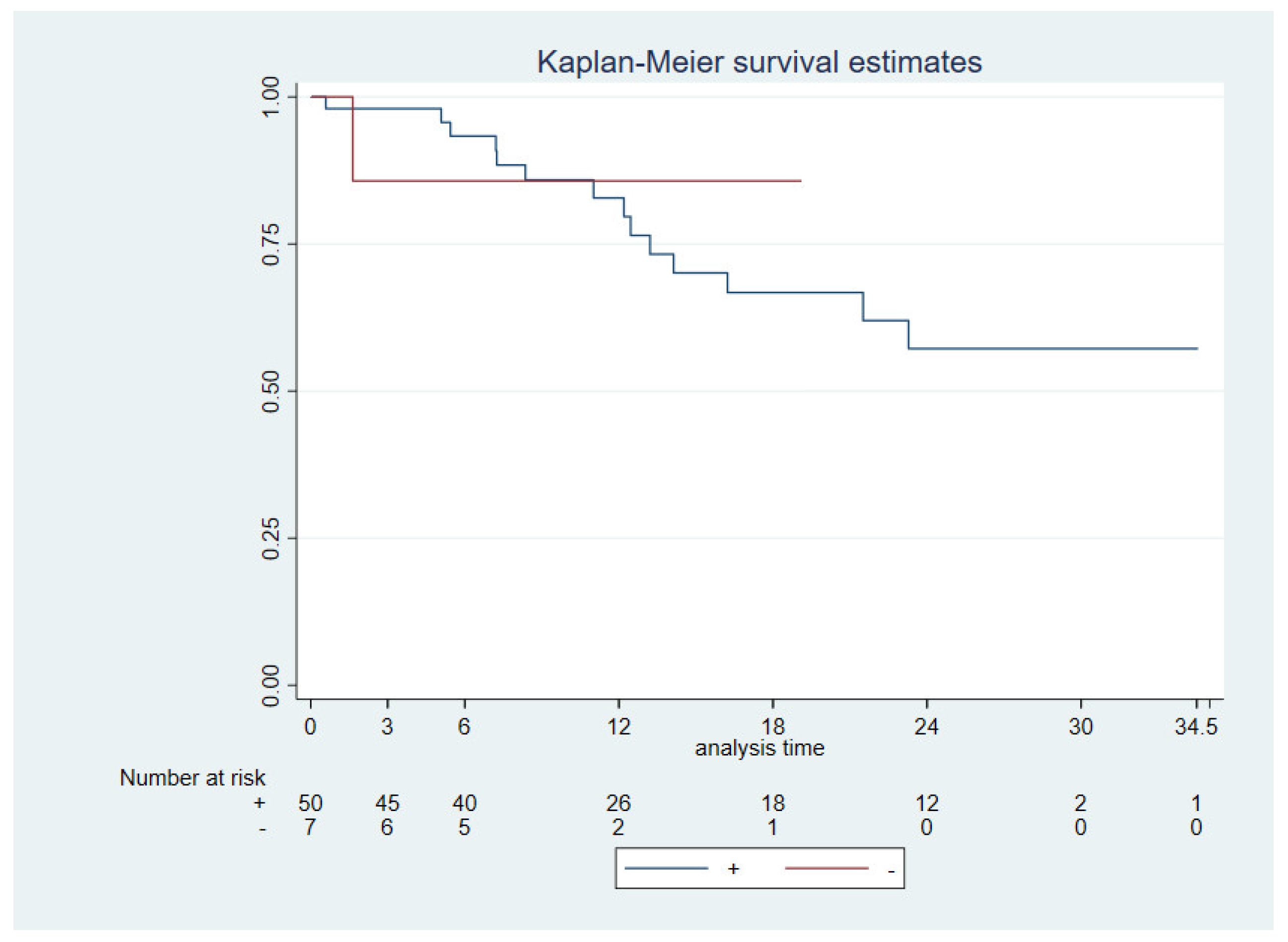
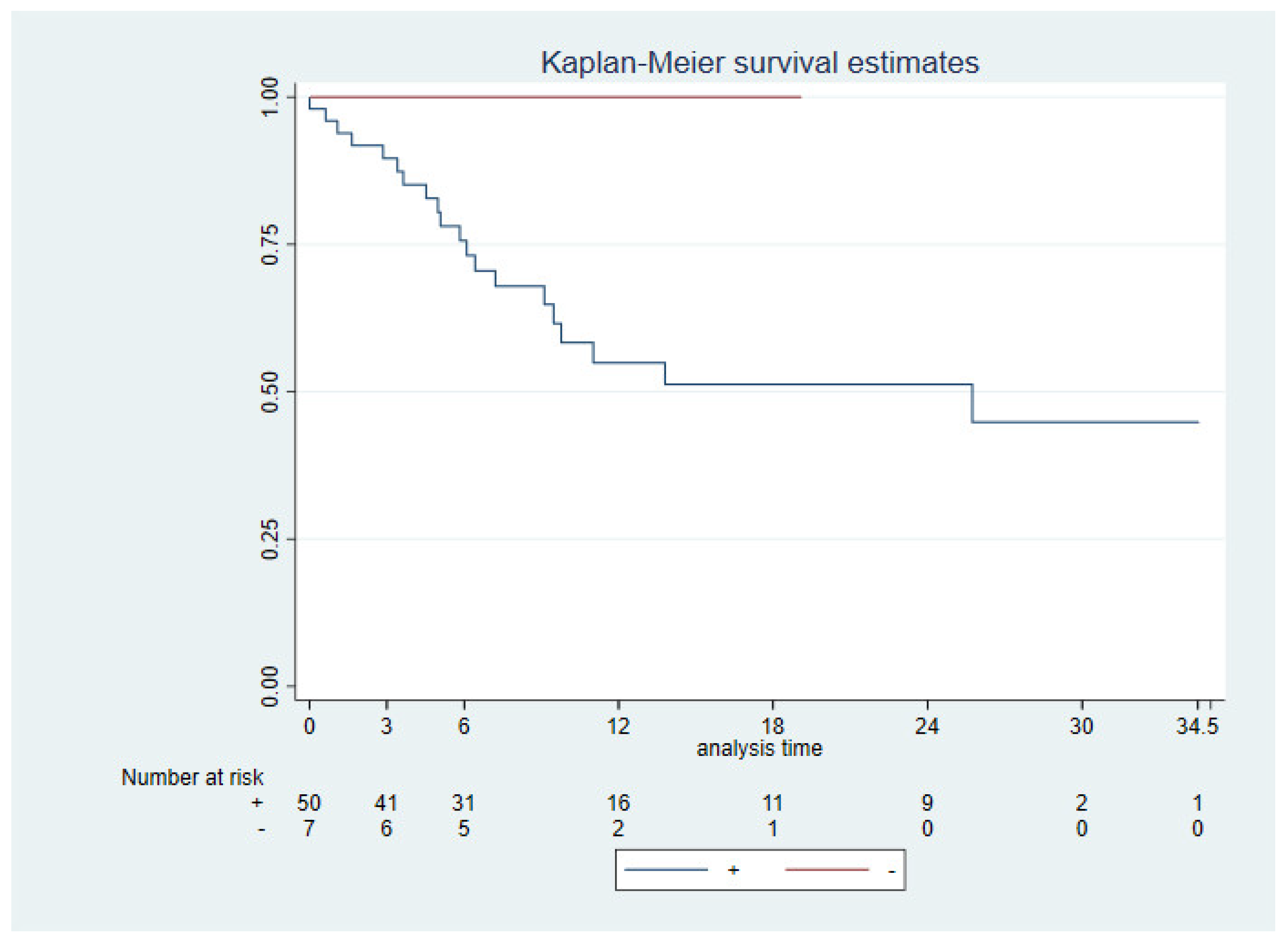
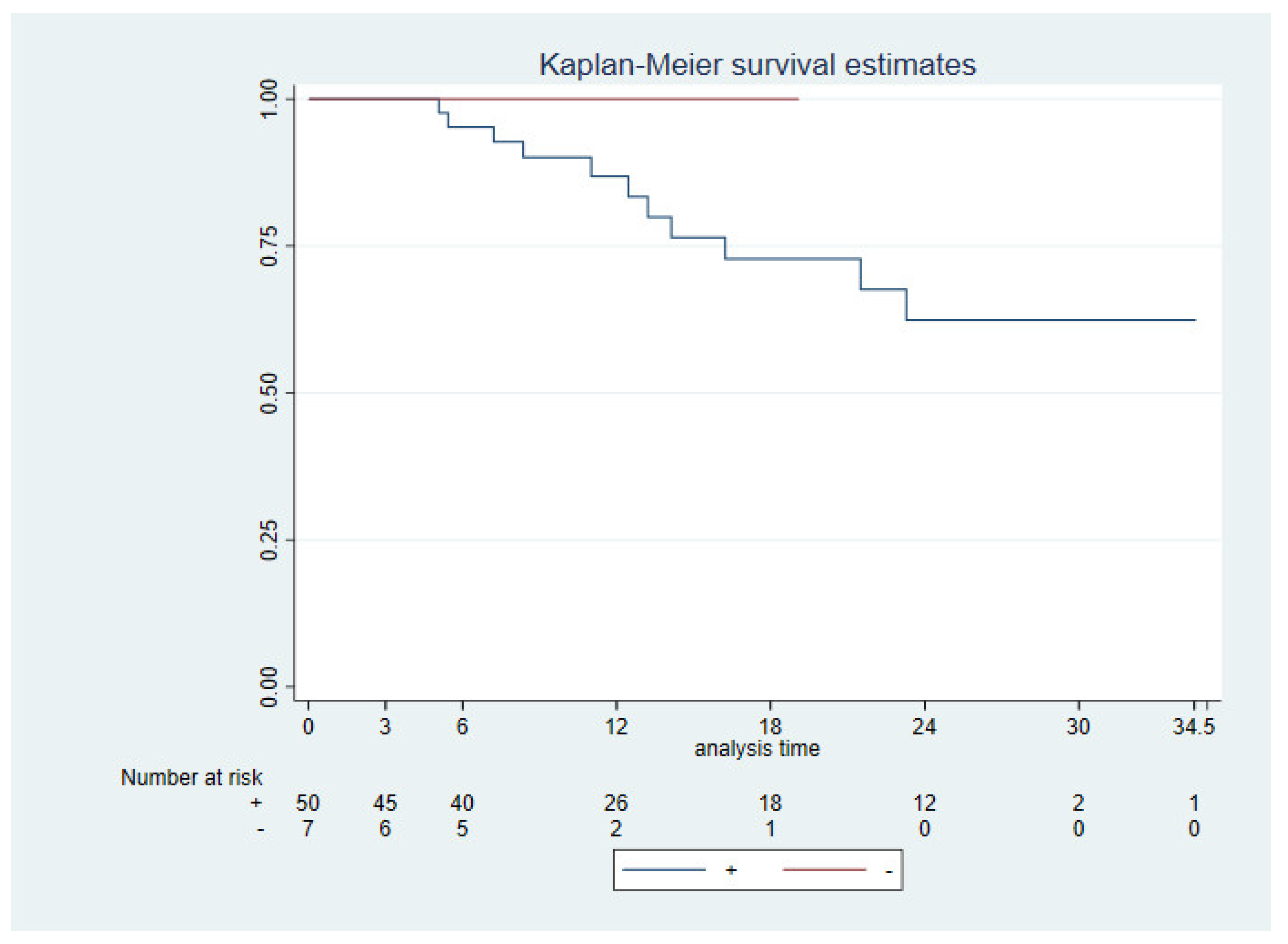
3.3. Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, K.S.; Ganry, L.; Fernandes, R.P. Oral Cavity Cancer. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.C.D.; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928, Erratum in Lancet 2020, 395, 272; Erratum in Lancet 2020, 395, 564; Erratum in Lancet 2021, 397, 2252. [Google Scholar] [CrossRef]
- Kiyota, N.; Hasegawa, Y.; Takahashi, S.C.D. A randomized, open-label, phase III clinical trial of nivolumab vs. therapy of investigator’s choice in recurrent squamous cell carcinoma of the head and neck: A subanalysis of Asian patients versus the global population in CheckMate 141. Oral Oncol. 2017, 73, 138–146. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.P.; Li, Z.; Kim, S.B.; et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 2021, 398, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Csőszi, T.; Özgüroğlu, M.; Matsubara, N.; Géczi, L.; Cheng, S.Y.; Fradet, Y.; Oudard, S.; Vulsteke, C.; Morales Barrera, R.; et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 931–945. [Google Scholar] [CrossRef]
- O’Brien, M.; Paz-Ares, L.; Marreaud, S.; Dafni, U.; Oselin, K.; Havel, L.; Esteban, E.; Isla, D.; Martinez-Marti, A.; Faehling, M.; et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): An interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022, 23, 1274–1286. [Google Scholar] [CrossRef]
- Ju, W.T.; Xia, R.H.; Zhu, D.W.; Dou, S.J.; Zhu, G.P.; Dong, M.J.; Wang, L.Z.; Sun, Q.; Zhao, T.C.; Zhou, Z.H.; et al. A pilot study of neoadjuvant combination of anti-PD-1 camrelizumab and VEGFR2 inhibitor apatinib for locally advanced resectable oral squamous cell carcinoma. Nat. Commun. 2022, 13, 5378. [Google Scholar] [CrossRef]
- Schoenfeld, J.D.; Hanna, G.J.; Jo, V.Y.; Rawal, B.; Chen, Y.H.; Catalano, P.S.; Lako, A.; Ciantra, Z.; Weirather, J.L.; Criscitiello, S.; et al. Neoadjuvant Nivolumab or Nivolumab Plus Ipilimumab in Untreated Oral Cavity Squamous Cell Carcinoma: A Phase 2 Open-Label Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1563–1570. [Google Scholar] [CrossRef]
- Wu, T.; Tang, C.; Tao, R.; Yong, X.; Jiang, Q.; Feng, C. PD-L1-Mediated Immunosuppression in Oral Squamous Cell Carcinoma: Relationship With Macrophage Infiltration and Epithelial to Mesenchymal Transition Markers. Front. Immunol. 2021, 12, 693881. [Google Scholar] [CrossRef]
- Qiao, X.-W.; Jiang, J.; Pang, X.; Huang, M.-C.; Tang, Y.-J.; Liang, X.; Tang, Y. The Evolving Landscape of PD-1/PD-L1 Pathway in Head and Neck Cancer. Front. Immunol. 2020, 11, 1721. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini-Spaltro, A.; Limarzi, F.; Gaudio, M.; Calpona, S.; Meccariello, G. PD-L1 expression in head and neck carcinoma by combined positive score: A comparison among preoperative biopsy, tumor resection, and lymph node metastasis. Virchows Arch. 2022, 481, 93–99. [Google Scholar] [CrossRef]
- Crosta, S.; Boldorini, R.; Bono, F.; Brambilla, V.; Dainese, E.; Fusco, N.; Gianatti, A.; L’Imperio, V.; Morbini, P.; Pagni, F. PD-L1 Testing and Squamous Cell Carcinoma of the Head and Neck: A Multicenter Study on the Diagnostic Reproducibility of Different Protocols. Cancers 2021, 13, 292. [Google Scholar] [CrossRef] [PubMed]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Bell, R.B.; Bifulco, C.B.; Burtness, B.; Gillison, M.L.; Harrington, K.J.; Le, Q.T.; Lee, N.Y.; Leidner, R.; Lewis, R.L.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J. Immunother. Cancer 2019, 7, 184. [Google Scholar] [CrossRef]
- Paver, E.C.; Cooper, W.A.; Colebatch, A.J.; Ferguson, P.M.; Hill, S.K.; Lum, T.; Shin, J.S.; O’Toole, S.; Anderson, L.; Scolyer, R.A.; et al. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: A guide to immunohistochemistry implementation and interpretation. Pathology 2021, 53, 141–156. [Google Scholar] [CrossRef]
- Wusiman, D.; Guo, L.; Huang, Z.; Li, Z.; Liu, S.; Ying, J.; Li, W.; An, C. The clinicopathological significance of PD-L1 expression assessed by the combined positive score (CPS) in head and neck squamous cell carcinoma. Pathol. Res. Pract. 2022, 236, 153934. [Google Scholar] [CrossRef]
- Miranda-Galvis, M.; Rumayor Piña, A.; Sales de Sá, R.; Almeida Leite, A.; Agustin Vargas, P.; Calsavara, V.F.; Lópes Pinto, C.A.; Teng, Y.; Kowalski, L.P. PD-L1 expression patterns in oral cancer as an integrated approach for further prognostic classification. Oral Dis. 2021, 27, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Lenouvel, D.; González-Moles, M.Á.; Ruiz-Ávila, I.; Chamorro-Santos, C.; González-Ruiz, L.; González-Ruiz, I.; Ramos-García, P. Clinicopathological and prognostic significance of PD-L1 in oral cancer: A preliminary retrospective immunohistochemistry study. Oral Dis. 2021, 27, 173–182. [Google Scholar] [CrossRef]
- Paolino, G.; Pantanowitz, L.; Barresi, V.; Pagni, F.; Munari, E.; Moretta, L.; Brunelli, M.; Bariani, E.; Vigliar, E.; Pisapia, P.; et al. PD-L1 evaluation in head and neck squamous cell carcinoma: Insights regarding specimens, heterogeneity and therapy. Pathol. Res. Pract. 2021, 226, 153605. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.H.; Olin, A.B.; Lelkaitis, G.; Hansen, A.E.; Andersen, F.L.; Johannesen, H.H.; Kjaer, A.; Fischer, B.M.; Specht, L.; Bentzen, S.M.; et al. Intratumor heterogeneity is biomarker specific and challenges the association with heterogeneity in multimodal functional imaging in head and neck squamous cell carcinoma. Eur. J. Radiol. 2021, 139, 109668. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Canteli, M.; Granda-Díaz, R.; Del Rio-Ibisate, N.; Allonca, E.; López-Alvarez, F.; Agorreta, J.; Garmendia, I.; Montuenga, L.M.; García-Pedrero, J.M.; Rodrigo, J.P. PD-L1 expression correlates with tumor-infiltrating lymphocytes and better prognosis in patients with HPV-negative head and neck squamous cell carcinomas. Cancer Immunol. Immunother. 2020, 69, 2089–2100. [Google Scholar] [CrossRef]
- Chen, J.; Gu, P.; Wu, H. Uncovering PD-L1 and CD8+ TILS Expression and Clinical Implication in Cervical Squamous Cell Carcinoma. Biomed. Res. Int. 2020, 2020, 8164365. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shu, P.; Fang, Y.; Yuan, W.; Zhang, Q.; Sun, J.; Fu, M.; Xue, A.; Gao, X.; Shen, K.; et al. Clinical and Prognostic Significance of Tumor-Infiltrating CD8+ T Cells and PD-L1 Expression in Primary Gastrointestinal Stromal Tumors. Front. Oncol. 2021, 11, 789915. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Nagatsuka, H.; Nakano, K.; Kogashiwa, Y.; Ebihara, Y.; Yano, M.; Yasuda, M. Significance of PD-L1 Expression in Tongue Cancer Development. Int. J. Med. Sci. 2018, 15, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Dave, K.; Ali, A.; Magalhaes, M. Increased expression of PD-1 and PD-L1 in oral lesions progressing to oral squamous cell carcinoma: A pilot study. Sci. Rep. 2020, 10, 9705. [Google Scholar] [CrossRef] [PubMed]
- Lenouvel, D.; González-Moles, M.Á.; Ruiz-Ávila, I.; Gonzalez-Ruiz, L.; Gonzalez-Ruiz, I.; Ramos-García, P. Prognostic and clinicopathological significance of PD-L1 overexpression in oral squamous cell carcinoma: A systematic review and comprehensive meta-analysis. Oral Oncol. 2020, 106, 104722. [Google Scholar] [CrossRef] [PubMed]
- de la Iglesia, J.V.; Slebos, R.J.C.; Martin-Gomez, L.; Wang, X.; Teer, J.K.; Tan, A.C.; Gerke, T.A.; Aden-Buie, G.; van Veen, T.; Masannat, J.; et al. Effects of Tobacco Smoking on the Tumor Immune Microenvironment in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2020, 26, 1474–1485. [Google Scholar] [CrossRef]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016, 1, e89829. [Google Scholar] [CrossRef]
- Weng, Y.M.; Peng, M.; Hu, M.X.; Yao, Y.; Song, Q.B. Clinical and molecular characteristics associated with the efficacy of PD-1/PD-L1 inhibitors for solid tumors: A meta-analysis. OncoTargets Ther. 2018, 11, 7529–7542. [Google Scholar] [CrossRef]
- Lin, Y.M.; Sung, W.W.; Hsieh, M.J.; Tsai, S.C.; Lai, H.W.; Yang, S.M.; Shen, K.H.; Chen, M.K.; Lee, H.; Yeh, K.T.; et al. High PD-L1 Expression Correlates with Metastasis and Poor Prognosis in Oral Squamous Cell Carcinoma. PLoS ONE 2015, 10, e0142656. [Google Scholar] [CrossRef]
- Wu, L.; Jiang, C.; Zhu, Z.; Sun, Y.; Zhang, T. Prognostic role of PD-L1 expression in patients with salivary gland carcinoma: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0272080. [Google Scholar] [CrossRef]
- Yang, W.F.; Wong, M.C.M.; Thomson, P.J.; Li, K.Y.; Su, Y.X. The prognostic role of PD-L1 expression for survival in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2018, 86, 81–90. [Google Scholar] [CrossRef]


| PD-L1 Expression (%) | ||||||
|---|---|---|---|---|---|---|
| Characteristic | CPS < 1 | CPS ≥ 1 | p | CPS 1–20 | CPS ≥ 20 | p |
| No. tumors | 7 (10.76%) | 58 (89.23%) | 25 (43.10%) | 33 (56.89%) | ||
| Age | 0.89 | 0.47 | ||||
| <60 | 2 (28.57%) | 18 (31.03%) | 9 (36%) | 9 (27.27%) | ||
| ≥60 | 5 (71.42%) | 40 (68.96%) | 16 (64%) | 24 (72.72%) | ||
| Sex | 0.92 | 0.33 | ||||
| Male | 3 (42.86%) | 26 (44.82%) | 13 (52%) | 13 (39.39%) | ||
| Female | 4 (57.14%) | 32 (55.17%) | 12 (48%) | 20 (60.60%) | ||
| Smoking status | 0.65 | 0.27 | ||||
| Smokers or ex-smokers | 3 (42.86%) | 30 (51.72%) | 15 (60%) | 15 (45.45%) | ||
| Non-smokers | 4 (57.14%) | 28 (48.27%) | 10 (40%) | 18 (54.54%) | ||
| Immune status | 0.96 | 0.43 | ||||
| Immunosuppressed | 2 (28.57%) | 17 (29.31%) | 6 (24%) | 11 (33.33%) | ||
| Not immunosuppressed | 5 (71.43%) | 41 (70.68%) | 19 (76%) | 22 (66.66%) | ||
| Primary vs. recurrence | 0.38 | 0.72 | ||||
| Primary | 7 (100.00%) | 52 (89.65%) | 22 (88%) | 30 (90.90%) | ||
| Recurrence | 0 (0.00%) | 6 (10%) | 3 (12%) | 3 (9.09%) | ||
| Location | 0.074 | 0.24 | ||||
| Tongue | 6 (85.71%) | 16 (27.59%) | 6 (24%) | 10 (30.30%) | ||
| Cervix | 0 (0.00%) | 1 (1.72%) | 0 (0.00%) | 1 (3.03%) | ||
| Gum | 0 (0.00%) | 12 (20.69%) | 7 (28%) | 5 (15.15%) | ||
| Buccal mucosa | 0 (0.00%) | 11 (18.97%) | 2 (8%) | 9 (27.27%) | ||
| Mouth floor | 1 (14.29%) | 11 (18.97%) | 7 (28%) | 4 (12.12%) | ||
| Retromolar trigone | 0 (0.00%) | 7 (12.07%) | 3 (12%) | 4 (12.12%) | ||
| T | <0.001 | 0.009 | ||||
| T1 | 5 (71.43%) | 6 (10.71%) | 5 (20%) | 1 (3.23%) | ||
| T2 | 1 (14.29%) | 16 (28.57%) | 2 (8%) | 14 (45.16%) | ||
| T3 | 1 (14.29%) | 11 (19.64%) | 5 (20%) | 6 (19.35%) | ||
| T4 | 0 (0.00%) | 23 (41.07%) | 13 (52%) | 10 (32.26%) | ||
| N status | 0.004 | 0.7 | ||||
| N0 | 7 (100.00%) | 24 (42.86%) | 11 (45.83%) | 13 (40.63%) | ||
| N+ | 0 (0.00%) | 32 (57.14%) | 13 (54.17%) | 19 (59.38%) | ||
| Stage | <0.001 | 0.14 | ||||
| I | 5 (71.43%) | 5 (8.77%) | 4 (16%) | 1 (3.13%) | ||
| II | 1 (14.29%) | 10 (17.54%) | 2 (8%) | 8 (25%) | ||
| III | 1 (14.29%) | 6 (10.53%) | 2 (8%) | 4 (12.5%) | ||
| IV | 0 (0.00%) | 36 (63.16%) | 17 (68%) | 19 (59.38%) | ||
| Differentiation grade | 0.12 | 0.43 | ||||
| Well | 2 (33.33%) | 4 (7.14%) | 2 (8%) | 2 (6.45%) | ||
| Moderate | 3 (50%) | 41 (73.21%) | 20 (80%) | 21 (67.74%) | ||
| Poor | 1 (16.66%) | 11 (19.64%) | 3 (12%) | 8 (25.81%) | ||
| ENE | 0.13 | 0.065 | ||||
| + | 0 (0.00%) | 13 (25.49%) | 3 (13.04%) | 10 (35.71%) | ||
| - | 7 (100.00%) | 38 (74.51%) | 20 (86.96%) | 18 (64.29%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leporace-Jiménez, F.; Portillo-Hernandez, I.; Jiménez-Almonacid, J.; Rodriguez, I.Z.; Mejía-Nieto, M.; Caballero Pedrero, P.; Aniceto, G.S. Revisiting the Role of PD-L1 Overexpression in Prognosis and Clinicopathological Features in Patients with Oral Squamous Cell Carcinoma. Onco 2024, 4, 131-142. https://doi.org/10.3390/onco4030011
Leporace-Jiménez F, Portillo-Hernandez I, Jiménez-Almonacid J, Rodriguez IZ, Mejía-Nieto M, Caballero Pedrero P, Aniceto GS. Revisiting the Role of PD-L1 Overexpression in Prognosis and Clinicopathological Features in Patients with Oral Squamous Cell Carcinoma. Onco. 2024; 4(3):131-142. https://doi.org/10.3390/onco4030011
Chicago/Turabian StyleLeporace-Jiménez, Fernando, Isabel Portillo-Hernandez, Justino Jiménez-Almonacid, Ignacio Zubillaga Rodriguez, María Mejía-Nieto, Pablo Caballero Pedrero, and Gregorio Sanchez Aniceto. 2024. "Revisiting the Role of PD-L1 Overexpression in Prognosis and Clinicopathological Features in Patients with Oral Squamous Cell Carcinoma" Onco 4, no. 3: 131-142. https://doi.org/10.3390/onco4030011
APA StyleLeporace-Jiménez, F., Portillo-Hernandez, I., Jiménez-Almonacid, J., Rodriguez, I. Z., Mejía-Nieto, M., Caballero Pedrero, P., & Aniceto, G. S. (2024). Revisiting the Role of PD-L1 Overexpression in Prognosis and Clinicopathological Features in Patients with Oral Squamous Cell Carcinoma. Onco, 4(3), 131-142. https://doi.org/10.3390/onco4030011





