The World of Immunotherapy Needs More Than PD-1/PD-L1—Two of the New Kids on the Block: LAG-3 and TIGIT
Abstract
Simple Summary
Abstract
1. Introduction
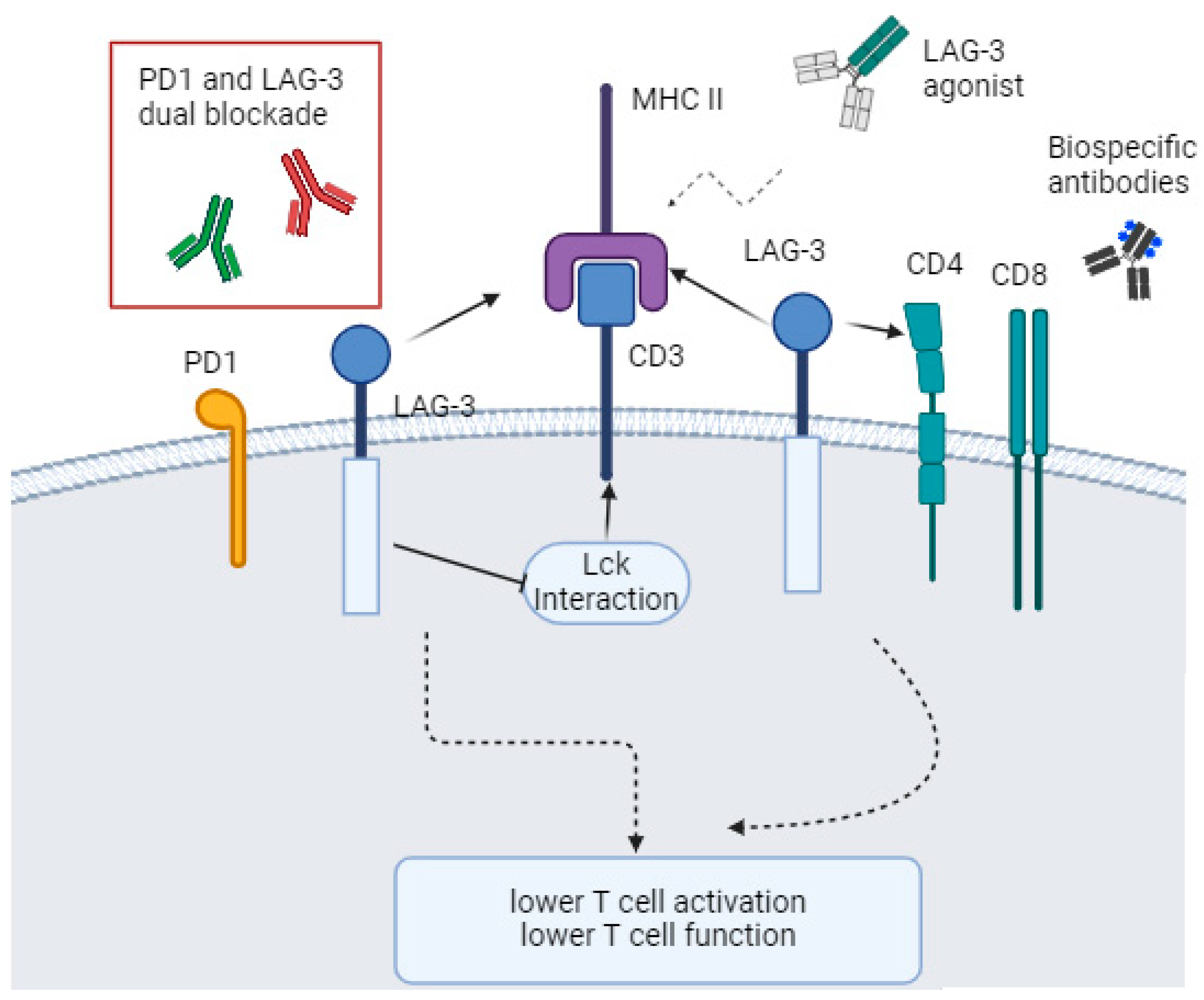
2. Lymphocyte-Activation Gene 3 (LAG-3)
3. T Cell Immunoglobulin and ITIM Domain (TIGIT)
4. Triple Blockade on the Way?
5. How to Assess LAG-3 and TIGIT Expression?
6. New Strategies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Valiullina, A.K.; Zmievskaya, E.A.; Ganeeva, I.A.; Zhuravleva, M.N.; Garanina, E.E.; Rizvanov, A.A.; Petukhov, A.V.; Bulatov, E.R. Evaluation of CAR-T Cells’ Cytotoxicity against Modified Solid Tumor Cell Lines. Biomedicines 2023, 11, 626. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Liu, C.; Wang, Z.; Wu, W.; Zhang, N.; Zhang, L.; Hu, J.; Luo, P.; Zhang, J.; et al. Immune Checkpoint Modulators in Cancer Immunotherapy: Recent Advances and Emerging Concepts. J. Hematol. Oncol. 2022, 15, 111. [Google Scholar] [CrossRef] [PubMed]
- Maritaz, C.; Broutin, S.; Chaput, N.; Marabelle, A.; Paci, A. Immune Checkpoint-Targeted Antibodies: A Room for Dose and Schedule Optimization? J. Hematol. Oncol. 2022, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zou, C.; Zhang, S.; Chu, T.S.M.; Zhang, Y.; Chen, W.; Zhao, C.; Yang, L.; Xu, Z.; Dong, S.; et al. Reshaping the Systemic Tumor Immune Environment (STIE) and Tumor Immune Microenvironment (TIME) to Enhance Immunotherapy Efficacy in Solid Tumors. J. Hematol. Oncol. 2022, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, N.; Tardiel-Cyril, D.R.; Davtyan, A.; Generali, D.; Roudi, R.; Li, Y. CTLA-4 in Regulatory T Cells for Cancer Immunotherapy. Cancers 2021, 13, 1440. [Google Scholar] [CrossRef] [PubMed]
- Rotte, A. Combination of CTLA-4 and PD-1 Blockers for Treatment of Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 255. [Google Scholar] [CrossRef] [PubMed]
- Bulaon, C.J.I.; Khorattanakulchai, N.; Rattanapisit, K.; Sun, H.; Pisuttinusart, N.; Strasser, R.; Tanaka, S.; Soon-Shiong, P.; Phoolcharoen, W. Antitumor Effect of Plant-Produced Anti-CTLA-4 Monoclonal Antibody in a Murine Model of Colon Cancer. Front. Plant Sci. 2023, 14, 1149455. [Google Scholar] [CrossRef]
- Bauché, D.; Mauze, S.; Kochel, C.; Grein, J.; Sawant, A.; Zybina, Y.; Blumenschein, W.; Yang, P.; Annamalai, L.; Yearley, J.H.; et al. Antitumor Efficacy of Combined CTLA4/PD-1 Blockade without Intestinal Inflammation Is Achieved by Elimination of FcγR Interactions. J. Immunother. Cancer 2020, 8, e001584. [Google Scholar] [CrossRef]
- Li, T.; Niu, M.; Zhou, J.; Wu, K.; Yi, M. The Enhanced Antitumor Activity of Bispecific Antibody Targeting PD-1/PD-L1 Signaling. Cell Commun. Signal. 2024, 22, 179. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination Strategies with PD-1/PD-L1 Blockade: Current Advances and Future Directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Prithviraj, P.; Anaka, M.; Bridle, K.R.; Crawford, D.H.G.G.; Dhungel, B.; Steel, J.C.; Jayachandran, A. Monitoring immune checkpoint regulators as predictive biomarkers in hepatocellular carcinoma. Front. Oncol. 2018, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Z.; Li, Y.; Zhao, W.; Wu, J.B.; Zhang, Z. PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy. Front. Pharmacol. 2021, 12, 731798. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 273409. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Hong, Z.; Zhang, C.; Wang, L.; Han, Z.; Ma, D. Immune Checkpoint Therapy for Solid Tumours: Clinical Dilemmas and Future Trends. Signal Transduct. Target. Ther. 2023, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Page, D.B.; Li, B.T.; Connell, L.C.; Schindler, K.; Lacouture, M.E.; Postow, M.A.; Wolchok, J.D. Toxicities of the Anti-PD-1 and Anti-PD-L1 Immune Checkpoint Antibodies. Ann. Oncol. 2015, 26, 2375–2391. [Google Scholar] [CrossRef]
- Wang, D.Y.; Johnson, D.B.; Davis, E.J. Toxicities Associated with PD-1/PD-L1 Blockade. Cancer J. 2018, 24, 36–40. [Google Scholar] [CrossRef]
- Su, C.; Wang, H.; Liu, Y.; Guo, Q.; Zhang, L.; Li, J.; Zhou, W.; Yan, Y.; Zhou, X.; Zhang, J. Adverse Effects of Anti-PD-1/PD-L1 Therapy in Non-Small Cell Lung Cancer. Front. Oncol. 2020, 10, 554313. [Google Scholar] [CrossRef]
- Wu, M.; Huang, Q.; Xie, Y.; Wu, X.; Ma, H.; Zhang, Y.; Xia, Y. Improvement of the Anticancer Efficacy of PD-1/PD-L1 Blockade via Combination Therapy and PD-L1 Regulation. J. Hematol. Oncol. 2022, 15, 24. [Google Scholar] [CrossRef]
- Dai, M.; Liu, M.; Yang, H.; Küçük, C.; You, H. New Insights into Epigenetic Regulation of Resistance to PD-1/PD-L1 Blockade Cancer Immunotherapy: Mechanisms and Therapeutic Opportunities. Exp. Hematol. Oncol. 2022, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, X.; Xu, L.; Li, Y.; Zeng, C. Current Insight into the Regulation of PD-L1 in Cancer. Exp. Hematol. Oncol. 2022, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Barrueto, L.; Caminero, F.; Cash, L.; Makris, C.; Lamichhane, P.; Deshmukh, R.R. Resistance to Checkpoint Inhibition in Cancer Immunotherapy. Transl. Oncol. 2020, 13, 100738. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of Resistance to Immune Checkpoint Inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jenkins, R.W.; Sullivan, R.J. Mechanisms of Resistance to Immune Checkpoint Blockade. Am. J. Clin. Dermatol. 2019, 20, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Russell, B.L.; Sooklal, S.A.; Malindisa, S.T.; Daka, L.J.; Ntwasa, M. The Tumor Microenvironment Factors That Promote Resistance to Immune Checkpoint Blockade Therapy. Front. Oncol. 2021, 11, 641428. [Google Scholar] [CrossRef] [PubMed]
- Relecom, A.; Merhi, M.; Inchakalody, V.; Uddin, S.; Rinchai, D.; Bedognetti, D.; Dermime, S. Emerging Dynamics Pathways of Response and Resistance to PD-1 and CTLA-4 Blockade: Tackling Uncertainty by Confronting Complexity. J. Exp. Clin. Cancer Res. 2021, 40, 74. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.; Saleh, K.; Chahine, C.; Khoury, R.; Khalife, N.; Cesne, A. Le LAG-3 Inhibitors: Novel Immune Checkpoint Inhibitors Changing the Landscape of Immunotherapy. Biomedicines 2023, 11, 1878. [Google Scholar] [CrossRef]
- Triebel, F. LAG-3: A Regulator of T-Cell and DC Responses and Its Use in Therapeutic Vaccination. Trends Immunol. 2003, 24, 619–622. [Google Scholar] [CrossRef]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a Novel Lymphocyte Activation Gene Closely Related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef]
- Turner, J.M.; Brodsky, M.H.; Irving, B.A.; Levin, S.D.; Perlmutter, R.M.; Littman, D.R. Interaction of the Unique N-Terminal Region of Tyrosine Kinase P56lck with Cytoplasmic Domains of CD4 and CD8 Is Mediated by Cysteine Motifs. Cell 1990, 60, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Zhang, X.; Chen, F.; Pan, Q.; Phiphatwatchara, P.; Zeng, Y.; Chen, H. The Promising Immune Checkpoint LAG-3: From Tumor Microenvironment to Cancer Immunotherapy. Genes Cancer 2018, 9, 176–189. [Google Scholar] [CrossRef]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A.A. LAG3 (CD223) as a Cancer Immunotherapy Target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef]
- Goding, S.R.; Wilson, K.A.; Xie, Y.; Harris, K.M.; Baxi, A.; Akpinarli, A.; Fulton, A.; Tamada, K.; Strome, S.E.; Antony, P.A. Restoring Immune Function of Tumor-Specific CD4+ T Cells during Recurrence of Melanoma. J. Immunol. 2013, 190, 4899–4909. [Google Scholar] [CrossRef]
- Grosso, J.F.; Kelleher, C.C.; Harris, T.J.; Maris, C.H.; Hipkiss, E.L.; De Marzo, A.; Anders, R.; Netto, G.; Getnet, D.; Bruno, T.C.; et al. LAG-3 Regulates CD8+ T Cell Accumulation and Effector Function in Murine Self- and Tumor-Tolerance Systems. J. Clin. Investig. 2007, 117, 3383–3392. [Google Scholar] [CrossRef]
- Sittig, S.P.; Køllgaard, T.; Grønbæk, K.; Idorn, M.; Hennenlotter, J.; Stenzl, A.; Gouttefangeas, C.; Straten, P. Clonal Expansion of Renal Cell Carcinoma-Infiltrating T Lymphocytes. Oncoimmunology 2013, 2, e26014. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mishra, A.K.; Kadoishi, T.; Wang, X.; Driver, E.; Chen, Z.; Wang, X.J.; Wang, J.H. Squamous Cell Carcinomas Escape Immune Surveillance via Inducing Chronic Activation and Exhaustion of CD8+ T Cells Co-Expressing PD-1 and LAG-3 Inhibitory Receptors. Oncotarget 2016, 7, 81341–81356. [Google Scholar] [CrossRef] [PubMed]
- Kouo, T.; Huang, L.; Pucsek, A.B.; Cao, M.; Solt, S.; Armstrong, T.; Jaffee, E. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol. Res. 2015, 3, 412–423. [Google Scholar] [CrossRef]
- Xu, F.; Liu, J.; Liu, D.; Liu, B.; Wang, M.; Hu, Z.; Du, X.; Tang, L.; He, F. LSECtin Expressed on Melanoma Cells Promotes Tumor Progression by Inhibiting Antitumor T-Cell Responses. Cancer Res. 2014, 74, 3418–3428. [Google Scholar] [CrossRef]
- Liu, W.; Tang, L.; Zhang, G.; Wei, H.; Cui, Y.; Guo, L.; Gou, Z.; Chen, X.; Jiang, D.; Zhu, Y.; et al. Characterization of a Novel C-Type Lectin-like Gene, LSECtin: Demonstration of Carbohydrate Binding and Expression in Sinusoidal Endothelial Cells of Liver and Lymph Node. J. Biol. Chem. 2004, 279, 18748–18758. [Google Scholar] [CrossRef]
- Bos, R.; Marquardt, K.L.; Cheung, J.; Sherman, L.A. Functional Differences between Low- and High-Affinity CD8+ T Cells in the Tumor Environment. Oncoimmunology 2012, 1, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Juno, J.A.; Stalker, A.T.; Waruk, J.L.M.; Oyugi, J.; Kimani, M.; Plummer, F.A.; Kimani, J.; Fowke, K.R. Elevated Expression of LAG-3, but Not PD-1, Is Associated with Impaired INKT Cytokine Production during Chronic HIV-1 Infection and Treatment. Retrovirology 2015, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Farsam, V.; Hassan, Z.M.; Zavaran-Hosseini, A.; Noori, S.; Mahdavi, M.; Ranjbar, M. Antitumor and Immunomodulatory Properties of Artemether and Its Ability to Reduce CD4+ CD25+ FoxP3+ T Reg Cells in Vivo. Int. Immunopharmacol. 2011, 11, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kusnadi, A.; Lee, E.J.; Kim, W.W.; Cho, B.C.; Lee, I.J.; Seong, J.; Ha, S.J. Tumor-Infiltrating Regulatory T Cells Delineated by Upregulation of PD-1 and Inhibitory Receptors. Cell. Immunol. 2012, 278, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Sugiura, D.; Okazaki, I.M.; Okazaki, T. LAG-3: From Molecular Functions to Clinical Applications. J. Immunother. Cancer 2020, 8, e001014. [Google Scholar] [CrossRef] [PubMed]
- Camisaschi, C.; Casati, C.; Rini, F.; Perego, M.; De Filippo, A.; Triebel, F.; Parmiani, G.; Belli, F.; Rivoltini, L.; Castelli, C. LAG-3 Expression Defines a Subset of CD4(+)CD25(High)Foxp3(+) Regulatory T Cells That Are Expanded at Tumor Sites. J. Immunol. 2010, 184, 6545–6551. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Z. The Effect of Immune Microenvironment on the Progression and Prognosis of Colorectal Cancer. Med. Oncol. 2014, 31, 82. [Google Scholar] [CrossRef] [PubMed]
- Takaya, S.; Saito, H.; Ikeguchi, M. Upregulation of Immune Checkpoint Molecules, PD-1 and LAG-3, on CD4+ and CD8+ T Cellsafter Gastric Cancer Surgery. Yonago Acta Med. 2015, 58, 39–44. [Google Scholar] [PubMed]
- Burugu, S.; Gao, D.; Leung, S.; Chia, S.K.; Nielsen, T.O. LAG-3+ Tumor Infiltrating Lymphocytes in Breast Cancer: Clinical Correlates and Association with PD-1/PD-L1+ Tumors. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 2977–2984. [Google Scholar] [CrossRef]
- Meng, Q.; Liu, Z.; Rangelova, E.; Poiret, T.; Ambati, A.; Rane, L.; Xie, S.; Verbeke, C.; Dodoo, E.; Del Chiaro, M.; et al. Expansion of Tumor-Reactive T Cells From Patients with Pancreatic Cancer. J. Immunother. 2016, 39, 81–89. [Google Scholar] [CrossRef]
- Huo, J.L.; Wang, Y.T.; Fu, W.J.; Lu, N.; Liu, Z.S. The Promising Immune Checkpoint LAG-3 in Cancer Immunotherapy: From Basic Research to Clinical Application. Front. Immunol. 2022, 13, 956090. [Google Scholar] [CrossRef]
- Melaiu, O.; Lucarini, V.; Giovannoni, R.; Fruci, D.; Gemignani, F. News on Immune Checkpoint Inhibitors as Immunotherapy Strategies in Adult and Pediatric Solid Tumors. Semin. Cancer Biol. 2022, 79, 18–43. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Bono, P.; Bhatia, S.; Melero, I.; Nyakas, M.S.; Svane, I.-M.; Larkin, J.; Gomez-Roca, C.; Schadendorf, D.; Dummer, R.; et al. Efficacy of BMS-986016, a Monoclonal Antibody That Targets Lymphocyte Activation Gene-3 (LAG-3), in Combination with Nivolumab in Pts with Melanoma Who Progressed during Prior Anti–PD-1/PD-L1 Therapy (Mel Prior IO) in All-Comer and Biomarker-Enriched Popu. Ann. Oncol. 2017, 28, v611–v612. [Google Scholar] [CrossRef]
- Wang, X.; Bao, Z.; Zhang, X.; Li, F.; Lai, T.; Cao, C.; Chen, Z.; Li, W.; Shen, H.; Ying, S. Effectiveness and Safety of PD-1/PD-L1 Inhibitors in the Treatment of Solid Tumors: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 59901–59914. [Google Scholar] [CrossRef]
- Chocarro, L.; Blanco, E.; Arasanz, H.; Fernández-Rubio, L.; Bocanegra, A.; Echaide, M.; Garnica, M.; Ramos, P.; Fernández-Hinojal, G.; Vera, R.; et al. Clinical Landscape of LAG-3-Targeted Therapy. Immuno-Oncol. Technol. 2022, 14, 100079. [Google Scholar] [CrossRef] [PubMed]
- Chocarro, L.; Bocanegra, A.; Blanco, E.; Fernández-Rubio, L.; Arasanz, H.; Echaide, M.; Garnica, M.; Ramos, P.; Piñeiro-Hermida, S.; Vera, R.; et al. Cutting-Edge: Preclinical and Clinical Development of the First Approved Lag-3 Inhibitor. Cells 2022, 11, 2351. [Google Scholar] [CrossRef] [PubMed]
- Lavie, D.; Timmerman, J.; Garcia Sanz, R.; Kim, W.S.; Kim, T.M.; Avigdor, A.; Dierickx, D.; Jagadeesh, D.; Molin, D.; Ozcan, M.; et al. KEYFORM-008: Coformulated Favezelimab and Pembrolizumab (MK4280A) versus Chemotherapy in Relapsed/Refractory Classical Hodgkin Lymphoma. J. Clin. Oncol. 2023, 41, TPS7585. [Google Scholar] [CrossRef]
- Aggarwal, V.; Workman, C.J.; Vignali, D.A.A. LAG-3 as the Third Checkpoint Inhibitor. Nat. Immunol. 2023, 24, 1415–1422. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Rha, S.Y.; Miller, W.H.; de Miguel, M.J.; Im, S.-A.; Lugowska, I.; Wermke, M.; Kotani, D.; Bauer, T.M.; Takashima, A.; Palcza, J.; et al. Phase 1 Trial of the Anti-LAG3 Antibody Favezelimab plus Pembrolizumab in Advanced Gastric Cancer. J. Clin. Oncol. 2023, 41, 394. [Google Scholar] [CrossRef]
- Garralda, E.; Sukari, A.; Lakhani, N.J.; Patnaik, A.; Lou, Y.; Im, S.A.; Golan, T.; Geva, R.; Wermke, M.; de Miguel, M.; et al. A First-in-Human Study of the Anti-LAG-3 Antibody Favezelimab plus Pembrolizumab in Previously Treated, Advanced Microsatellite Stable Colorectal Cancer. ESMO Open 2022, 7, 100639. [Google Scholar] [CrossRef] [PubMed]
- Mencel, J.; Turkes, F.; Barber, L.; Challoner, B.; Buzzetti, M.; Tran, A.; Chen, H.; McCafferty, N.; Woolston, A.; Crux, R.; et al. P-111 PD1 and LAG3 Inhibition as Second+ Line Treatment after EGFR Antibody-Containing Therapy in RAS/BRAF Wildtype, MMRp Metastatic Colorectal Cancer. Ann. Oncol. 2023, 34, S53–S54. [Google Scholar] [CrossRef]
- Schöffski, P.; Tan, D.S.W.; Martín, M.; Ochoa-De-Olza, M.; Sarantopoulos, J.; Carvajal, R.D.; Kyi, C.; Esaki, T.; Prawira, A.; Akerley, W.; et al. Phase I/II Study of the LAG-3 Inhibitor Ieramilimab (LAG525) ± Anti-PD-1 Spartalizumab (PDR001) in Patients with Advanced Malignancies. J. Immunother. Cancer 2022, 10, e003776. [Google Scholar] [CrossRef] [PubMed]
- Rotte, A.; Sahasranaman, S.; Budha, N. Targeting TIGIT for Immunotherapy of Cancer: Update on Clinical Development. Biomedicines 2021, 9, 1277. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zheng, Q.; Xin, N.; Wang, W.; Zhao, C. CD155, an Onco-Immunologic Molecule in Human Tumors. Cancer Sci. 2017, 108, 1934–1938. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The Surface Protein TIGIT Suppresses T Cell Activation by Promoting the Generation of Mature Immunoregulatory Dendritic Cells. Nat. Immunol. 2008, 10, 48–57. [Google Scholar] [CrossRef]
- Bhandaru, M.; Rotte, A. Monoclonal Antibodies for the Treatment of Melanoma: Present and Future Strategies. Methods Mol. Biol. 2019, 1904, 83–108. [Google Scholar] [CrossRef]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT Co-Inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, W.; Li, H.; Chen, Y.; Tian, H.; Li, L.; Zhang, L.; Gao, C.; Zheng, J. Immunoreceptor TIGIT Inhibits the Cytotoxicity of Human Cytokine-Induced Killer Cells by Interacting with CD155. Cancer Immunol. Immunother. 2016, 65, 305–314. [Google Scholar] [CrossRef]
- Kurtulus, S.; Sakuishi, K.; Ngiow, S.F.; Joller, N.; Tan, D.J.; Teng, M.W.L.; Smyth, M.J.; Kuchroo, V.K.; Anderson, A.C. TIGIT Predominantly Regulates the Immune Response via Regulatory T Cells. J. Clin. Investig. 2015, 125, 4053–4062. [Google Scholar] [CrossRef]
- Judge, S.J.; Darrow, M.A.; Thorpe, S.W.; Gingrich, A.A.; O’Donnell, E.F.; Bellini, A.R.; Sturgill, I.R.; Vick, L.V.; Dunai, C.; Stoffel, K.M.; et al. Analysis of Tumor-Infiltrating NK and T Cells Highlights IL-15 Stimulation and TIGIT Blockade as a Combination Immunotherapy Strategy for Soft Tissue Sarcomas. J. Immunother. Cancer 2020, 8, e001355. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.; Gu, Z.; Jiang, Z.; Zhao, S.; Song, Y.; Yu, J. Targeting TIGIT for Cancer Immunotherapy: Recent Advances and Future Directions. Biomark. Res. 2024, 12, 7. [Google Scholar] [CrossRef]
- Liu, Z.; Zeng, H.; Jin, K.; Yu, Y.; You, R.; Zhang, H.; Liu, C.; Su, X.; Yan, S.; Chang, Y.; et al. TIGIT and PD-1 Expression Atlas Predicts Response to Adjuvant Chemotherapy and PD-L1 Blockade in Muscle-Invasive Bladder Cancer. Br. J. Cancer 2022, 126, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Joller, N.; Lozano, E.; Burkett, P.R.; Patel, B.; Xiao, S.; Zhu, C.; Xia, J.; Tan, T.G.; Sefik, E.; Yajnik, V.; et al. Treg Cells Expressing the Coinhibitory Molecule TIGIT Selectively Inhibit Proinflammatory Th1 and Th17 Cell Responses. Immunity 2014, 40, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Lucca, L.E.; Dominguez-Villar, M. Modulation of Regulatory T Cell Function and Stability by Co-Inhibitory Receptors. Nat. Rev. Immunol. 2020, 20, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Abreu, D.R.; Hussein, M.; Cobo, M.; Patel, A.J.; Secen, N.; Lee, K.H.; Massuti, B.; Hiret, S.; Yang, J.C.H.; et al. Tiragolumab plus Atezolizumab versus Placebo plus Atezolizumab as a First-Line Treatment for PD-L1-Selected Non-Small-Cell Lung Cancer (CITYSCAPE): Primary and Follow-up Analyses of a Randomised, Double-Blind, Phase 2 Study. Lancet Oncol. 2022, 23, 781–792. [Google Scholar] [CrossRef]
- Rudin, C.M.; Liu, S.V.; Soo, R.A.; Lu, S.; Hong, M.H.; Lee, J.S.; Bryl, M.; Dumoulin, D.W.; Rittmeyer, A.; Chiu, C.H.; et al. SKYSCRAPER-02: Tiragolumab in Combination with Atezolizumab Plus Chemotherapy in Untreated Extensive-Stage Small-Cell Lung Cancer. J. Clin. Oncol. 2024, 42, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Brazel, D.; Ou, S.H.I.; Nagasaka, M. Tiragolumab (Anti-TIGIT) in SCLC: Skyscraper-02, a Towering Inferno. Lung Cancer Targets Ther. 2023, 14, 1–9. [Google Scholar] [CrossRef]
- Niu, J.; Maurice-Dror, C.; Lee, D.H.; Kim, D.W.; Nagrial, A.; Voskoboynik, M.; Chung, H.C.; Mileham, K.; Vaishampayan, U.; Rasco, D.; et al. First-in-Human Phase 1 Study of the Anti-TIGIT Antibody Vibostolimab as Monotherapy or with Pembrolizumab for Advanced Solid Tumors, Including Non-Small-Cell Lung Cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 169–180. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Hsu, C.-H.; Li, D.; Burgoyne, A.; Cotter, C.; Badhrinarayanan, S.; Wang, Y.; Yin, A.; Rao Edubilli, T.; et al. Results from the MORPHEUS-Liver Study: Phase Ib/II Randomized Evaluation of Tiragolumab (Tira) in Combination with Atezolizumab (Atezo) and Bevacizumab (Bev) in Patients with Unresectable, Locally Advanced or Metastatic Hepatocellular Carcinoma (UHCC). J. Clin. Oncol. 2023, 41, 4010. [Google Scholar] [CrossRef]
- Ge, Z.; Peppelenbosch, M.P.; Sprengers, D.; Kwekkeboom, J. TIGIT, the Next Step Towards Successful Combination Immune Checkpoint Therapy in Cancer. Front. Immunol. 2021, 12, 699895. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Tian, W.; Wang, Z.; Zhang, J.; Zhou, R. Co-Inhibition of TIGIT and PD-1/PD-L1 in Cancer Immunotherapy: Mechanisms and Clinical Trials. Mol. Cancer 2023, 22, 93. [Google Scholar] [CrossRef]
- Vesely, M.D.; Zhang, T.; Chen, L. Resistance Mechanisms to Anti-PD Cancer Immunotherapy. Annu. Rev. Immunol. 2022, 40, 45–74. [Google Scholar] [CrossRef]
- Li, X.; Wang, R.; Fan, P.; Yao, X.; Qin, L.; Peng, Y.; Ma, M.; Asley, N.; Chang, X.; Feng, Y.; et al. A Comprehensive Analysis of Key Immune Checkpoint Receptors on Tumor-Infiltrating t Cells from Multiple Types of Cancer. Front. Oncol. 2019, 9, 1066. [Google Scholar] [CrossRef]
- Chauvin, J.M.; Pagliano, O.; Fourcade, J.; Sun, Z.; Wang, H.; Sander, C.; Kirkwood, J.M.; Chen, T.H.T.; Maurer, M.; Korman, A.J.; et al. TIGIT and PD-1 Impair Tumor Antigen-Specific CD8+ T Cells in Melanoma Patients. J. Clin. Investig. 2015, 125, 2046–2058. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Oh, D.-Y.; Pelster, M.; Wainberg, Z.A.; Sison, E.A.R.; Scott, J.R.; Ronayne, J.; Wishengrad, D.; Rhee, J.; Nuyten, D.S.A.; et al. EDGE-Gastric Arm A1: Phase 2 Study of Domvanalimab, Zimberelimab, and FOLFOX in First-Line (1L) Advanced Gastroesophageal Cancer. J. Clin. Oncol. 2023, 41, 433248. [Google Scholar] [CrossRef]
- Pawłowska, A.; Skiba, W.; Suszczyk, D.; Kuryło, W.; Jakubowicz-Gil, J.; Paduch, R.; Wertel, I. The Dual Blockade of the TIGIT and PD-1/PD-L1 Pathway as a New Hope for Ovarian Cancer Patients. Cancers 2022, 14, 5757. [Google Scholar] [CrossRef]
- Raphael, I.; Kumar, R.; McCarl, L.H.; Shoger, K.; Wang, L.; Sandlesh, P.; Sneiderman, C.T.; Allen, J.; Zhai, S.; Campagna, M.L.; et al. TIGIT and PD-1 Immune Checkpoint Pathways Are Associated with Patient Outcome and Anti-Tumor Immunity in Glioblastoma. Front. Immunol. 2021, 12, 637146. [Google Scholar] [CrossRef]
- Rohrberg, K.S.; Brandão, M.; Castanon Alvarez, E.; Felip, E.; Gort, E.H.; Hiltermann, T.J.J.N.; Izumi, H.; Kim, D.-W.; Kim, S.-W.; Paz-Ares, L.G.; et al. Safety, Pharmacokinetics (PK), Pharmacodynamics (PD) and Preliminary Efficacy of AZD2936, a Bispecific Antibody Targeting PD-1 and TIGIT, in Checkpoint Inhibitor (CPI)-Experienced Advanced/Metastatic Non-Small-Cell Lung Cancer (NSCLC): First Report of ART. J. Clin. Oncol. 2023, 41, 9050. [Google Scholar] [CrossRef]
- Rishiq, A.; Bsoul, R.; Pick, O.; Mandelboim, O. Studying TIGIT Activity against Tumors through the Generation of Knockout Mice. Oncoimmunology 2023, 12, 2217735. [Google Scholar] [CrossRef] [PubMed]
- Monteran, L.; Ershaid, N.; Scharff, Y.; Zoabi, Y.; Sanalla, T.; Ding, Y.; Pavlovsky, A.; Zait, Y.; Langer, M.; Caller, T.; et al. Combining TIGIT Blockade with MDSC Inhibition Hinders Breast Cancer Bone Metastasis by Activating Antitumor Immunity. Cancer Discov. 2024, OF1–OF24. [Google Scholar] [CrossRef]
- Han, J.H.; Cai, M.; Grein, J.; Perera, S.; Wang, H.; Bigler, M.; Ueda, R.; Rosahl, T.W.; Pinheiro, E.; LaFace, D.; et al. Effective Anti-Tumor Response by TIGIT Blockade Associated with FcγR Engagement and Myeloid Cell Activation. Front. Immunol. 2020, 11, 573405. [Google Scholar] [CrossRef]
- Zhao, K.; Jiang, L.; Si, Y.; Zhou, S.; Huang, Z.; Meng, X. TIGIT Blockade Enhances Tumor Response to Radiotherapy via a CD103 + Dendritic Cell-Dependent Mechanism. Cancer Immunol. Immunother. 2023, 72, 193–209. [Google Scholar] [CrossRef]
- Kim, T.W.; Bedard, P.L.; Lorusso, P.; Gordon, M.S.; Bendell, J.; Oh, D.Y.; Ahn, M.J.; Garralda, E.; D’Angelo, S.P.; Desai, J.; et al. Anti-TIGIT Antibody Tiragolumab Alone or with Atezolizumab in Patients with Advanced Solid Tumors: A Phase 1a/1b Nonrandomized Controlled Trial. JAMA Oncol. 2023, 9, 1574–1582. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Lu, Z.; Gao, S.; Wang, J.-Y.; Sun, J.-M.; Liu, T.; Fan, Q.; Cai, J.; Ge, F.; Li, S.; et al. SKYSCRAPER-08: A Phase III, Randomized, Double-Blind, Placebo-Controlled Study of First-Line (1L) Tiragolumab (Tira) + Atezolizumab (Atezo) and Chemotherapy (CT) in Patients (Pts) with Esophageal Squamous Cell Carcinoma (ESCC). J. Clin. Oncol. 2024, 42, 245. [Google Scholar] [CrossRef]
- Study Details|A Study of Tiragolumab in Combination with Atezolizumab Compared with Placebo in Combination with Atezolizumab in Patients with Previously Untreated Locally Advanced Unresectable or Metastatic PD-L1-Selected Non-Small Cell Lung Cancer. Available online: https://clinicaltrials.gov/study/NCT04294810 (accessed on 22 June 2024).
- Yamamoto, N.; Koyama, T.; Sato, J.; Yoshida, T.; Sudo, K.; Iwasa, S.; Kondo, S.; Yonemori, K.; Kawasaki, A.; Satake, K.; et al. Phase I Study of the Anti-TIGIT Antibody Tiragolumab in Combination with Atezolizumab in Japanese Patients with Advanced or Metastatic Solid Tumors. Cancer Chemother. Pharmacol. 2024, 1–7. [Google Scholar] [CrossRef]
- Thibaudin, M.; Limagne, E.; Hampe, L.; Ballot, E.; Truntzer, C.; Ghiringhelli, F. Targeting PD-L1 and TIGIT Could Restore Intratumoral CD8 T Cell Function in Human Colorectal Cancer. Cancer Immunol. Immunother. 2022, 71, 2549–2563. [Google Scholar] [CrossRef]
- Shao, Q.; Wang, L.; Yuan, M.; Jin, X.; Chen, Z.; Wu, C. TIGIT Induces (CD3+) T Cell Dysfunction in Colorectal Cancer by Inhibiting Glucose Metabolism. Front. Immunol. 2021, 12, 688961. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, Y.; Tan, J.; Xu, L.; Li, Y. Targeting LAG-3, TIM-3, and TIGIT for Cancer Immunotherapy. J. Hematol. Oncol. 2023, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Gilmour, C.; Patnaik, S.; Wang, L.L. Combinatorial Blockade for Cancer Immunotherapy: Targeting Emerging Immune Checkpoint Receptors. Front. Immunol. 2023, 14, 1264327. [Google Scholar] [CrossRef]
- Yang, R.; Huang, S.; Huang, C.; Fay, N.S.; Wang, Y.; Putrevu, S.; Wright, K.; Zaman, M.S.; Cai, W.; Huang, B.; et al. Fc-Competent Multispecific PDL-1/TIGIT/LAG-3 Antibodies Potentiate Superior Anti-Tumor T Cell Response. Sci. Rep. 2023, 13, 9865. [Google Scholar] [CrossRef] [PubMed]
- Ulas, E.B.; Hashemi, S.M.S.; Houda, I.; Kaynak, A.; Veltman, J.D.; Fransen, M.F.; Radonic, T.; Bahce, I. Predictive Value of Combined Positive Score and Tumor Proportion Score for Immunotherapy Response in Advanced NSCLC. JTO Clin. Res. Rep. 2023, 4, 100532. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Rashid, S.; Al-Bozom, I.A. PD−L1 Immunostaining: What Pathologists Need to Know. Diagn. Pathol. 2021, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, C.; Wang, F.; Zhang, H.; Xiao, W.; Li, H.; Lu, Z.; Pan, Z.; Wu, X.; Zhang, R. Right-and Left-Sided Stage III Colon Cancers Present Different Prognostic Outcomes of Oxaliplatin-Based Adjuvant Chemotherapy after Curative Resection. Cancer Manag. Res. 2018, 10, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, R.; Miura, S.; Muto, S.; Toyomasu, Y.; Fujimoto, Y.; Ieguchi, K.; Onishi, N.; Shimizu, T.; Watanabe, M.; Takayanagi, D.; et al. Novel Quantitative Immunohistochemical Analysis for Evaluating PD-L1 Expression with Phosphor-Integrated Dots for Predicting the Efficacy of Patients with Cancer Treated with Immune Checkpoint Inhibitors. Front. Immunol. 2023, 14, 1260492. [Google Scholar] [CrossRef] [PubMed]
- Vranic, S.; Gatalica, Z. PD-L1 Testing by Immunohistochemistry in Immuno-Oncology. Biomol. Biomed. 2023, 23, 15. [Google Scholar] [CrossRef]
- Butterfield, L.H. The Society for Immunotherapy of Cancer Biomarkers Task Force Recommendations Review. Semin. Cancer Biol. 2018, 52, 12–15. [Google Scholar] [CrossRef]
- Taube, J.M.; Akturk, G.; Angelo, M.; Engle, E.L.; Gnjatic, S.; Greenbaum, S.; Greenwald, N.F.; Hedvat, C.V.; Hollmann, T.J.; Juco, J.; et al. The Society for Immunotherapy of Cancer Statement on Best Practices for Multiplex Immunohistochemistry (IHC) and Immunofluorescence (IF) Staining and Validation. J. Immunother. Cancer 2020, 8, e000155. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; McCune, B.; Locke, D.; Hedvat, C.; Wojcik, J.B.; Schroyer, C.; Yan, J.; Johnson, K.; Sanders-Cliette, A.; Samala, S.; et al. Development of a LAG-3 Immunohistochemistry Assay for Melanoma. J. Clin. Pathol. 2023, 76, 591–598. [Google Scholar] [CrossRef]
- Wojcik, J.B.; Desai, K.; Avraam, K.; Vandebroek, A.; Dillon, L.M.; Giacomazzi, G.; Rypens, C.; Benci, J.L. Measurement of LAG-3 Expression Across Multiple Staining Platforms with the 17B4 Antibody Clone. Arch. Pathol. Lab. Med. 2023, 147, 1307–1314. [Google Scholar] [CrossRef]
- Arimura, K.; Hiroshima, K.; Nagashima, Y.; Nakazawa, T.; Ogihara, A.; Orimo, M.; Sato, Y.; Katsura, H.; Kanzaki, M.; Kondo, M.; et al. LAG3 Is an Independent Prognostic Biomarker and Potential Target for Immune Checkpoint Inhibitors in Malignant Pleural Mesothelioma: A Retrospective Study. BMC Cancer 2023, 23, 1206. [Google Scholar] [CrossRef]
- Ulase, D.; Behrens, H.M.; Krüger, S.; Heckl, S.M.; Ebert, U.; Becker, T.; Röcken, C. LAG3 in Gastric Cancer: It’s Complicated. J. Cancer Res. Clin. Oncol. 2023, 149, 10797–10811. [Google Scholar] [CrossRef] [PubMed]
- Annibali, O.; Bianchi, A.; Grifoni, A.; Tomarchio, V.; Tafuri, M.; Verri, M.; Avvisati, G.; Crescenzi, A. A Novel Scoring System for TIGIT Expression in Classic Hodgkin Lymphoma. Sci. Rep. 2021, 11, 7059. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Blessin, N.C.; Simon, R.; Kluth, M.; Fischer, K.; Hube-Magg, C.; Makrypidi-Fraune, G.; Wellge, B.; Mandelkow, T.; Debatin, N.F.; et al. Expression of the Immune Checkpoint Receptor TIGIT in Hodgkin’s Lymphoma. BMC Cancer 2018, 18, 1209. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Xiao, K.; Li, K.; Xue, P.; Zhu, S. Prognostic Role of TIGIT Expression in Patients with Solid Tumors: A Meta-Analysis. J. Immunol. Res. 2021, 2021, 5440572. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, J.; Xu, X.; Wang, H.; Zhang, C.; Yin, S.; He, Y. Peritumoral TIGIT+ CD20+ B Cell Infiltration Indicates Poor Prognosis but Favorable Adjuvant Chemotherapeutic Response in Gastric Cancer. Int. Immunopharmacol. 2022, 108, 108735. [Google Scholar] [CrossRef] [PubMed]
- Tavana, S.; Mokhtari, Z.; Sanei, M.H.; Heidari, Z.; Dehghanian, A.R.; Faghih, Z.; Rezaei, M. Clinicopathological Significance and Prognostic Role of LAG3 + Tumor-Infiltrating Lymphocytes in Colorectal Cancer; Relationship with Sidedness. Cancer Cell Int. 2023, 23, 23. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, Y.; Wu, X.; Liu, Z.; Liu, X. A Novel Strategy to Fuel Cancer Immunotherapy: Targeting Glucose Metabolism to Remodel the Tumor Microenvironment. Front. Oncol. 2022, 12, 931104. [Google Scholar] [CrossRef]
- Rahman, A.; Janic, B.; Rahman, T.; Singh, H.; Ali, H.; Rattan, R.; Kazi, M.; Ali, M.M. Immunotherapy Enhancement by Targeting Extracellular Tumor PH in Triple-Negative Breast Cancer Mouse Model. Cancers 2023, 15, 4931. [Google Scholar] [CrossRef]
- Pilon-Thomas, S.; Kodumudi, K.N.; El-Kenawi, A.E.; Russell, S.; Weber, A.M.; Luddy, K.; Damaghi, M.; Wojtkowiak, J.W.; Mulé, J.J.; Ibrahim-Hashim, A.; et al. Neutralization of Tumor Acidity Improves Antitumor Responses to Immunotherapy. Cancer Res. 2016, 76, 1381–1390. [Google Scholar] [CrossRef]
- Scott, K.E.N.; Cleveland, J.L. Lactate Wreaks Havoc on Tumor-Infiltrating T and NK Cells. Cell Metab. 2016, 24, 649–650. [Google Scholar] [CrossRef] [PubMed]
- Davern, M.; Donlon, N.E.; O’Connell, F.; Gaughan, C.; O’Donovan, C.; Habash, M.; Sheppard, A.D.; MacLean, M.; Dunne, M.R.; Moore, J.; et al. Acidosis Significantly Alters Immune Checkpoint Expression Profiles of T Cells from Oesophageal Adenocarcinoma Patients. Cancer Immunol. Immunother. 2023, 72, 55–71. [Google Scholar] [CrossRef] [PubMed]


| Clinical Trial | Status | Summary | Reference |
|---|---|---|---|
| KEYFORM-008 | Ongoing | Favezelimab/pembrolizumab with bendamustine or gemcitabine in participants with PDL1-refractory, relapsed, or refractory classical Hodgkin lymphoma. | [57] |
| RELATIVITY-047 (NCT03470922) | Ongoing | Relatlimab in combination with nivolumab in treating unresectable melanoma or melanoma that has spread. | [56,59] |
| NCT03044613 | Ongoing | Nivolumab (anti-PD-1) or Anti-PD1/Anti LAG-3- (relatlimab) as a neoadjuvant in patients with resectable distal esophageal/gastroesophageal junction cancer. | [60] |
| iSCORE (NCT03867799) | Ongoing | Antitumoral benefit of nivolumab (anti-PD1) and relatlimab (anti-LAG3) therapy in patients with metastatic colorectal, RAS/BRAF wildtype and without microsatellite instability | [62] |
| NCT02460224 | Completed | Assessment of safety and antitumoral effects of the LAG-3 inhibitor, ieramilimab with the anti-PD-1 antibody, spartalizumab, in advanced/metastatic solid tumors. | [63] |
| Clinical Trial | Status | Summary | Reference |
|---|---|---|---|
| CITYSCAPE (NCT03563716) | Ongoing | Efficacy study of tiragolumab plus atezolizumab compared with atezolizumab in chemotherapy-naive patients with locally advanced unresectable or metastatic PD-L1-selected non-small cell lung cancer. | [76] |
| SKYSCRAPER-01 (NCT04294810) | Ongoing | Compared the efficacy and safety of tiragolumab plus atezolizumab compared with atezolizumab alone as a first line treatment with PD-L1 high locally advanced, unresectable or metastatic non-small cell lung cancer. | [96] |
| SKYSCRAPER-02 (NCT04256421) | Ongoing | Evaluated the efficacy of tiragolumab plus atezolizumab and carboplatin and etoposide compared with atezolizumab and etoposide in participants with chemotherapy-naive extensive-stage small cell lung cancer. | [77,78] |
| KEYVIBE-001 (NCT02964013) | Ongoing | Safety, efficacy, and pharmacokinetics study of vibostolimab isolated or in combination with pembrolizumab or pembrolizumab plus pemetrexed and carboplatin in metastatic solid tumors for which there is no therapy | [79] |
| Morpheus-Liver (NCT04524871) | Ongoing | Study on locally advanced or metastatic hepatocellular carcinoma patients who have not received prior systemic therapy for their disease. | [80] |
| EDGE-Gastric (NCT05329766) | Ongoing | Treatment combinations with and without chemotherapy in participants with locally advanced unresectable or metastatic gastric, GEJ, and esophageal adenocarcinoma. Chemotherapy will consist of FOLFOX (oxaliplatin, leucovorin, fluorouracil). | [86] |
| ARTEMIDE-01 (NCT04995523) | Ongoing | Phase I/II study designed to evaluate if experimental anti-TIGIT/anti-PD-1 bispecific antibody rilvegostomig is safe, tolerable and efficacious in participants with advanced or metastatic non-small cell lung cancer. | [89] |
| SKYSCRAPER-08 | Ongoing | A phase III study of atezolizumab plus tiragolumab in combination with paclitaxel and cisplatin compared with paclitaxel and cisplatin as a first-line treatment in patients with unresectable locally advanced, unresectable recurrent, or metastatic esophageal squamous cell carcinoma. | [95] |
| GO30103 (NCT02794571) | Ongoing | Evaluated the safety, tolerability of tiragolumab alone or in combination with atezolizumab and/or other therapies in locally advanced, recurrent, or metastatic incurable tumors. | [94] |
| jRCT2080224926 | Completed | Tiragolumab and atezolizumab intravenously every 21 days until unacceptable toxicity or disease progression. | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gama, J.M.; Teixeira, P.; Caetano Oliveira, R. The World of Immunotherapy Needs More Than PD-1/PD-L1—Two of the New Kids on the Block: LAG-3 and TIGIT. Onco 2024, 4, 116-130. https://doi.org/10.3390/onco4030010
Gama JM, Teixeira P, Caetano Oliveira R. The World of Immunotherapy Needs More Than PD-1/PD-L1—Two of the New Kids on the Block: LAG-3 and TIGIT. Onco. 2024; 4(3):116-130. https://doi.org/10.3390/onco4030010
Chicago/Turabian StyleGama, João Martins, Paulo Teixeira, and Rui Caetano Oliveira. 2024. "The World of Immunotherapy Needs More Than PD-1/PD-L1—Two of the New Kids on the Block: LAG-3 and TIGIT" Onco 4, no. 3: 116-130. https://doi.org/10.3390/onco4030010
APA StyleGama, J. M., Teixeira, P., & Caetano Oliveira, R. (2024). The World of Immunotherapy Needs More Than PD-1/PD-L1—Two of the New Kids on the Block: LAG-3 and TIGIT. Onco, 4(3), 116-130. https://doi.org/10.3390/onco4030010










