Abstract
Introduction: The digital era has provided the development of innovative health devices that enable the precise characterization of health and disease, facilitating diagnoses and interventions. This study aimed to systematically review and verify the quality of mobile applications (apps) available for the monitoring and assessment of breast cancer-related lymphedema (BCRL). Methods: A systematic search was conducted in the Apple App Store and Google Play Store for apps related to BCRL monitoring and assessment. Two independent reviewers extracted descriptive data and evaluated app quality using the validated User Mobile App Rating Scale (uMARS). Results: Out of 630 apps screened, four met the inclusion criteria and were analyzed. Two Korean apps targeted patients, providing educational content, self-assessment tools, and bilingual interfaces. Two British apps, LymVol and LymphaTech Lite, focused on volumetric measurement and clinical use, although LymVol lacked compatibility with recent Android versions. Quality assessment using the uMARS indicated that the included applications performed consistently across the evaluated domains, despite low download numbers and the absence of user ratings. Conclusions: Although mobile apps have the potential to enhance lymphedema monitoring and assessment, more accessible and scientifically validated tools are needed to ensure effective use by healthcare professionals and patients. Developers are encouraged to create accessible, linguistically inclusive smartphone apps that incorporate standardized assessment protocols and regular updates to ensure usability and accuracy. Rigorous validation studies covering reproducibility, diagnostic accuracy, and real-world clinical outcomes should be conducted by researchers to guarantee safety and reliability.
1. Introduction
Breast cancer-related lymphedema (BCRL) is a common complication of cancer treatment related to surgical approach and adjuvant therapies (e.g., chemotherapy or radiotherapy) [1]. Its occurrences are relatively underestimated and poorly understood, which can negatively impact the quality of life of female survivors [1]. Assessing and monitoring patients enables early detection of their conditions and implementation of specialized and assertive intervention [2]. The absence of a unified tool to assess BCRL demands the use of different objective methodologies, such as computed tomography and lymphoscintigraphy. However, the lack of validated standards challenges the definition of the prevalence and associated repercussions [3].
International guidelines recommend performing volumetric techniques to confirm a lymphedema diagnosis. These techniques are based on circumferential measurements using perimetry or water displacement, perometry, and optoelectronic volumetry [3,4,5]. Volumetry analysis using perimetry shows fewer limitations, including a low error rate, lower cost, and shorter time expenditure [4]. Moreover, volumetric assessment via perimetry employs formulas and calculations that are reliable and widely accepted by professionals. However, despite its effectiveness, this method can be time-consuming and complex, thereby limiting its practicality in routine clinical settings [5].
In the digital era, scientific and technological advances have facilitated the development of innovative health technologies, enabling the precise characterization of health conditions, diagnoses, and interventions through mobile devices [6]. Smartphone applications, in particular, can support this clinical measurement by assisting in data collection, performing calculations, and streamlining the process of assessing and monitoring lymphedema [7,8,9].
Digital health technologies have also expanded the possibilities for remote care and follow-up, offering resources such as health education, therapeutic exercise guidance, clinical information, and access to medical care [10,11]. There is moderate-to-strong evidence that app-based, internet-based, and telerehabilitation interventions positively influence outcomes in musculoskeletal conditions, including patient-reported measures of impairment and quality of life [12].
A mobile health app (mHealth) is a software for mobile devices that processes health data for patients or health professionals for clinical purposes [13]. Healthcare professionals value simplicity in mHealth applications, recognizing their potential to support clinical practice, even in the face of concerns regarding insufficient medical information, non-standardized processes, and accountability issues [14]. mHealth applications can indeed contribute to improving the quality of care and are already being incorporated into daily practice [15]. Furthermore, professionals express optimism regarding the use of these tools to promote participatory care, enhancing patient communication and engagement [16].
On the other hand, while mHealth applications offer new opportunities for health promotion and disease self-management, healthcare professionals face challenges related to quality, regulation, and commercial impacts [17]. In this context, professionals play a crucial role in the development of mHealth applications aimed at prevention, particularly breast cancer, as their involvement ensures the quality and integration of essential features such as self-examination tutorials, reminders, data recording, and education on risk factors [18].
Despite the growing number of studies on the feasibility of mHealth interventions in breast cancer survivors, the objectives of recently published systematic reviews have focused on identifying the impact of mHealth interventions on patients with breast cancer [19,20,21], including their effects on mental health [22]. However, little is known about the use and availability of tools specifically designed for the assessment and monitoring of lymphedema. Given these limitations in tracking lymphedema progression and the advent of digital technologies, the present study aimed to systematically review and evaluate the quality of applications developed to monitor and assess BCRL. Considering the limitations of monitoring to monitor lymphedema progression and the advent of digital technologies, this study aimed to systematically review and evaluate the quality of apps designed for monitoring and assessing BCRL.
2. Materials and Methods
This systematic review, whose protocol is registered on the Open Science Framework (OSF) under the number 5c36n “https://osf.io/5c36n/ (accessed on 5 November 2025)”, examined the available apps for monitoring and assessing BCRL. The searches were conducted on 28 February 2025, in the Apple App Store (iOS) and Google Play Store (Android), using the following English-language search terms: “Lymphedema”, “lymphatic drainage”, “post-breast cancer lymphedema”, “lymphatic”, and “swelling in the arms”. The searches were performed independently by two researchers, without geographic restrictions, to capture all potentially relevant apps.
Following the identification of the apps included in this review, complementary searches were performed in PubMed, Scopus, Cochrane, Web of Science, and Embase to retrieve studies assessing the validity or clinical applicability of these tools, to verify whether they had been tested within research or clinical practice contexts. For these searches, the names of the included apps will be used as keywords, combined with terms related to psychometric and clinical assessment (validation OR validity OR reliability OR usability OR feasibility OR “clinical trial” OR applicability OR implementation).
2.1. Eligibility Criteria and App Selection
The study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementery Material). Eligibility criteria were defined to ensure that the review captured only mobile health applications with direct applicability to the evaluation and/or monitoring of breast cancer-related lymphedema, while preserving broad initial inclusion to avoid missing potentially relevant tools. Apps had to be functional on widely accessible platforms, since feasibility and scalability in real-world contexts were central considerations. Cost and language were not restricted. Exclusion criteria were established as a second level of screening to eliminate apps that were not aligned with the scope of the review, such as those requiring external hardware or lacking specific features for lymphedema care.
Inclusion criteria
- Applications related to lymphedema;
- Availability for download and functionality on at least one of the two major app stores (App Store or Google Play).
Exclusion criteria
- Apps without any feature specifically designed for the evaluation or monitoring of breast cancer-related lymphedema;
- Apps requiring additional external devices or resources beyond a smartphone for their operation;
- Apps that were not functional after download or could not be accessed for testing.
App identification and screening were performed independently by two researchers, based initially on the title and description of each app. In cases where eligibility could not be determined, available images from the app stores were examined. Any discrepancies between reviewers were resolved through a consensus meeting involving a third researcher. Finally, descriptive data were extracted from the included apps, which were subsequently downloaded for quality assessment.
2.2. Data Extraction
The data extraction was also conducted independently by two researchers. Descriptive data was recorded, including the name of the app, developer, available features, price, country of origin, number of downloads, version number, star rating of users, and most recent updates available. Furthermore, the User Mobile App Rating Scale (uMARS) was employed to assess the quality of the apps. The researchers created a login and password when necessary to access the app interface and assessed the features and functionalities available for each app.
2.3. Quality Assessment
The uMARS was developed for the evaluation and classification of the quality of mHealth apps and has excellent internal consistency and satisfactory intra-examiner reliability [23,24]. Its instrument comprises questions distributed into five subscales: four subscales with 20 questions designed to assess the objective quality of the app—engagement, functionality, aesthetics, and information quality—and one subscale with 6 questions for subjective quality. Moreover, there is a final discursive question for pertinent comments from the evaluator [24,25].
The objective quality assessment was independently conducted by the researchers using the uMARS. For optimal use, recommendations from authors were followed, comprising a calibration exercise using the uMARS scale training slides [26]. Subsequently, a consensus meeting established a shared interpretation of the uMARS items, avoiding discrepancies in question interpretation [24].
Three menstrual cycle apps were selected for individual assessment in a calibration test following a meeting to evaluate the inter-rater reproducibility. Menstrual cycle apps were selected for the calibration test due to their widespread availability and frequent use by the general population. This stage aimed solely to train the researchers in the consistent application of the uMARS and to evaluate inter-rater reproducibility, allowing practical experience across all uMARS subscales (engagement, functionality, aesthetics, and information) before assessing the target apps. The data reliability was calculated using the intraclass correlation coefficient (ICC) with a 95% confidence interval, employing a two-way mixed-effects model with absolute agreement. The minimum requirement to proceed with the assessment of the apps was set as a moderate level (ICC > 0.6) [27]. The researchers achieved satisfactory reproducibility in the engagement and functionality subscales, with ICCs of 0.85 and 0.77, respectively. The aesthetics and information ICC subscales were 0.6 and 0.67, respectively, indicating moderate reproducibility. The subjective subscale was not analyzed concerning reproducibility.
3. Results
The initial search of the app stores identified 628 applications, and two additional apps were retrieved from other sources, totaling 630. After removing duplicates, 506 apps were screened according to the eligibility criteria. Of these, 485 were excluded, and 23 were considered potentially eligible (21 from the app store search and 2 from other sources). Following secondary screening, four apps fulfilled all eligibility criteria and were included in the final analysis: three identified from the app stores and one retrieved from other sources. Following the identification of the included apps, complementary searches were conducted in PubMed, Scopus, Web of Science, Embase, and Cochrane. The reasons for exclusion at each stage and the complementary search are summarized in Figure 1.
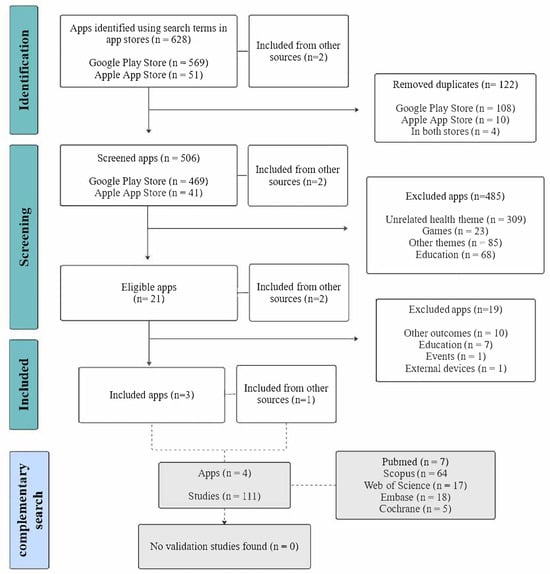
Figure 1.
Flowchart illustrating the selection process of mobile applications and the complementary database search.
The results of the descriptive analysis of the apps are presented in Table 1. Two apps included were from South Korea: “Lymphedema Self-Management” (림프부종 자가관리 or Lymphedema) that offered an option for Korean or English language on the interface; and “Myeongsimboam—Breast Cancer Edition” (명심보암—유방암편). The more recent versions of these apps, updated in November and December 2023, respectively, were downloaded at no cost.

Table 1.
Description of the included mobile applications (apps).
The “LymVol—Lymphedema Limb Volu” app on the Google Play store was also available on the Apple App store as “LymVol Limb: Volume Calculator”. This app was from England and needed to be purchased, costing $17.71 and $24.79, in the respective app stores. The most recent update was registered in September 2019.
The Korean apps “Myeongsimboam—Breast Cancer Edition” (명심보암—유방암편) and “Lymphedema Self-Management” (림프부종 자가관리) were designed for patients. Their interfaces present information about breast cancer, protocols for rehabilitation and management of lymphedema, exercise diaries, and self-assessment tools. The information is presented in text, images, and videos. The “Myeongsimboam—Breast Cancer Edition” app (명심보암—유방암편) is affiliated with the Cancer Rehabilitation Center at Ulsan University Hospital. The “Lymphedema self-management” app (림프부종 자가관리) showed several scientific references, which reinforce the information’s credibility.
The British application, developed by the Lymphedema Training Academy, was designed for healthcare professionals and provides volumetric calculations for both upper and lower limbs based on circumferential measurements in centimetres (cm), taken from proximal to distal points on each limb. Additionally, the app includes a body mass index calculator and detailed guidance on how to perform limb measurements accurately and effectively, thereby supporting measurement precision and consistency. A noted limitation of the application is its incompatibility with recent versions of the Android operating system.
The “Myungsimboam—Breast Cancer” application (명심보암—유방암편) does not feature a tool for volumetric calculation. Instead, it offers information on perimeter measurement techniques, indicating specific anatomical landmarks: at the wrist line and 5 cm above it, as well as at the elbow line, 15 cm and 10 cm above, and 10 cm below.
The “LymphaTech Lite” application is a digital tool designed to support the measurement and monitoring of limb volume, offering various subscription plans that include both free and paid features. The free version enables users to select upper or lower limbs for assessment and to choose between the right, left, or both limbs. Circumferential measurements are manually entered at 10 cm intervals, based on which the application calculates limb volume and the volumetric difference between limbs.
Paid plans expand the app’s functionality. The single-user plan allows for the storage of measurement data, the generation of personalized reports, and data export. The clinical practice plan, targeted at healthcare professionals, supports the management of multiple patients and includes features such as measurement tracking, adjustable perimetry point intervals (starting from 4 cm), and the creation and export of detailed reports.
According to the app store description, the application integrates with a Bluetooth-enabled perimetry device (Bluetooth Tape Measure) developed by LymphaTech. This device automatically captures limb circumference data and transmits it to the app via Bluetooth, enhancing the precision and efficiency of data collection.
The average star rating of users reported in the app stores was unavailable for the four apps, probably due to the low number of downloads. The “LymphaTech Lite” had 1+ downloads, “Lymphedema Self-Management” (림프부종 자가관리) had 5+ downloads, “LymVol” had 50+ downloads, and “Myeongsimboam—Breast Cancer Edition” (명심보암—유방암편) had 100+ downloads.
The quality of the apps assessed by uMARS indicated that the included applications performed consistently across the evaluated domains. The app scores were 4.43 for the “LymphaTech Lite”, 4.35 for the “Lymphedema Self-Management” (림프부종 자가관리), 3.87 for the LymVol, and 3.77 for the “Myeongsimboam—Breast Cancer Edition” (명심보암—유방암편). Quality analysis is presented in Table 2.

Table 2.
Quality assessment of mobile applications (apps) using the User Mobile App Rating Scale (uMARS).
4. Discussion
This study observed a limited number of available apps designed for the assessment and monitoring of patients with BCRL in online stores. Among the eligible applications, four met the inclusion criterion of calculating the volume of the affected limb to support the measurement of lymphedema. The British app LymVol was developed for healthcare professionals to monitor upper or lower limb lymphedema, although not specifically for BCRL, whereas LymphaTech allows use by professionals or patients seeking only the assessment feature. In contrast, the Korean apps were aimed at patients with BCRL and, in addition to supporting lymphedema assessment, provided educational content on breast cancer, exercise diaries, management strategies, and treatment options. Although these apps achieved relatively high scores on the uMARS assessment, no validation studies were identified during the literature search to confirm their reliability or clinical efficacy.
Conventional methods for lymphedema assessment and monitoring include water displacement, perometry, optoelectronic volumetry, lymphoscintigraphy, bioimpedance spectroscopy, and computed tomography. Although these techniques are considered reliable, they often present variability in protocols and are limited by high costs and restricted accessibility [3,5]. Mobile applications offer a more affordable and widely accessible alternative, particularly for use outside specialized centers. However, none of the apps reviewed have undergone clinical validation, limiting their current applicability in evidence-based practice.
LymVol, available on the Play Store and developed by the Lymphedema Training Academy, requires payment for download and access to its features. Similarly, while LymphaTech Lite provides some functionalities free of charge, essential resources for monitoring the progression of lymphedema are accessible only through subscription to paid plans. In this context, a potential explanation lies in economic barriers. Patients with limited financial resources may be unable to afford access to these technologies, which constitutes a significant limitation to their use and contributes to disparities in healthcare access and outcomes [28].
The Korean applications demonstrated a more informative and comprehensive approach compared to others, which were primarily limited to assessment functionalities. Despite this, indicators such as the number of downloads, user star ratings, and frequency of software updates suggest a generally low level of user engagement across all evaluated apps. Language barriers may further contribute to limited adherence, highlighting a limitation in accessibility. The Web Content Accessibility Guidelines recommend the availability of apps in multiple languages. These guidelines ensure access to the web and emphasize the need to provide text alternatives, video descriptions, and support for assistive technologies, enhancing the feasibility [29].
Widespread adoption of mHealth depends on overcoming existing barriers. Incorporating human-centered design and involving healthcare professionals increases the usability and ease of use of these tools [30]. Lowering financial barriers through strategies such as tiered pricing models or providing basic app functionalities for free or at minimal cost has been recognized as an effective approach to improving equity of access in low-resource settings [31]. Additionally, apps with multilingual or localization support better serve diverse linguistic groups, helping to bridge gaps when dominant languages would otherwise limit use [32]. These considerations align with the World Health Organization’s Global Strategy on Digital Health 2020–2025, which emphasizes the importance of digital health in achieving universal health coverage and reducing health disparities [33].
The challenge to download the “LymVol—Lymphedema Limb Volume” app could be due to the absence of software updates for the latest versions of Android. The last update was registered five years before the search of this study, still featuring version 1.0, which compromises the dissemination of the app, hindering the use of the app among professionals in clinical practice. Considering the increased evolution of mHealth apps, specific categories were delineated to mitigate the risks and foster the development of these tools. These categories include usability, guarantee, privacy, security, suitability and relevance, transparency and content, technical support, and updates [34]. Emphasizing the importance of app maintenance to ensure optimal utilization of the technology.
A lack of information regarding the sources, scientific and up-to-date support, and validation studies was observed, although the content of the apps appeared to be well-founded. Many scales were developed to assess the usability and quality of mHealth apps [35]. Nevertheless, standardization in evaluation methods is needed to guarantee consistency and reliability. The absence of regulations for health technology assessment impairs the identification of the best resources for users since it does not have the methodological rigor of traditional studies. Digital health technology has great potential, depending on the benefits established by scientific evidence [36].
Due to the low number of users and downloads of the included apps, evaluations and star ratings were not available. Despite these limitations, the apps demonstrated moderate to good quality when analyzed using uMARS. Reviews and ratings are valuable tools for assessing perceived quality, helping developers understand audience behaviour, expectations, and feedback, while also guiding necessary updates to enhance functionality and engagement [37]. It is worth noting that such evaluations serve not only to inform end-users, but also to support ongoing improvement by providing actionable insights into overall app performance and usability [38].
Despite the quality observed through the uMARS, none of the included applications have validation studies demonstrating their clinical applicability. The search conducted in the databases indicates that, although promising, these applications still face a significant limitation: the scarcity of technical and scientific evaluations, which undermines their reliability and robustness for clinical use. Clinical validation is essential to ensure that artificial intelligence algorithms are accurate, generalizable, and capable of delivering real patient benefits, and is conducted through external testing, diagnostic studies, and ideally randomized clinical trials that assess their impact on clinical outcomes [39].
According to its developers, although the uMARS is a straightforward and reliable instrument for evaluating mHealth quality, its effective application requires prior training and relevant health-related experience [24]. A letter to the editor suggested that establishing a clear conceptual definition for app quality assessment, alongside a theoretical framework to evaluate this quality, could enhance the robustness of evaluations performed using the uMARS [40]. Ko et al. (2023) [41] compared the perceived functionality reported by end-users with that assessed by healthcare professionals using the uMARS, demonstrating that discrepancies in ratings may arise depending on the evaluator. With the rapid growth of digital health tools, it is increasingly important to assess end-users’ capacity to engage effectively with these services. Such evaluation should consider user characteristics, digital accessibility, health and digital literacy, and equity in access to technology [42].
Moumane, Idri, and Abran (2016) [43] emphasize that usability assessment in mobile applications should combine objective and subjective measures, accounting for technical factors such as screen size, resolution, and connectivity, as well as contextual elements that influence user experience. The study further underscores the necessity of empirical validation guided by international standards, including ISO 9241 and ISO 25062, to ensure that applications are effective, efficient, and satisfactory. Therefore, while the uMARS provides valuable insights, it cannot replace comprehensive usability analysis and formal validation of applications, highlighting the critical implications of the present findings [43].
Different artificial intelligence models offer substantial benefits to healthcare, improving clinical decision-making and diagnostic accuracy, while also supporting advancements in health education, risk stratification, and treatment strategies [44]. Nevertheless, the authors emphasize the urgent need for validation studies to establish the true effectiveness and potential of these technologies. Akbar, Coiera, and Magrabi (2019) [45], in a scoping review spanning multiple databases, identified significant safety concerns regarding the quality of information provided by health applications. These issues primarily involved inaccuracies or incompleteness of content, as well as responses that failed to meet consumer needs. The review also highlighted a critical gap resulting from insufficient validation and limited involvement of healthcare specialists. Some of these limitations carried the potential for direct patient harm. The authors concluded that the safety of health applications constitutes a growing public health concern.
Despite the recognized need for validation, standardization in evaluation processes remains lacking. Sedhom et al. (2021) [46] assessed 22 popular oncology mHealth apps in a cohort study and found that most failed to meet high-quality standards across multiple domains and user requirements, performing poorly in both technical and clinical areas, providing limited benefit, and falling short of user expectations. In the context of mHealth expansion for skin cancer risk assessment, Sangers et al. (2022) [47] investigated the diagnostic accuracy of an app designed to detect skin lesions, revealing considerable imprecision despite its promising potential. The authors highlight the importance of studies that examine not only prospective validation but also real-world implementation among lay populations, emphasizing the necessity of rigorous technical investigations to ensure the applicability and reliability of mHealth tools. The ability of the reviewed apps to capture patient-reported outcomes (PROMs) in a standardized and validated manner remains uncertain, representing a limitation in their use for comprehensive BCRL management.
In addition to the applications analyzed in this review, the app LymphaTech deserves attention. Although developed for mobile devices, it requires the use of specific external equipment and access through a trained team or user account, which prevented full exploration of its functionalities and, consequently, its inclusion in the present analysis. Despite this limitation, the application has a validation study demonstrating high reproducibility, with intraclass correlation coefficients (ICCs) ≥ 0.99, comparable to those obtained with the perometer [48]. These findings highlight the potential of LymphaTech for facilitating the assessment of various health conditions, particularly in lymphedema monitoring, and suggest promising avenues for clinical application. Although LymphaTech has demonstrated strong validation, its reliance on external equipment and specialized accounts limits accessibility. This highlights a core challenge in evaluating mHealth tools: many effective applications are difficult to access or compare due to differences in functionalities, requirements, and user constraints. Consequently, this study focused on applications that operate independently on smartphones, allowing for standardized comparisons while acknowledging that this approach may not capture the full range of available technologies.
Restricting the inclusion to apps available in major app stores may have excluded relevant applications, limiting the generalizability of the findings. In addition, the evaluation was based on the versions of the applications available at the time of data collection, and given the frequent updates and modifications typical of mobile applications, the results may not fully reflect their current or future iterations. Although the uMARS tool provides a structured framework for assessing app quality, the outcomes may still be influenced by the evaluators’ prior experience and familiarity with health-related technologies.
Finally, although we employed a robust methodology with a rigorous process of identification and selection of applications, none of the included apps had published clinical validation studies, such as evaluations of diagnostic accuracy, reproducibility, or their impact on patient outcomes. This lack of validation constitutes a critical limitation for their integration into evidence-based practice. Moreover, it highlights a clear mismatch between the speed of app development for the market and the slower progress in conducting clinical validation studies. Future research in mobile technologies for lymphoedema assessment should therefore prioritize the incorporation of clinical validation in the development and evaluation of new applications.
5. Conclusions
Despite the large number of apps identified in online stores, only four of the 23 eligible applications met all inclusion criteria, highlighting a gap in the availability of adequate tools for the assessment and monitoring of BCRL. The included apps differed in terms of functionality and accessibility, with notable challenges, including a lack of regular software updates, financial constraints, and language limitations. Furthermore, the absence of standardized assessment protocols and limited scientific validation were consistent limitations across the apps. The quality assessment using the uMARS indicated that the included applications performed consistently across the evaluated domains. However, methodological inconsistencies and the lack of empirical validation limit conclusions regarding their broader clinical applicability.
Based on these findings, future developers should prioritize the creation of accessible, linguistically inclusive apps that function independently on smartphones, ensuring broad usability for patients and healthcare providers. Apps should integrate standardized assessment protocols and provide regular updates to maintain relevance and accuracy. Researchers are encouraged to conduct rigorous validation studies, including reproducibility testing, diagnostic accuracy, and real-world clinical outcomes, to ensure reliability and safety. Regulators and professional bodies may consider establishing guidelines and frameworks for mHealth app evaluation, focusing on usability, scientific validation, data security, and equity of access. Implementing these measures can enhance the integration of mHealth tools into clinical practice.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedinformatics5040062/s1, Reference [49] are cited in the supplementary materials. File S1: PRISMA Checklist.
Author Contributions
N.T.: Conceptualization, Data curation, Investigation, Methodology, Project administration, Validation, Visualization, Writing—original draft and final version. M.G.A.L.: Data curation, Investigation, Writing—original draft and final version. H.A.d.S.L.: Supervision, Investigation, Writing—original draft and final version. D.D.: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing—original draft and final version. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Fundação de Amparo à Pesquisa de Pernambuco (FACEPE), process no. IBPG-0497-4.08/23.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank the Federal University of Pernambuco and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for supporting their postgraduate scholarship.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| apps | mobile applications |
| BCRL | breast cancer-related lymphedema |
| uMARS | User Mobile App Rating Scale |
| mHealth | mobile health app |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ICC | intraclass correlation coefficient |
References
- Abouelazayem, M.; Elkorety, M.; Monib, S. Breast Lymphedema After Conservative Breast Surgery: An Up-to-date Systematic Review. Clin. Breast Cancer 2021, 21, 156–161. [Google Scholar] [CrossRef]
- McEvoy, M.P.; Gomberawalla, A.; Smith, M.; Boccardo, F.M.; Holmes, D.; Djohan, R.; Thiruchelvam, P.; Klimberg, S.; Dietz, J.; Feldman, S. The prevention and treatment of breast cancer-related lymphedema: A review. Front. Oncol. 2022, 12, 1062472. [Google Scholar] [CrossRef]
- Donahue, P.M.C.; MacKenzie, A.; Filipovic, A.; Koelmeyer, L. Advances in the prevention and treatment of breast cancer-related lymphedema. Breast Cancer Res. Treat. 2023, 200, 1–14. [Google Scholar] [CrossRef]
- De Vrieze, T.; Gebruers, N.; Tjalma, W.A.; Nevelsteen, I.; Thomis, S.; De Groef, A.; Dams, L.; Van der Gucht, E.; Belgrado, J.-P.; Vandermeeren, L.; et al. What is the best method to determine excessive arm volume in patients with breast cancer-related lymphoedema in clinical practice? Reliability, time efficiency and clinical feasibility of five different methods. Clin. Rehabil. 2019, 33, 1221–1232. [Google Scholar] [CrossRef]
- Levenhagen, K.; Davies, C.; Perdomo, M.; Ryans, K.; Gilchrist, L. Diagnosis of Upper Quadrant Lymphedema Secondary to Cancer: Clinical Practice Guideline from the Oncology Section of the American Physical Therapy Association. Phys. Ther. 2017, 97, 729–745. [Google Scholar] [CrossRef]
- Bhavnani, S.P.; Narula, J.; Sengupta, P.P. Mobile technology and the digitization of healthcare. Eur. Heart J. 2016, 37, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Hameeteman, M.; Verhulst, A.C.; Vreeken, R.D.; Maal, T.J.J.; Ulrich, D.J.O. 3D stereophotogrammetry in upper-extremity lymphedema: An accurate diagnostic method. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Magalhães, A.; Oliveira, H.P. A Kinect-Based System to Assess Lymphedema Impairments in Breast Cancer Patients. In Pattern Recognition and Image Analysis; Paredes, R., Cardoso, J.S., Pardo, X.M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 228–236. [Google Scholar] [CrossRef]
- Yahathugoda, C.; Weiler, M.J.; Rao, R.; De Silva, L.; Dixon, J.B.; Weerasooriya, M.V.; Weil, G.J.; Budge, P.J. Use of a Novel Portable Three-Dimensional Imaging System to Measure Limb Volume and Circumference in Patients with Filarial Lymphedema. Am. J. Trop. Med. Hyg. 2017, 97, 1836–1842. [Google Scholar] [CrossRef]
- Hou, Y.; Feng, S.; Tong, B.; Lu, S.; Jin, Y. Effect of pelvic floor muscle training using mobile health applications for stress urinary incontinence in women: A systematic review. BMC Women’s Health 2022, 22, 400. [Google Scholar] [CrossRef] [PubMed]
- Lara-Palomo, I.C.; Gil-Martínez, E.; Ramírez-García, J.D.; Capel-Alcaraz, A.M.; García-López, H.; Castro-Sánchez, A.M.; Antequera-Soler, E. Efficacy of e-Health Interventions in Patients with Chronic Low-Back Pain: A Systematic Review with Meta-Analysis. Telemed. J. E-Health Off. J. Am. Telemed. Assoc. 2022, 28, 1734–1752. [Google Scholar] [CrossRef]
- Nagel, J.; Wegener, F.; Grim, C.; Hoppe, M.W. Effects of Digital Physical Health Exercises on Musculoskeletal Diseases: Systematic Review with Best-Evidence Synthesis. JMIR MHealth UHealth 2024, 12, e50616. [Google Scholar] [CrossRef]
- Maas, L.; Freye, M.; Pan, C.C.; Dassow, H.H.; Niess, J.; Jahnel, T. The Definitions of Health Apps and Medical Apps from the Perspective of Public Health and Law: Qualitative Analysis of an Interdisciplinary Literature Overview. JMIR MHealth UHealth 2022, 10, e37980. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, X.; Zhang, X.M. Healthcare professionals’ and patients’ assessments of listed mobile health apps in China: A qualitative study. Front. Public Health 2023, 11, 1220160. [Google Scholar] [CrossRef]
- Tsirintani, M. Use of Mobile Health by Health Care Professionals in Greece: A Validation Study. Stud. Health Technol. Inform. 2025, 328, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Guardado, S.; Mylonopoulou, V.; Rivera-Romero, O.; Patt, N.; Bansi, J.; Giunti, G. An Exploratory Study on the Utility of Patient-Generated Health Data as a Tool for Health Care Professionals in Multiple Sclerosis Care. Methods Inf. Med. 2023, 62, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Grundy, Q. A Review of the Quality and Impact of Mobile Health Apps. Annu. Rev. Public Health 2022, 43, 117–134. [Google Scholar] [CrossRef]
- Altmannshofer, S.; Flaucher, M.; Beierlein, M.; Eskofier, B.M.; Beckmann, M.W.; A Fasching, P.; Huebner, H. A content-based review of mobile health applications for breast cancer prevention and education: Characteristics, quality and functionality analysis. Digit. Health 2024, 10, 20552076241234627. [Google Scholar] [CrossRef]
- Jongerius, C.; Russo, S.; Mazzocco, K.; Pravettoni, G. Research-Tested Mobile Apps for Breast Cancer Care: Systematic Review. JMIR MHealth UHealth 2019, 7, e10930. [Google Scholar] [CrossRef]
- Flaucher, M.; Zakreuskaya, A.; Nissen, M.; Mocker, A.; A Fasching, P.; Beckmann, M.W.; Eskofier, B.M.; Leutheuser, H. Evaluating the Effectiveness of Mobile Health in Breast Cancer Care: A Systematic Review. Oncologist 2023, 28, e847–e858. [Google Scholar] [CrossRef]
- Frid, S.; Amat-Fernández, C.; Fuentes-Expósito, M.Á.; Muñoz-Mateu, M.; Valachis, A.; Sisó-Almirall, A.; Grau-Corral, I. Mapping the Evidence on the Impact of mHealth Interventions on Patient-Reported Outcomes in Patients with Breast Cancer: A Systematic Review. JCO Clin. Cancer Inform. 2024, 8, e2400014. [Google Scholar] [CrossRef]
- Horn, A.; Jírů-Hillmann, S.; Widmann, J.; Montellano, F.A.; Salmen, J.; Pryss, R.; Wöckel, A.; Heuschmann, P.U. Systematic review on the effectiveness of mobile health applications on mental health of breast cancer survivors. J. Cancer Surviv. Res. Pract. 2023, 19, 1–17. [Google Scholar] [CrossRef]
- Stoyanov, S.R.; Hides, L.; Kavanagh, D.J.; Zelenko, O.; Tjondronegoro, D.; Mani, M. Mobile App Rating Scale: A New Tool for Assessing the Quality of Health Mobile Apps. JMIR MHealth UHealth 2015, 3, e3422. [Google Scholar] [CrossRef]
- Stoyanov, S.R.; Hides, L.; Kavanagh, D.J.; Wilson, H. Development and Validation of the User Version of the Mobile Application Rating Scale (uMARS). JMIR MHealth UHealth 2016, 4, e5849. [Google Scholar] [CrossRef]
- Gralha, S.R.; Bittencourt ONda, S. Portuguese Translation and validation of the user rating scale for mobile applications in the health area (uMARS). Res. Soc. Dev. 2023, 12, e8912642056. [Google Scholar] [CrossRef]
- MARS Training Video. 2016. Available online: https://www.youtube.com/watch?v=25vBwJQIOcE (accessed on 21 May 2024).
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Bertolazzi, A.; Quaglia, V.; Bongelli, R. Barriers and facilitators to health technology adoption by older adults with chronic diseases: An integrative systematic review. BMC Public Health. 2024, 24, 506. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A. Web Content Accessibility Guidelines (WCAG) 2.2. 5 October 2023. Available online: https://www.w3.org/TR/WCAG22/ (accessed on 30 July 2024).
- van Heerden, A.; Harris, D.M.; van Rooyen, H.; Barnabas, R.V.; Ramanathan, N.; Ngcobo, N.; Mpiyakhe, Z.; Comulada, W.S. Perceived mHealth barriers and benefits for home-based HIV testing and counseling and other care: Qualitative findings from health officials, community health workers, and persons living with HIV in South Africa. Soc. Sci. Med. 1982 2017, 183, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Stephan, L.S.; Almeida, E.D.; Guimaraes, R.B.; Ley, A.G.; Mathias, R.G.; Assis, M.V.; Leiria, T.L.L. Processes and Recommendations for Creating mHealth Apps for Low-Income Populations. JMIR MHealth UHealth 2017, 5, e6510. [Google Scholar] [CrossRef]
- Ashworth, H.; Ebrahim, S.; Ebrahim, H.; Bhaiwala, Z.; Chilazi, M. A Free, Open-Source, Offline Digital Health System for Refugee Care. JMIR Med. Inform. 2022, 10, e33848. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy on Digital Health 2020–2025, 1st ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Llorens-Vernet, P.; Miró, J. Standards for Mobile Health-Related Apps: Systematic Review and Development of a Guide. JMIR MHealth UHealth 2020, 8, e13057. [Google Scholar] [CrossRef]
- Azad-Khaneghah, P.; Neubauer, N.; Miguel Cruz, A.; Liu, L. Mobile health app usability and quality rating scales: A systematic review. Disabil. Rehabil. Assist. Technol. 2021, 16, 712–721. [Google Scholar] [CrossRef]
- Marengo, L.L.; Kozyreff, A.M.; Moraes Fda, S.; Maricato, L.I.G.; Barberato-Filho, S. Tecnologias móveis em saúde: Reflexões sobre desenvolvimento, aplicações, legislação e ética. Rev. Panam. Salud Pública 2023, 46, e37. [Google Scholar] [CrossRef]
- Haggag, O.; Grundy, J.; Abdelrazek, M.; Haggag, S. A large scale analysis of mHealth app user reviews. Empir. Softw. Eng. 2022, 27, 196. [Google Scholar] [CrossRef]
- Wilks, C.R.; Gurtovenko, K.; Rebmann, K.; Williamson, J.; Lovell, J.; Wasil, A.R. A systematic review of dialectical behavior therapy mobile apps for content and usability. Borderline Pers. Disord. Emot. Dysregulation 2021, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Choi, J.; Byeon, J.S. Key Principles of Clinical Validation, Device Approval, and Insurance Coverage Decisions of Artificial Intelligence. Korean J. Radiol. 2021, 22, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Baptista, S.; Oldenburg, B.; O’Neil, A. Response to “Development and Validation of the User Version of the Mobile Application Rating Scale (uMARS).”. JMIR MHealth UHealth 2017, 5, e16. [Google Scholar] [CrossRef]
- Ko, S.; Lee, J.; An, D.; Woo, H. Menstrual Tracking Mobile App Review by Consumers and Health Care Providers: Quality Evaluations Study. JMIR MHealth UHealth 2023, 11, e40921. [Google Scholar] [CrossRef]
- Kim, H.; Schnall, R.; Yoon, N.; Koh, S.J.; Lee, J.; Cheon, J.H. Development and Validation of a Mobile-Centered Digital Health Readiness Scale (mDiHERS): Health Literacy and Equity Scale. J. Med. Internet Res. 2024, 26, e58497. [Google Scholar] [CrossRef]
- Moumane, K.; Idri, A.; Abran, A. Usability evaluation of mobile applications using ISO 9241 and ISO 25062 standards. SpringerPlus 2016, 5, 548. [Google Scholar] [CrossRef] [PubMed]
- Neha, F.; Bhati, D.; Shukla, D.K. Generative AI Models (2018–2024): Advancements and Applications in Kidney Care. BioMedInformatics 2025, 5, 18. [Google Scholar] [CrossRef]
- Akbar, S.; Coiera, E.; Magrabi, F. Safety concerns with consumer-facing mobile health applications and their consequences: A scoping review. J. Am. Med. Inform. Assoc. JAMIA 2019, 27, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Sedhom, R.; McShea, M.J.; Cohen, A.B.; Webster, J.A.; Mathews, S.C. Mobile app validation: A digital health scorecard approach. NPJ Digit. Med. 2021, 4, 111. [Google Scholar] [CrossRef] [PubMed]
- Sangers, T.; Reeder, S.; van der Vet, S.; Jhingoer, S.; Mooyaart, A.; Siegel, D.M.; Nijsten, T.; Wakkee, M. Validation of a Market-Approved Artificial Intelligence Mobile Health App for Skin Cancer Screening: A Prospective Multicenter Diagnostic Accuracy Study. Dermatology 2022, 238, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Binkley, J.M.; Weiler, M.J.; Frank, N.; Bober, L.; Dixon, J.B.; Stratford, P.W. Assessing Arm Volume in People during and after Treatment for Breast Cancer: Reliability and Convergent Validity of the LymphaTech System. Phys. Ther. 2020, 100, 457–467. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).