Abstract
Background/Objectives: Dementia is a leading cause of cognitive decline, with significant challenges for early detection and timely intervention. The lack of effective, user-centred technologies further limits clinical response, particularly in underserved areas. This study aimed to develop and describe a co-design process for creating a Diagnostic and Statistical Manual of Mental Disorders (DSM-5)-compliant, AI-powered Smart Assistant (SmartApp) to monitor neurocognitive decline, while ensuring accessibility, clinical relevance, and responsible AI integration. Methods: A co-design framework was applied using a novel combination of Agile principles and the Double Diamond Model (DDM). More than twenty iterative Scrum sprints were conducted, involving key stakeholders such as clinicians (psychiatrist, psychologist, physician), designers, students, and academic researchers. Prototype testing and design workshops were organised to gather structured feedback. Feedback was systematically incorporated into subsequent iterations to refine functionality, usability, and clinical applicability. Results: The iterative process resulted in a SmartApp that integrates a DSM-5-based screening tool with 24 items across key cognitive domains. Key features include longitudinal tracking of cognitive performance, comparative visual graphs, predictive analytics using a regression-based machine learning module, and adaptive user interfaces. Workshop participants reported high satisfaction with features such as simplified navigation, notification reminders, and clinician-focused reporting modules. Conclusions: The findings suggest that combining co-design methods with Agile/DDM frameworks provides an effective pathway for developing AI-powered clinical tools as per responsible AI standards. The SmartApp offers a clinically relevant, user-friendly platform for dementia screening and monitoring, with potential to support vulnerable populations through scalable, responsible digital health solutions.
1. Introduction
The global rise in dementia cases is estimated to be 30–40% in the next decade, which is attributed to potentially modifiable risk factors such as physical inactivity, smoking and unhealthy dietary habits [1]. Targeted interventions addressing these risk factors could delay or even prevent the onset of dementia, particularly benefiting vulnerable populations who are more likely to engage in such high-risk behaviours [2]. The widespread accessibility of the internet via mobile devices holds the potential to extend preventive healthcare to those with limited access to traditional services [3]. Recent advancements in innovative technologies present automated diagnostic systems for disease management in patients with Parkinson’s disease, hepatitis, carcinoma and heart failure [4]. However, such tools are considered to be less developed in dementia diagnosis and progression monitoring [5]. One potential barrier to the application of technological solutions to support clinical methods is that it is relatively unclear whether these technologies can consistently deliver needed diagnoses in a responsible manner, albeit with improved care outcomes, which requires comprehensive clinical engagement. The co-design approach of designing technology emphasises engagement and empathy with key subject matter experts to be part of the design process [6]. The co-design process comprises effective communication and collaboration to provide consistent feedback and suggestions on rapid prototyping cycles. This process incorporates well with the Agile framework [7], where technology designers/developers work in interdisciplinary teams and prioritise regular development of functional software.
Recent research suggests a trend towards implementing digital technology to improve home-based care, specifically post-COVID-19 pandemic, where the co-design approach is considered to be most effective [8]. However, despite the potential of assistive technology, several applications are not designed with the dementia population in mind, leading to usability barriers. Nevertheless, the co-design process established that people with dementia are capable of using touchscreen technology, and their participation in the design process ensures a more intuitive and user-friendly app.
Responsible AI practices—emphasising transparency, explainability and ethical safeguards—are essential when applying AI to cognitively vulnerable populations such as people with dementia, where AI tools can accurately diagnose various forms of dementia, including vascular dementia, Lewy body dementia and frontotemporal dementia. However, none of the recent research provides predictability of the increasing frequency of noticeable symptoms. The latest literature suggests that mobile computational technologies in health (mHealth) have recently gained popularity [9], which offer the promise of personalised care and remote health messaging and services at low cost on a global scale [10]. As a result, numerous healthcare applications have surged, reaching over 90,000 [11]. However, few of these applications have been developed based on solid evidence or evaluated for effectiveness through randomised controlled trials and co-design. While the conceptual design and architecture of mHealth interventions are critical to their usability and uptake, guidelines for designing these tools for vulnerable populations remain scarce [12]. In order to bridge this gap, this study presents a comprehensive co-design development study for a novel mobile phone application (named SmartApp) by applying an iterative development approach.
In this research paper, we illustrate how we implemented the co-design methods to involve dementia care providers/clinicians, designers and academicians. The cohesive approach utilised in this study also incorporates the Double Diamond Model (DDM) [13], which is highly recommended for healthcare applications such as dementia identification and fits very well with the operational execution of co-design mechanisms and Agile principles. This article is divided into five key sections, providing a theoretical and methodological approach in Section 2, the details of project execution in Section 3 and discussions with implications in Section 4. Section 5 provides a conclusion of this study, emphasising the contribution and providing future possibilities of extension and applicability of this approach.
2. Methods/Theoretical Framework
A collaborative approach is essential in the development of technology for medical therapies and patient care solutions, ensuring that user expectations, needs, and preferences are met [14]. This helps to improve protocol adherence, deliver meaningful outcomes, and reduce healthcare costs. Without proper collaborative efforts, research programs focusing on healthcare technology are more likely to fail to provide meaningful patient impact and value [15]. For a successful implementation of technology development, it is crucial that the researchers in health informatics have access to healthcare service providers or patient engagement departments as partners [16]. Several different methods and frameworks exist for the effective development of healthcare technology; however, for aging design issues, the DDM is considered highly suitable [13]. DDM divides the design process into four phases, Discover, Define, Develop and Deliver (as depicted in Figure 1), which helps improve the effective design process management and planning. DDM has been embraced in medical and clinical research to ensure that research reflects patient priorities and meaningful outcomes [14]. By integrating DDM into the research design, the team involved in this study was able to identify issues that may otherwise have gone unnoticed. DDM ensures patient perspectives are incorporated, which leads to more meaningful and impactful research outcomes [17]. This study included four different categories of members, as highlighted in Figure 1 and detailed in Table 1, including clinicians (a clinical psychologist, a principal psychiatrist and a general physician), designers (a software developer, a user interface designer and a mobile app designer), postgraduate students and an academic researcher specialising in Artificial Intelligence (AI). By fostering sustained DDM mechanisms, the research team was able to enhance competence, build capacity, and ensure the final research product is useful and applicable to the patient population. Integrating Responsible AI principles in the development process, such as with DDM and a co-design mechanism, particularly through user involvement and iterative refinement, ensures alignment with human values and facilitates trust calibration [18,19].
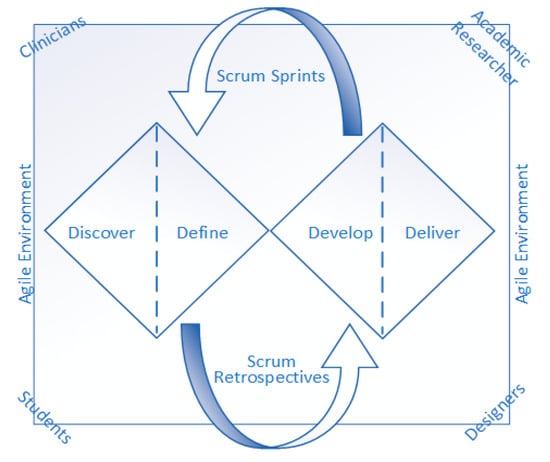
Figure 1.
Scrum-DDM co-design environment for SmartApp development.

Table 1.
Workshop participants’ details.
Developing the solution, particularly for individuals dealing with cognitively impaired patients, required a tailored method of involvement and co-design [20]. Hence, it is necessary to balance facilitating meaningful involvement alongside being mindful of individuals’ capabilities, capacities, preferences and availability. In this study, the research and development team utilised the DDM strategy along with the Agile principles supported by the Scrum framework [21] to ensure an effective co-design environment. Incorporating an effective co-design strategy early in the research process broadens the range of ideas and saves resources by aligning research with patient priorities from the outset [22]. Furthermore, including clinicians in data-intensive projects helps navigate ethical challenges and promotes better data governance [8,23]. This study employs a novel combination of Agile principles by incorporating the Scrum methodology and DDM methodology, as depicted in Figure 1. The four distinct themes of the DDM model, Discover, Define, Develop and Deliver, helped to explore user needs and experiences through interviews; visualise the patient’s experience with a user journey map; extract design themes; and design prototypes and improve the deliverable end product with the help of Scrum Sprints and Retrospective. Operationally integrated DDM phases aligned with Scrum Sprints helped strengthen the co-design approach. The team conducted short-span iterative Scrum Sprints (i.e., loops) to cater to the needs and translate them within the progress of the development using Scrum Retrospectives (i.e., change requests/feedback).
In addition to the Agile framework principles from the Scrum methodology, along with the DDM framework, the team ensured three vital aspects to maintain rigour and ensure usability: (1) Continuous collaboration with clinicians, (2) Freedom/Flexibility to Changes in each Sprint, and (3) frequent improvements.
Continuous Collaboration with Clinicians: While developing healthcare solutions, ensuring that patient expectations, needs and preferences becomes highly important [15]. Hence, in the development phase of the solution, the research team involved end-user clinicians as advisors/mentors to be the key source of primary information to enhance accuracy and reliability. For the frequent involvement of clinicians with the research and development team, multiple meetings were organised, and the feedback loop was maintained through emails and other means of communication. Our interface design incorporates elements of transparency and real-time user feedback, allowing clinicians to track patients’ cognitive changes. This mirrors the trust-load modelling approach recommended by Wang et al. [24], which underscores the importance of probabilistic transparency in Responsible AI for effective human–AI collaboration. The combination of the Scrum–DDM co-design approach assisted the team in ensuring the requirements were met efficiently and effectively by following the Agile Manifesto’s ‘Individuals and interactions, working software and Customer collaboration’ [25].
Complete Freedom/Flexibility to Changes in each Scrum Sprint: As this study utilised the Scrum methodology, freedom/flexibility to change is essential as a key manifesto of Agile principles. Agile change management mechanisms allow the change to be implemented with flexibility, prioritising the feedback of the people involved [26]. As a result, the changes incorporated during each sprint help to develop the functionality that is more likely to be acceptable to the users [25]. The key extracts of feedback, suggested changes and action items that were incorporated as part of this study are highlighted in Table 2 in Section 3.

Table 2.
Feedback and resulting software modifications from workshops 1 to 3.
Continuous Improvement: Responding to change is one of the key manifestoes [25]; therefore, whatever possible changes were collected from continued communication and collaboration with clinicians, the design team incorporated them as several major and minor amendments (detailed in Table 2). Continuous improvements have been made to the design and the flow of the SmartApp system, as depicted in Figure 1, Figure 2 and Figure 3. The extent of the end-user modifications adopted in the development process can be observed by comparing Figure 1 (the research/design team’s prototype) with Figure 2 (where the modifications have been made following the first co-design workshop) and Figure 3 (where the final design is updated based on feedback from the co-design workshops).
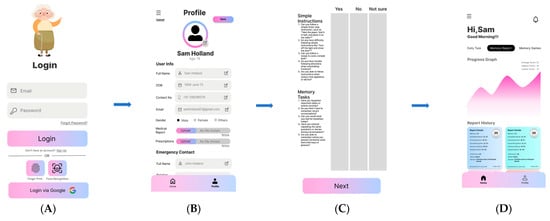
Figure 2.
Initial conceptual design. (A) login interface; (B) profile interface; (C) questions interface; (D) results interface.
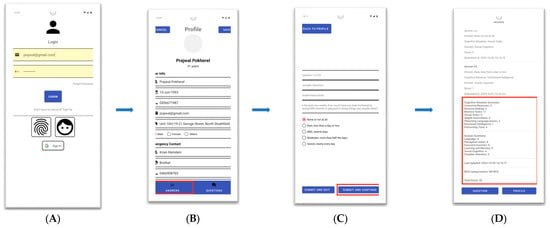
Figure 3.
Iteration 2 altered design. (A) login interface; (B) profile interface; (C) questions interface; (D) results interface.
The latest research literature highlights that recent software applications have not yet achieved an acceptable level of user requirements in clinical domains [27] and lack congruency with major psychiatric classification systems such as the Diagnostic and Statistical Manual of Mental Disorders [28]. The systematic design and development process incorporating creative and participative principles and tools as part of this study suggests that new healthcare technology solutions should incorporate such standards [29]; hence, this research is based on DSM-5 criteria that include multiple screening criteria from 13 different cognitive domains [28]. The participants in the co-design workshops were selected through purposive sampling based on their professional expertise and availability. This approach ensured that only highly experienced individuals shaped the initial prototype before exposing it to patient risk. The team included clinicians (a psychiatrist, psychologist and general physician), academic researchers, designers/developers and postgraduate students, as detailed in Table 1. Inclusion criteria included at least 5 years of experience in clinical or software design roles. While this phase excluded direct involvement of patients and caregivers due to ethical and safety considerations, future iterations will incorporate end-user testing involving individuals living with dementia and their carers. This will provide a more generalisable assessment of the SmartApp’s usability in real-world contexts. The baseline core work was carried out by the student team and supervised by the design team. The clinicians were consulted thoroughly as part of the process. The activities were primarily led by an academic researcher/AI. Ethical approval for this project was granted by the University of New England, Armidale, NSW, Australia (UNE HRE number HE24-143).
The SmartApp screening component was built upon the DSM-5 TR framework and incorporates 24 core items derived from the DSM-defined cognitive and behavioural domains, such as memory, executive function, attention, language and visuospatial abilities. Each item was formulated into a user-friendly multiple-choice question, where responses were scored on a five-point Likert scale ranging from ‘Not at all’ to ‘Severely.’ The AI engine, embedded in SmartApp, processed these scores in real time to generate a visual profile of neurocognitive performance and flag potential decline. The system also utilised time-series analysis to monitor changes across repeated attempts, thereby supporting clinicians in understanding longitudinal symptom progression.
It is important to note that the SmartApp is not a digital replication or the replacement of the Mini-Mental State Examination (MMSE). While both the MMSE and the SmartApp partially share the overarching goal of screening for cognitive decline, their design foundations differ substantially. The MMSE is a fixed-format tool assessing a limited set of cognitive functions such as orientation, memory and language. In contrast, the SmartApp is grounded in the DSM-5 TR framework for neurocognitive disorders, encompassing 24 items across DSM-5-defined cognitive and behavioural domains. The application also integrates AI-driven analytics, longitudinal tracking of performance over repeated assessments, and clinician-focused comparative visualisations. These features extend the scope of cognitive screening beyond the MMSE’s structure, enabling broader assessment and predictive modelling in a mobile, user-centred format, which provide flexibility to monitor longitudinal data.
3. Results of the Co-Design Process
Following the Agile principle, under the Scrum methodology, the design and development process was broken down into three main iterations and multiple Sprints to provide an initial conceptual design, improve the design and update the final design of the interfaces that the general public could use for improved usability.
Iteration 1: Initial conceptual design: The design team, including clinicians, designers, students and an AI academic researcher (as detailed in Table 1), worked on the initial conceptual design of SmartApp, as presented in Figure 2, including the user login interface in Figure 2A, user profile interface in Figure 2B, screening questions for neurocognitive decline (NCD) categorisations interface in Figure 2C and results of NCD categorisation in Figure 2D. The feedback from Workshop 1 is presented in Table 2, which shows the response and instructions received from the clinical team, comprising a psychiatrist, a psychologist and a general physician.
Iteration 2: Improvement on initial design: Following the feedback and instructions of the clinical team, the design team worked on improving the initial conceptual design and updated the interfaces, as presented in Figure 3, including user login, profile, questions and results interfaces. The feedback from Workshop 1 is presented in Table 2, which shows the response and instructions received from the clinical team comprising a psychiatrist, a psychologist and a general physician.
Iteration 3: Final Update of Design: After the feedback from Workshop 2, the design team updated the layout and the flow of the SmartApp’s execution, which resulted in the final update of the interface design. Figure 4 presents layouts approved by the clinicians for the Beta version of the SmartApp. Figure 4A highlights the red box on the login button to reflect the update in the backend process, as recommended by the academic researcher, to incorporate the information security parameters. Figure 4B presents more features added to the side menu in the user profile section, including the log-out option highlighted in the red box. Figure 4C shows the question-by-question responses of the user as recommended by the clinicians. Similarly, on the suggestion of the clinicians, Figure 4D presents the comparative graph layout of two different users on the clinician module.
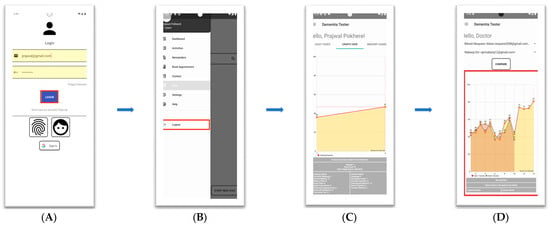
Figure 4.
Iteration 3 updated design. (A) Login interface; (B) profile interface; (C) questions interface; (D) results interface.
As highlighted in recent ethical AI literature, embedding provable ethical frameworks in AI systems is critical, especially in medical contexts. The SmartApp’s clinician feedback loop and DSM-5 alignment reflect a step toward architecturally enforced ethics, in line with the Ethical Firewall approach proposed by Thurzo, A. [30]. The feedback collected from the academic, design, health sciences and student teams thoroughly helped to carry out the co-design process. A ‘moderate match’ of expectations of the health sciences team to the ‘highly appreciated’ outcome of the prototype was achieved through continuous involvement of the team, and frequent updates resulted in a usable end product. Some of the feedback collected from workshops 1–3 and actions taken to address it are presented in Table 2, respectively.
The last and fourth workshop was primarily based on demonstrating the final prototype design and its execution, which was the reflection of all the feedback provided on the custom design and usability of the SmartApp. All four teams were fully satisfied with the current version of the SmartApp and discussed the imminent delivery to complete the project. As detailed in Table 2, the feedback was entirely positive, and all the participating members agreed that all the issues raised and recommendations provided on the usability of SmartApp were fully incorporated.
4. Discussion and Implications
The combination of Agile principles with Scrum and DDM followed an iterative co-design mechanism for the development of the SmartApp to provide predictive analysis on the neurocognitive decline of elderly people, especially those who are at risk of dementia. This approach involved key stakeholders, including clinicians, designers, an academic researcher and students, in the design and development phases. The co-design process emphasised user involvement in defining application requirements and testing the identified features, ensuring effective usability. By following the DDM approach and applying Agile principles, the research team was able to continuously improve the app by collecting and integrating feedback at every stage, making real-time adjustments during each development Scrum Sprint. While the iterative process effectively engaged clinicians, designers and researchers, this discussion extends beyond the immediate outcomes of the SmartApp to explore broader implications for healthcare technology development. This innovative process provides several key insights to be discussed that present valuable contributions to scale such an approach to other domains as well.
- Key Takeaways from the Co-Design Process:
One of the key takeaways from the co-design process was the innovative combination of DDM and Agile that provided an iterative co-design approach to ensure continuous feedback and integration of end-user requirements. This methodology proved especially beneficial in designing for a cognitively impaired user base, emphasising simplicity, adaptability, and inclusivity. The involvement of clinicians not only enhanced the clinical relevance of the app but also mitigated potential ethical and usability challenges. This iterative feedback loop mechanism can serve as a model for future healthcare projects, particularly those addressing vulnerable populations.
Similarly, another takeaway from the adopted co-design process was the critical role of sustained collaboration among multidisciplinary stakeholders. Engaging clinicians as advisors not only ensured the app’s clinical rigour but also facilitated ethical considerations and practical applicability. Additionally, involving practising clinicians during iterative prototyping helped to address potential usability barriers early in the design process, fostering a sense of ownership and trust. This experience underscores the importance of harnessing an environment where feedback is actively sought and integrated, enabling the development of technology that aligns closely with user needs and expectations.
The feedback from co-design workshops emphasised the importance of intuitive interfaces and personalised features, such as notification-based task reminders and comparative visual graphs (as discussed in Section 3). These enhancements not only improved user satisfaction but also highlighted the necessity of addressing diverse user needs through flexible design. Lessons learned here can inform broader guidelines for designing technology for populations with varying levels of digital literacy.
- Ethical Considerations and Responsible AI:
The co-design process adopted in this study aligns with Responsible AI frameworks by ensuring ethical safeguards are embedded at every stage. Principles such as transparency, explainability, and trust-building were prioritised during interface design and clinician engagement. These values echo the foundational elements of ethical AI systems proposed in recent frameworks such as the Ethical Firewall architecture [30] and probabilistic transparency models for human–AI collaboration [24]. Our study also aligns with emerging research emphasising responsible digital health innovations, as also advocated by Gangemi et al. [31] in their evaluation of AI-based dementia tools.
The SmartApp ensures data privacy through multiple mechanisms, including encrypted data transmission (TLS 1.3), role-based access control (RBAC) for clinician and user modules, and authentication via secure token-based login. Audit logs are maintained for all clinician data access events to ensure traceability and compliance. Sensitive data is stored on Google Firebase with Firestore database-level encryption and daily backups. Only authorised clinicians can access comparative patient data, and all logs are auditable under institutional data governance protocols.
- Usability and Accessibility:
While this study focused on dementia care, the Agile–DDM approach is adaptable to other healthcare domains. For example, similar principles could be applied to develop digital solutions for chronic disease management, rehabilitation, or mental health monitoring. Future implementations should explore these domains to validate the generalisability of this approach. Furthermore, the integration of predictive analytics and personalised interventions underscores the potential for such methodologies to improve user-centred care.
The scalability of this methodology also lies in its ability to accommodate varying levels of complexity within healthcare settings. By leveraging iterative sprints and stakeholder feedback, the Agile–DDM approach can be tailored to address specific challenges across diverse medical conditions. Moreover, its flexibility makes it particularly suitable for resource-constrained environments, where maximising impact with minimal resources is crucial. Expanding its application to underserved populations could significantly enhance equitable access to healthcare innovations, paving the way for broader systemic improvements.
The iterative development process allowed the SmartApp’s functionality to evolve through numerous changes based on the feedback received from workshops and regular user testing sessions. This method aligns with the core agile principles and the DDM approach, which prioritises adaptability and collaboration [25]. By continuously involving participants in the development process, the research team was able to address usability challenges specific to individuals with cognitive impairments, such as simplifying navigation, optimising visual elements and ensuring flexibility in task completion. The feedback collected from co-design workshops led to the addition of several key features, including Google sign-up options, total score calculations for neurocognitive decline (NCD) screening and comparison graphs for multiple users. The implementation of a FireBase database and ML-based prediction models further enhanced the technical capabilities, allowing for more robust data management and personalised predictions for NCD progression.
Another critical insight was the role of adaptive design in ensuring accessibility for a wide range of users, including those with cognitive impairments. The inclusion of features like simplified navigation, customizable settings and support for multiple interaction modes proved essential in overcoming barriers to usability. By focusing on these aspects, future healthcare applications can be designed to accommodate the unique needs of vulnerable populations, thereby promoting inclusivity and fostering greater adoption of digital health tools.
- Limitations and Opportunities:
Despite its strengths, this study has limitations. The SmartApp’s current evaluation is limited to feedback from a small group of stakeholders and simulated usability tests. Future research for this development is planned to involve larger-scale usability testing, including patients and caregivers, to assess the SmartApp’s effectiveness in real-world scenarios. While the usability evaluation in this phase was primarily qualitative and workshop-based, we tracked basic metrics during testing sessions, including average task completion time (approx. 3–5 min for initial screening) and error reporting (none reported in the final iteration). Although the evaluation was not formally controlled, structured observations were used to record timing, interface errors and feedback. However, we acknowledge the need to use validated usability instruments such as the System Usability Scale (SUS), which is already underway and will be available in future publications. Additionally, while the technical component of the SmartApp incorporates ML models for predictive analysis, the scope of these models remains limited. Expanding the ML capabilities to include more robust algorithms and real-world datasets could significantly enhance the utility. The current predictive analytics module of the SmartApp uses a simple linear regression model trained and tested on synthetic data. While the model demonstrated over 90% accuracy, these results must be interpreted cautiously, as synthetic data may not reflect the complexity of real-world dementia progression. We recognise this as a key limitation and plan to retrain and validate the ML module using real patient datasets upon ethical approval. Moreover, future versions will explore more advanced models such as XGBoost and LSTM to capture non-linear patterns in neurocognitive decline.
These limitations also highlight several opportunities for future exploration. For instance, conducting longitudinal studies to evaluate the long-term impact of such systems on patient outcomes could provide valuable insights into their clinical efficacy. Furthermore, exploring partnerships with healthcare organisations and policymakers could facilitate broader implementation and adoption. The involved research team is confident that subsequent iterations of the SmartApp can achieve greater impact and utility within the healthcare ecosystem.
- Theoretical and Practical Contributions:
From a theoretical perspective, this study bridges the gap between user-centred design principles and agile development methodologies, demonstrating their combined effectiveness in healthcare innovation. Practically, it provides a roadmap for integrating diverse stakeholder input into the design of clinical tools, ensuring relevance, usability, and scalability. The unique combination of DDM and Agile principles can serve as a benchmark for other healthcare technology initiatives.
This development study of the SmartApp, utilising the co-design approach with the involvement of four different teams, clearly highlights the importance of iterative collaborative design principles. The execution of this project via the Scrum–DDM approach helped to enhance teamwork by improving communication and cooperation among team members, which ultimately led to increased productivity over time. This methodology promotes short-term problem-solving, reduces project risks, and facilitates greater customer involvement, enabling the delivery of more functional products or services on a frequent basis. The participating team agreed that the Scrum–DDM approach is particularly useful for complex projects where predicting every outcome is challenging [32]. It provides a framework of practices that promote visibility, allowing practitioners to monitor project stages, make corrections and implement improvements to keep the project aligned with its goals [33]. Key advantages of using the Scrum–DDM approach include adaptability, transparency, continuous feedback, ongoing improvement and motivation.
This study implemented weekly sprints, which provided tighter control in addressing changing requirements, delivering meaningful functionality and guaranteeing progress in each iteration. Sprint planning sessions were convened by the academic researcher and were attended by members of the design team and the student team on the Scrum and DDM principles. The academic researcher facilitated all the Scum meetings and workshops and served as a customer proxy during the planning sessions, fulfilling the role of the Scrum product owner. In Scrum, the product owner is tasked with defining and prioritising product requirements, and in this case, the academic researcher served this role due to their close work with clinicians in capturing the SmartApp’s requirements.
More than 20 Scrum Sprints, on top of four co-design workshops, were held with clinicians, designers, students and an academic researcher, tailored to the improved usability of the SmartApp. Each co-design cycle consisted of weekly Scrum Sprints lasting approximately 5–7 days. The team used collaborative digital platforms such as Trello for Sprint planning, Google Meet and Zoom for real-time feedback sessions, and shared documents via Google Drive to maintain logs of design iterations and feedback. Feedback was collected using a combination of observation notes, structured surveys with Likert-based ratings, and semi-structured interviews conducted during workshops. Each item of feedback was assigned to a Sprint backlog and tracked using Trello cards until implemented and approved in retrospectives. Clinicians were involved in feedback loops at every second or third Sprint to ensure critical input on usability and clinical relevance. For example, specific changes recommended during the co-design workshops (e.g., visual graphs and notification reminders) were partially endorsed by 2/3 of the clinical team, but eventually, after the design team incorporated them with flexible/user-friendly design, 100% consensus was achieved, which is evidence of user satisfaction and clinical relevance. Each of these meetings lasted approximately one hour and included discussion among the team, feedback collection, and information exchange on the prototype testing. In order to carry out the ‘Discover & Define’ stage of the DDM framework, Scrum meetings 1–3 featured discussions on potential project scope and features, leading to the creation of the baseline for SmartApp conceptual architecture. During these initial meetings, visual working prototypes were utilised to demonstrate interactive screens that displayed key design elements. The initial series of meetings was conducted after the kick-off meeting of the project. In meetings 4–7, the team spent time discussing finalising the design of the user interface, complying with the requirements of the user module and the clinician module. Likewise, in meetings 8–11, the team finalised the dementia/NCD screening questions on DSM-5 framework compliance. As part of the discussion, other protocols were also discussed, e.g., the Mini-Mental State Examination (MMSE) [34] and the six-item cognitive impairment test (6-CIT) [35]. In order to carry out the ‘Develop’ stage of the DDM framework, the team utilised the Scrum Sprints more thoroughly as compared to the overall Scrum strategy for the overall breakdown of the project phases. For meetings 12–14, the time was mainly utilised for the outcomes of the dementia screen application and its visual presentation on SmartApp interfaces. Meetings 15–17, primarily focused on finalising the machine learning (ML) module and its integration in the SmartApp. Finally, in order to carry out the ‘Deliver’ stage of the DDM framework, meetings 18–20 were utilised for the testing and the go-live state for further testing. All the participant teams observed the final screening testing as performed by the SmartApp. All participants, who were involved in the whole process, followed the consent provided and ethical approval granted by the Ethics Committee from the University of New England, NSW, Australia (UNE HRE number HE24-143).
One of the key findings from the co-design workshops was that end users preferred a flexible interface and a gradual approach to answering the 24 dementia screening questions, leading to the implementation of a notification-based system for reattempting the remaining questions. Moreover, the addition of features such as visual graphs for tracking cognitive performance and ML models for predictive analytics significantly improved functionality and usability. The synthetic data utilised for the testing and validation of the ML model was split into 80% for training and 20% for testing, which yielded above 90% accuracy. The current implementation of ML models is based on a simple regression algorithm [36], with a plan to incorporate more recent ML models in the future updates of SmartApp.
The practical implications of this work extend beyond the immediate development of the SmartApp. By showcasing a structured yet flexible approach to co-design, this study offers actionable insights for practitioners seeking to develop innovative healthcare technologies. The emphasis on iterative feedback and cross-disciplinary collaboration ensures that the resulting tools are not only clinically sound but also aligned with user expectations. This dual focus on theoretical rigour and practical application positions the Agile–DDM methodology as a valuable framework for advancing digital health solutions.
Given the long-term aim of scaling SmartApp for clinical use, we are also designing training modules tailored for users with low digital literacy, including video tutorials and simplified onboarding steps. For example, an illustrated step-by-step guide in the local language is currently under development for regional deployments. For clinical deployment, we plan to run comparative usability and impact studies to assess clinical outcomes, adoption rates and satisfaction scores. These assessments will draw on protocols similar to those discussed by [27], with a focus on quantifiable improvements in early dementia identification.
- Future Research Directions:
By extending the discussion to address these broader implications and lessons learned, this study contributes to the growing body of literature on healthcare technology design, providing valuable insights for both researchers and practitioners. Building on this work, there are several future research directions, e.g., piloting the SmartApp with a larger cohort of dementia patients and caregivers to validate its usability and clinical effectiveness; exploring additional functionalities, such as IoT integration for real-time monitoring and expanded ML-based predictive capabilities; and investigating the applicability of this co-design methodology to other healthcare contexts, particularly for underserved or marginalised populations, etc. Similar to this project, other app development projects can follow the same trajectory to develop digital solutions for chronic disease management, rehabilitation or mental health monitoring.
Future research should also explore the ethical considerations associated with AI-driven healthcare tools, particularly concerning data privacy and algorithmic transparency. By addressing these issues, subsequent developments can ensure greater acceptance and trust among users. Additionally, integrating emerging technologies, such as wearables and sensor networks, could further enhance the functionality and impact of healthcare applications developed through this co-design framework.
5. Conclusions
The co-design process, based on a combination of Scrum methodology in compliance with Agile principles and the Double Diamond Model (DDM) framework, was pivotal in the effective development of the SmartApp to monitor neurocognitive decline, especially for people who are at risk of dementia. This approach ensured that the end users’ needs and preferences were central to the design and functionality of the application. By actively involving the key stakeholders, including a team of clinicians (including a psychologist, a psychiatrist and a general physician), a team of software designers, a team of postgraduate students and an academic researcher, the team was able to produce a user-friendly and clinically relevant tool that addresses the specific cognitive challenges of the target population. The iterative feedback loops provided valuable insights that were promptly incorporated into the functionality, improving both usability and clinical accuracy. By extending the discussion to address these broader implications and lessons learned, this study contributes to the growing body of literature on healthcare technology design, providing valuable insights for both researchers and practitioners. The findings of this development study can be generalised to build other disease management technologies. Future directions for the SmartApp include pilot testing with a larger population to validate its effectiveness and the integration of additional technologies, such as IoT sensors, to expand its functionality. The next phase of validation includes formal usability testing with quantitative metrics and user satisfaction scores.
This project serves as a model for developing healthcare applications using Scrum-DDM-based co-design methods, particularly for vulnerable populations, and highlights the importance of involving end-users in all stages of the development process. Beyond its immediate outcomes, this study demonstrates the broader applicability of the Agile–DDM approach to healthcare technology development. The lessons learned underscore the importance of flexibility, collaboration and iterative refinement in addressing the challenges inherent in designing for complex medical contexts. By fostering inclusivity and leveraging innovative methodologies, this research contributes to the growing body of knowledge on co-design practices and their potential to transform patient-centred care. Future iterations of the SmartApp will build on this foundation, incorporating additional features, expanding clinical testing and exploring applications in other healthcare domains. The insights gained from this work provide a robust framework for advancing the design and implementation of digital health tools, ultimately improving patient outcomes and fostering innovation in healthcare technology.
Author Contributions
Conceptualization, F.U.D.; methodology, F.U.D., N.G., N.S., T.H., P.J.T. and N.S.; software, N.G.; validation, F.U.D., N.S. (Namrata Shetty), T.H., P.J.T. and N.S. (Niusha Shafiabady); formal analysis, N.S. (Namrata Shetty), T.H. and P.J.T.; investigation, F.U.D., N.S. (Namrata Shetty), T.H., P.J.T. and N.S. (Niusha Shafiabady); resources, F.U.D. and N.S. (Namrata Shetty); data curation, F.U.D., N.G. and N.S. (Niusha Shafiabady); writing—original draft preparation, F.U.D., N.G.; writing—review and editing, N.S. (Namrata Shetty), T.H., P.J.T. and N.S. (Niusha Shafiabady); visualisation, F.U.D., N.G.; supervision, N.S. (Namrata Shetty), T.H., P.J.T. and N.S. (Niusha Shafiabady); project administration, F.U.D. and N.S. (Niusha Shafiabady); funding acquisition, F.U.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of New England, Armidale, NSW, Australia, grant number: A23/3782-8582119.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of New England, NSW, Australia (HRE approval #: HE24-143, November 2024). All procedures were performed in compliance with UNE’s institutional guidelines and as per the approval that has been granted by the Ethics Committee. Informed consent was obtained, and the privacy has been thoroughly maintained.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author as per the institutional guidelines and as per the approval that has been granted by the Ethics Committee.
Acknowledgments
The authors acknowledge the resources provided by the University of New England, Armidale, NSW, Australia, to complete this research work and associated publication.
Conflicts of Interest
Fareed Ud Din is employed at School of Science and Technology, Univesty of New England, Armidla, 2350, NSW, Australia. Nabaraj Giri is employed at School of Science and Technology, University of New England. Namrata Shetty is employed at Omega HealthCare, Fairfield, NSW, Australia. Tom Hilton is employed at School of Science and Technology, University of New England, Armidale, NSW, Australia. Niusha Shafiabady is employed at Discipline of IT, Australian Catholic University, North Sydney NSW, Australia. Phillip J Tully is employed at School Psychology, Deakin University, Melbourne, VIC, Australia.
References
- Montero-Odasso, M.; Ismail, Z.; Livingston, G. One third of dementia cases can be prevented within the next 25 years by tackling risk factors. The case “for” and “against”. Alzheimer’s Res. Res. Res. Res. Therapy 2020, 12, 81. [Google Scholar] [CrossRef]
- Stringhini, S.; Sabia, S.; Shipley, M.; Brunner, E.; Nabi, H.; Kivimaki, M.; Singh-Manoux, A. Association of socioeconomic position with health behaviors and mortality. JAMA 2010, 303, 1159–1166. [Google Scholar] [CrossRef]
- Meier, C.A.; Fitzgerald, M.C.; Smith, J.M. eHealth: Extending, enhancing, and evolving healthcare. Annu. Rev. Biomed. Eng. 2013, 15, 359–382. [Google Scholar] [CrossRef]
- Asakawa, T.; Sugiyama, K.; Nozaki, T.; Sameshima, T.; Kobayashi, S.; Wang, L.; Hong, Z.; Chen, S.; Li, C.; Namba, H. Can the latest computerized technologies revolutionize conventional assessment tools and therapies for a neurological disease? The example of Parkinson’s disease. Neurol. Med.-Chir. 2019, 59, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Paraskevaidi, M.; Martin-Hirsch, P.L.; Martin, F.L. Progress and challenges in the diagnosis of dementia: A critical review. ACS Chem. Neurosci. 2018, 9, 446–461. [Google Scholar] [CrossRef] [PubMed]
- Liedtka, J. Learning to use design thinking tools for successful innovation. Strategy Leadersh. 2011, 39, 13–19. [Google Scholar] [CrossRef]
- Agile Manifesto. The 12 Principles Behind the Agile Manifesto. 2001. Available online: https://www.agilealliance.org/agile101/12-principles-behind-the-agile-manifesto (accessed on 13 September 2024).
- Fox, S.; Brown, L.J.E.; Antrobus, S.; Brough, D.; Drake, R.J.; Jury, F.; Leroi, I.; Parry-Jones, A.R.; Machin, M. Co-design of a smartphone app for people living with dementia by applying agile, iterative co-design principles: Development and usability study. JMIR Publ. 2022, 10, e24483. [Google Scholar] [CrossRef]
- Giri, A.; Ud Din, F. Role of data as an interface between primary, secondary and tertiary care: Evidence from literature. Inform. Health 2025, 2, 63–72. [Google Scholar] [CrossRef]
- Nilsen, W.; Kumar, S.; Shar, A.; Varoquiers, C.; Wiley, T.; Riley, W.T.; Pavel, M.; Atienza, A.A. Advancing the science of mHealth. J. Health Commun. 2012, 17 (Suppl. S1), 5–10. [Google Scholar] [CrossRef]
- Statista. Number of MHealth Apps Available in the Apple App Store from 1st Quarter 2015 to 1st Quarter 2021. 2021. Available online: https://www.statista.com/statistics/779910/health-apps-available-ios-worldwide/ (accessed on 2 April 2025).
- Stowell, E.; Lyson, M.C.; Saksono, H.; Wurth, R.C.; Jimison, H.; Pavel, M.; Parker, A.G. Designing and evaluating mHealth interventions for vulnerable populations: A systematic review. In Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems, Montreal, QC, Canada, 21–26 April 2018. [Google Scholar] [CrossRef]
- Ford, K.L.; West, A.B.; Bucher, A.; Osborn, C.Y. Personalized digital health communications to Increase COVID-19 vaccination in underserved populations: A double diamond approach to behavioral design. Front. Digit. Health 2022, 4, 831093. [Google Scholar] [CrossRef]
- Jackson, T.; Pinnock, H.; Liew, S.M.; Horne, E.; Ehrlich, E.; Fulton, O.; Worth, A.; Sheikh, A.; De Simoni, A. Patient and public involvement in research: From tokenistic box-ticking to valued team members. BMC Med. 2020, 18, 79. [Google Scholar] [CrossRef]
- Smits, D.-W.; Van Meeteren, K.; Klem, M.; Alsem, M.; Ketelaar, M. Designing a tool to support patient and public involvement in research projects: The involvement matrix. Res. Involv. Engagem. 2020, 6, 30. [Google Scholar] [CrossRef]
- Maccarthy, J.; Guerin, S.; Wilson, A.G.; Dorris, E.R. Facilitating public and patient involvement in basic and preclinical health research. PLoS ONE 2019, 14, e0216600. [Google Scholar] [CrossRef]
- Hoddinott, P.; Pollock, A.; O’CAthain, A.; Boyer, I.; Taylor, J.; MacDonald, C.; Oliver, S.; Donovan, J.L. How to incorporate patient and public perspectives into the design and conduct of research. F1000Research 2018, 7, 752. [Google Scholar] [CrossRef] [PubMed]
- Pagel, C.; Jesper, E.; Thomas, J.; Blackshaw, E.; Rakow, T.; Pearson, M.; Spiegelhalter, D. Understanding children’s heart surgery data: A cross-disciplinary approach to co-develop a website. Ann. Thorac. Surg. 2017, 104, 342–352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sobczak, K. The Power of Science Communication. 2018. Available online: https://imnis.org.au/2018/05/the-power-of-science-communication/ (accessed on 3 May 2025).
- Shen, S.; Doyle-Thomas, K.A.R.; Beesley, L.; Karmali, A.; Williams, L.; Tanel, N.; McPherson, A.C. How and why should we engage parents as co-researchers in health research? A scoping review of current practices. Health Expect. 2017, 20, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Torrente, G.; de Souza, T.Q.; Tonaki, L.; Cardoso, A.P.; Manickchand Junior, L.; da Silva, G.O. Scrum framework and health solutions: Management and results. In Nurses and Midwives in the Digital Age; IOS Press: Amsterdam, The Netherlands, 2021; pp. 290–294. [Google Scholar]
- Brett, J.O.; Staniszewska, S.; Mockford, C.; Herron-Marx, S.; Hughes, J.; Tysall, C.; Suleman, R. A systematic review of the impact of patient and public involvement on service users, researchers, and communities. Patient-Patient-Centered Outcomes Res. 2014, 7, 387–395. [Google Scholar] [CrossRef]
- Beier, K.; Schweda, M.; Schicktanz, S. Taking patient involvement seriously: A critical ethical analysis of participatory approaches in data-intensive medical research. BMC Med. Inform. Decis. Mak. 2019, 19, 90. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Xue, C. Collaborative Decision Making with Responsible AI: Establishing Trust and Load Models for Probabilistic Transparency. Electronics 2024, 13, 3004. [Google Scholar] [CrossRef]
- Beck, K.; Beedle, M.; van Bennekum, A.; Cockburn, A.; Cunningham, W.; Fowler, M.; Thomas, D. Manifesto for Agile Software Development. Agile Alliance. 2001. Available online: https://agilemanifesto.org/ (accessed on 23 April 2025).
- Kranzen, M. Agile Change Management: Valuable Insights for Project Adaptability. Prosci. 2024. Available online: https://www.prosci.com/blog/agile-change-management (accessed on 23 April 2025).
- Fabio, R.A.; Plebe, A.; Ascone, C.; Suriano, R. Psychometric properties and validation of the critical reasoning assessment. Personal. Individ. Differ. 2025, 246, 113344. [Google Scholar] [CrossRef]
- DSM-5. American Psychiatric Association. Organization of DSM-5. Available online: https://www.psychiatry.org/File%20Library/Psychiatrists/Practice/DSM/APA_DSM_Organization-of-DSM-5.pdf (accessed on 2 April 2025).
- Khadgi, M.; Ud Din, F. Towards AI/ML-powered Hybrid Project Management Strategy for the Healthcare Sector. In Proceedings of the 9th International Conference on Machine Learning and Soft Computing, Tokyo, Japan, 24–26 January 2025; Volume 1, pp. 1–6. [Google Scholar]
- Thurzo, A. Provable AI Ethics and Explainability in Medical and Educational AI Agents: Trustworthy Ethical Firewall. Electronics 2025, 14, 1294. [Google Scholar] [CrossRef]
- Gangemi, A.; Fabio, R.A.; Suriano, R.; De Luca, R.; Marra, A.; Tomo, M.; Quartarone, A.; Calabrò, R.S. Does Transcranial Direct Current Stimulation Affect Potential P300-Related Events in Vascular Dementia? Considerations from a Pilot Study. Biomedicines 2024, 12, 1290. [Google Scholar] [CrossRef] [PubMed]
- Stopa, G.R.; Rachid, C.L. Scrum: Metodologia ágil como ferramenta de gerenciamento de projetos. CES Rev. 2019, 33, 302–323. [Google Scholar]
- Schwaber, K. Agile Project Management with Scrum; Microsoft Press: Redmond, WA, USA, 2004. [Google Scholar]
- Arevalo-Rodriguez, I.; Smailagic, N.; Roqué-Figuls, M.; Ciapponi, A.; Sanchez-Perez, E.; Giannakou, A.; Pedraza, O.L.; Cosp, X.B.; Cullum, S. Mini-Mental State Examination (MMSE) for the early detection of dementia in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. 2021, 7, CD010783. [Google Scholar] [CrossRef] [PubMed]
- Salis, F.; Pili, D.; Collu, M.; Serchisu, L.; Laconi, R.; Mandas, A. Six-item cognitive impairment test (6-CIT)’s accuracy as a cognitive screening tool: Best cut-off levels in emergency department setting. Front. Med. 2023, 10, 1186502. [Google Scholar] [CrossRef]
- Su, X.; Yan, X.; Tsai, C.L. Linear regression. Wiley Interdiscip. Rev. Comput. Stat. 2012, 4, 275–294. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).