Efficient Photocathode of an Ultrathin Organic p-n Bilayer Comprising p-Type Zinc Phthalocyanine and n-Type Fullerene for Hydrogen Peroxide Production
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qi, Y.; Zhang, B.; Zhang, G.; Zheng, Z.; Xie, T.; Chen, S.; Ma, G.; Li, C.; Domen, K.; Zhang, F. Efficient overall water splitting of a suspended photocatalyst boosted by metal-support interaction. Joule 2024, 8, 193–203. [Google Scholar] [CrossRef]
- Lin, L.; Ma, Y.; Vequizo, J.J.M.; Nakabayashi, M.; Gu, C.; Tao, X.; Yoshida, H.; Pihosh, Y.; Nishina, Y.; Yamakata, A.; et al. Efficient and stable visible-light-driven Z-scheme overall water splitting using an oxysulfide H2 evolution photocatalyst. Nat. Commun. 2024, 15, 397. [Google Scholar] [CrossRef]
- Xin, X.; Li, Y.; Zhang, Y.; Wang, Y.; Chi, X.; Wei, Y.; Diao, C.; Su, J.; Wang, R.; Guo, P.; et al. Large electronegativity differences between adjacent atomic sites activate and stabilize ZnIn2S4 for efficient photocatalytic overall water splitting. Nat. Commun. 2024, 15, 337. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, Q.; Liu, Y.; Zheng, Z.; Cheng, H.; Huang, B.; Wang, P. Photocatalytic Overall Water Splitting with a Solar-to-Hydrogen Conversion Efficiency Exceeding 2% through Halide Perovskite. Angew. Chem. Int. Ed. 2024, 63, e202411016. [Google Scholar] [CrossRef]
- Takata, T.; Liu, L.; Hisatomi, T.; Domen, K. Best Practices for Assessing Performance of Photocatalytic Water Splitting Systems. Adv. Mater. 2024, 36, 2406848. [Google Scholar] [CrossRef]
- Kondo, Y.; Mizutani, S.; Kuwahara, Y.; Mori, K.; Sekino, T.; Yamashita, H. Perfluoroalkyl-functionalization of zirconium-based metal–organic framework nanosheets for photosynthesis of hydrogen peroxide from dioxygen and water. J. Mater. Chem. A 2025, 13, 3701–3710. [Google Scholar] [CrossRef]
- Xu, X.; Sui, Y.; Chen, W.; Huang, W.; Li, X.; Li, Y.; Liu, D.; Gao, S.; Wu, W.; Pan, C.; et al. The photocatalytic H2O2 production by metal-free photocatalysts under visible-light irradiation. Appl. Catal. B 2024, 341, 123271. [Google Scholar] [CrossRef]
- Liu, T.; Pan, Z.; Vequizo, J.J.M.; Kato, K.; Wu, B.; Yamakata, A.; Katayama, K.; Chen, B.; Chu, C.; Domen, K. Overall photosynthesis of H2O2 by an inorganic semiconductor. Nat. Commun. 2022, 13, 1034. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, Y.; Isobe, Y.; Shiraishi, Y.; Sakamoto, H.; Tanaka, S.; Ichikawa, S.; Hirai, T. Carbon Nitride–Aromatic Diimide–Graphene Nanohybrids: Metal-Free Photocatalysts for Solar-to-Hydrogen Peroxide Energy Conversion with 0.2% Efficiency. J. Am. Chem. Soc. 2016, 138, 10019–10025. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, W.; Deng, C.; Sheng, H.; Zhao, J. Accelerated Photocatalytic Carbon Dioxide Reduction and Water Oxidation under Spatial Synergy. Angew. Chem. Int. Ed. 2024, 63, e202317969. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, C.; Chen, R.; Chen, X.; Ding, J.; Zhang, J.; Wan, H.; Guan, G. Constructing the facet junction on solid solution BiOBr1-xClx for efficient photocatalytic CO2 reduction with H2O. J. Alloys Compd. 2024, 985, 174022. [Google Scholar] [CrossRef]
- Nakamoto, T.; Iguchi, S.; Naniwa, S.; Tanaka, T.; Teramura, K. Surface Modifications of Heterogeneous Photocatalysts for Photocatalytic Conversion of CO2 by H2O as the Electron Donor. ChemCatChem 2024, 16, e202400594. [Google Scholar] [CrossRef]
- Cao, F.; Zhang, X.; Niu, X.; Lin, X.; Wu, T.; Zhong, S.; Lin, H.; Zhao, L.; Bai, S. Upgrading Single S-Scheme Heterojunction to Multi-S-Scheme Ones for Better Synergy of Photocatalytic CO2 Reduction and H2O Oxidation: The Third Component Location Matters. ACS Catal. 2024, 14, 12529–12540. [Google Scholar] [CrossRef]
- Riedl, H.J.; Pfleiderer, G. Preparation from Organic Compounds by the Alkyl-Anthraquinone Process. U.S. Patent Application No. C01B15/023, US2158525A, 16 May 1939. [Google Scholar]
- Ciriminna, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Hydrogen Peroxide: A Key Chemical for Today’s Sustainable Development. ChemSusChem 2016, 9, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Huang, J.-R.; Liu, J.-C.; Huang, N.-Y.; Chen, X.-M.; Liao, P.-Q. Integration of Plasmonic Ag(I) Clusters and Fe(II) Porphyrinates into Metal–Organic Frameworks for Efficient Photocatalytic CO2 Reduction Coupling with Photosynthesis of Pure H2O2. Angew. Chem. Int. Ed. 2024, 63, e202412553. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Fukui, K.; Kawai, Y.; Nagai, K.; Kato, H. A water splitting system using organo-photocathode and titanium dioxide photoanode capable of bias-free H2 and O2 evolution. Chem. Commun. 2016, 52, 7735–7737. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Ikezoi, K.; Chisaka, M.; Abe, T. Photocatalytic and photoelectrochemical production of hydrogen peroxide under acidic conditions in organic p-n bilayer/bismuth vanadate system. Int. J. Electrochem. Sci. 2022, 17, 221143. [Google Scholar] [CrossRef]
- Abe, T.; Chiba, J.; Ishidoya, M.; Nagai, K. Organophotocatalysis System of p/n Bilayers for Wide Visible Light-Induced Molecular Hydrogen Evolution. RSC Adv. 2012, 2, 7992–7996. [Google Scholar] [CrossRef]
- Abe, T.; Hiyama, Y.; Fukui, K.; Sahashi, K.; Nagai, K. Efficient p-zinc phthalocyanine/n-fullerene organic bilayer electrode for molecular hydrogen evolution induced by the full visible-light energy. Int. J. Hydrogen Energy 2015, 40, 9165–9170. [Google Scholar] [CrossRef]
- Abe, T.; Miyakushi, S.; Nagai, K.; Norimatsu, T. Study of the factors affecting the photoelectrode characteristics of a perylene/phthalocyanine bilayer working in the water phase. Phys. Chem. Chem. Phys. 2008, 10, 1562–1568. [Google Scholar] [CrossRef]
- Morikawa, T.; Adachi, C.; Tsutsui, T.; Saito, S. Multilayer-Type Organic Solar Cells Using Phthalocyanines and Perylene Derivatives. Nippon Kagaku Kaishi 1990, 1990, 962–967. [Google Scholar] [CrossRef]
- Capobianchi, A.; Tucci, M. Ruthenium phthalocyanine thin films for photovoltaic applications. Thin Solid Films 2004, 451, 33–36. [Google Scholar] [CrossRef]
- Abe, T.; Nakamura, K.; Ichinohe, H.; Nagai, K. Evaluation of photoanodic output on carbon cluster/phthalocyanine films with respect to the types of n-type conductors employed. J. Mater. Sci. 2012, 47, 1071–1076. [Google Scholar] [CrossRef]
- Eisenberg, G.M. Colorimetric Determination of Hydrogen Peroxide. Ind. Eng. Chem. Anal. Ed. 1943, 15, 327–328. [Google Scholar] [CrossRef]
- Swason, H.E.; Tatge, E. Standard X-ray diffraction powder patterns. Natl. Bur. Stand. Circ. 1953, 539, 33. [Google Scholar]
- Tanaka, S.; Hanada, T.; Ono, K.; Watanabe, K.; Yoshino, K.; Hiromitsu, I. Improvement of power conversion efficiency of phthalocyanine/C60 heterojunction solar cells by inserting a lithium phthalocyanine layer at the indium-tin oxide/phthalocyanine interface. Appl. Phys. Lett. 2010, 97, 253306. [Google Scholar] [CrossRef]
- Abe, T.; Tobinai, S.; Taira, N.; Chiba, J.; Itoh, T.; Nagai, K. Molecular Hydrogen Evolution by Organic p/n Bilayer Film of Phthalocyanine/Fullerene in the Entire Visible-Light Energy Region. J. Phys. Chem. C 2011, 115, 7701–7705. [Google Scholar] [CrossRef]
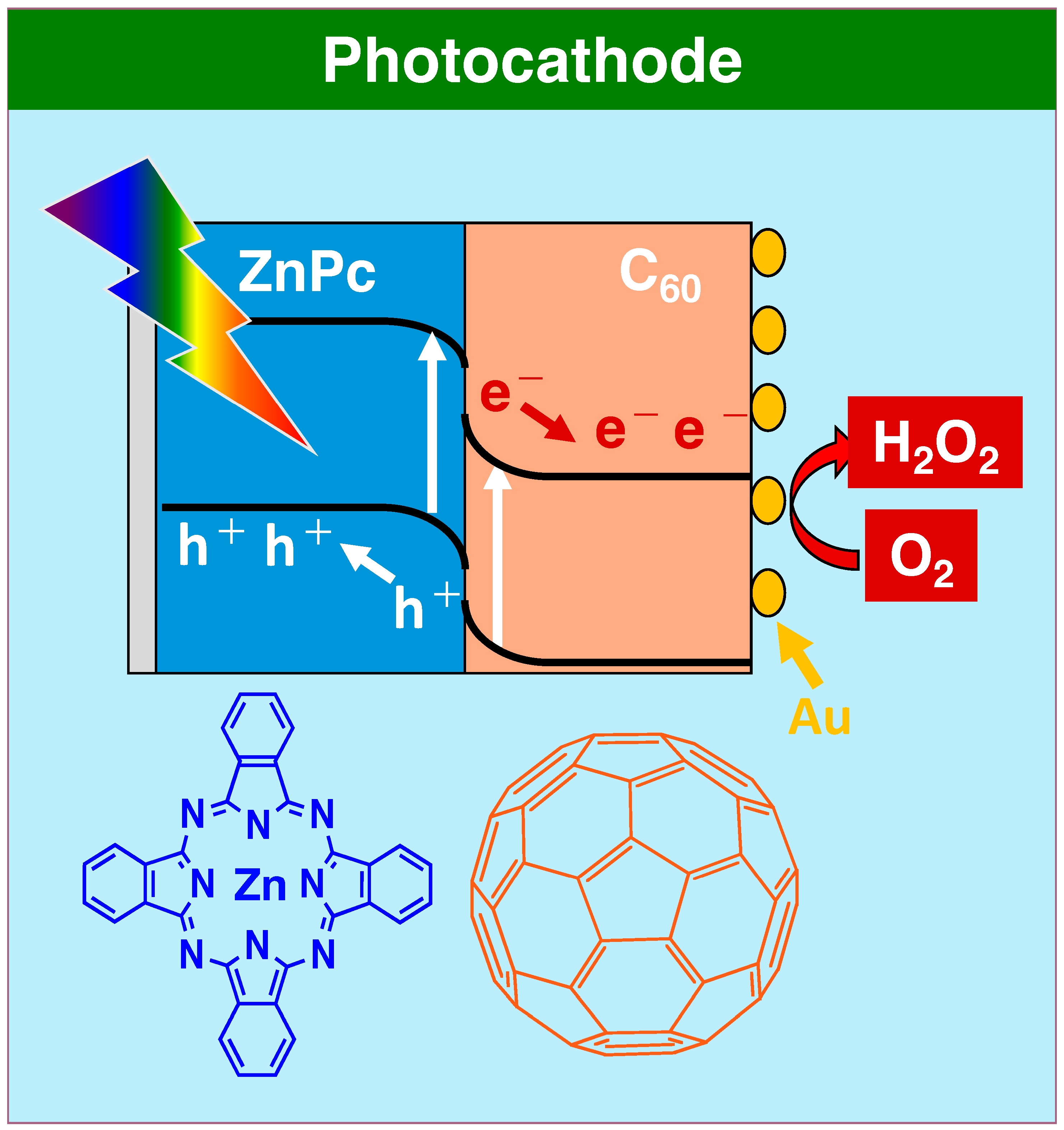

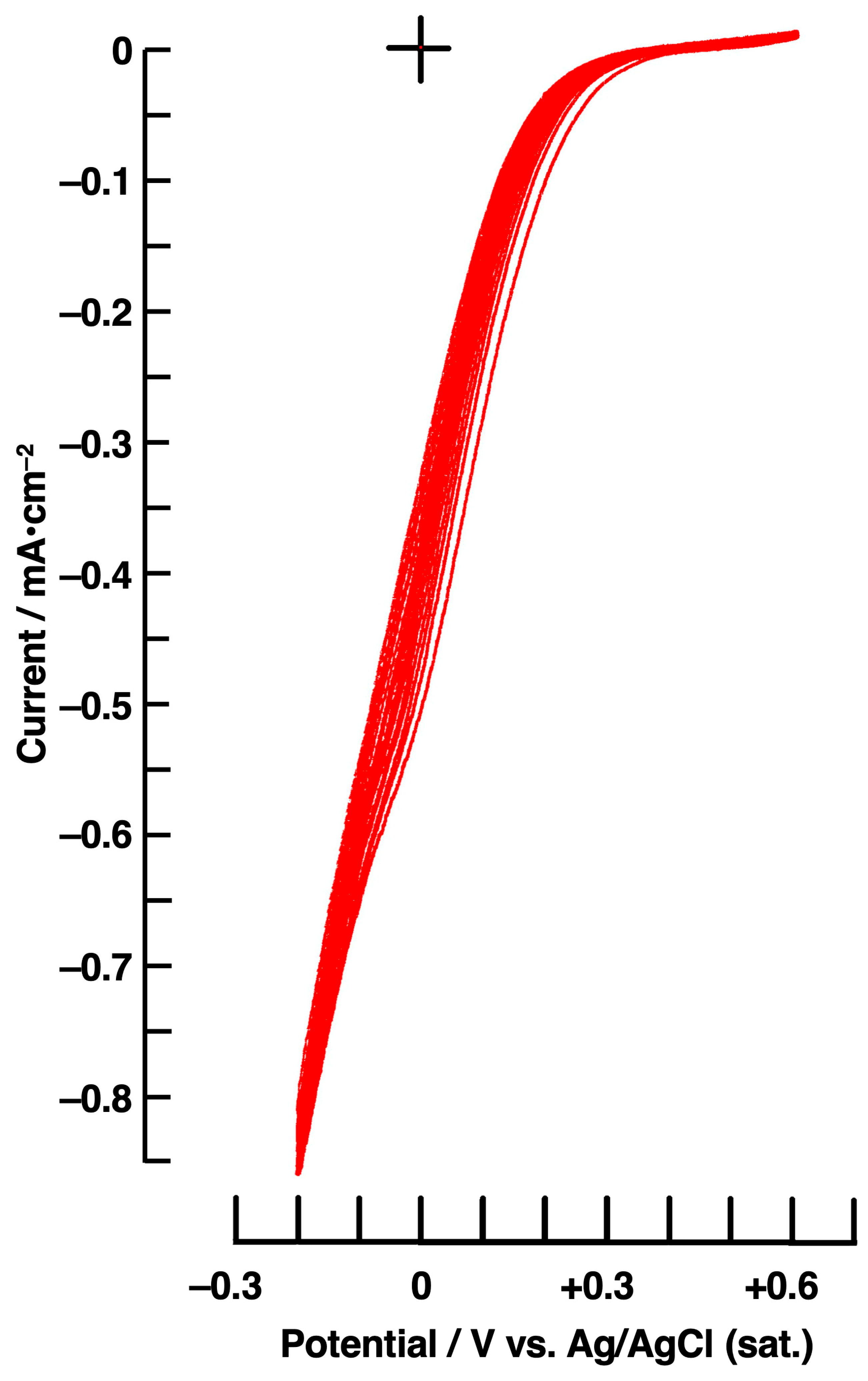
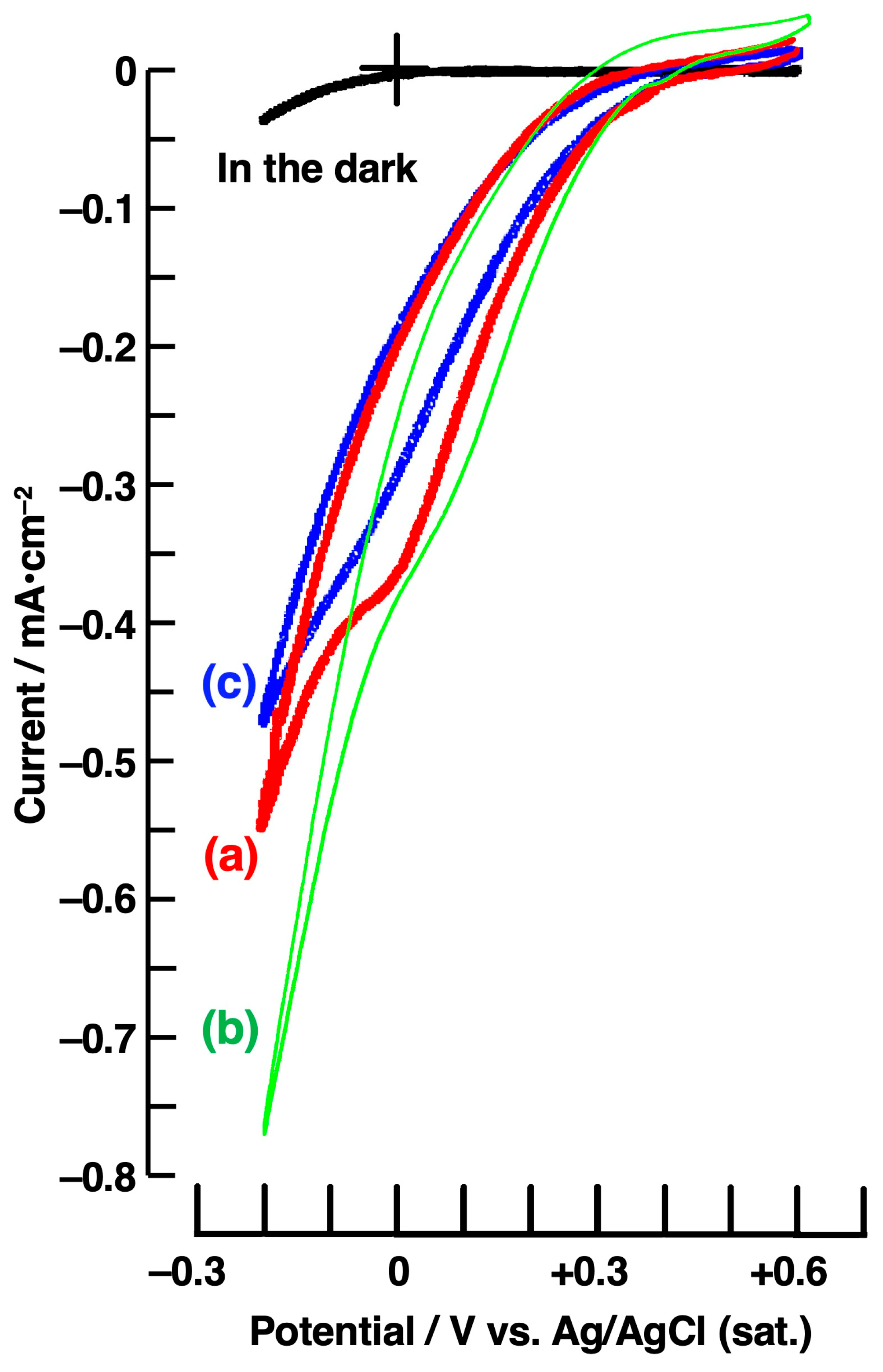
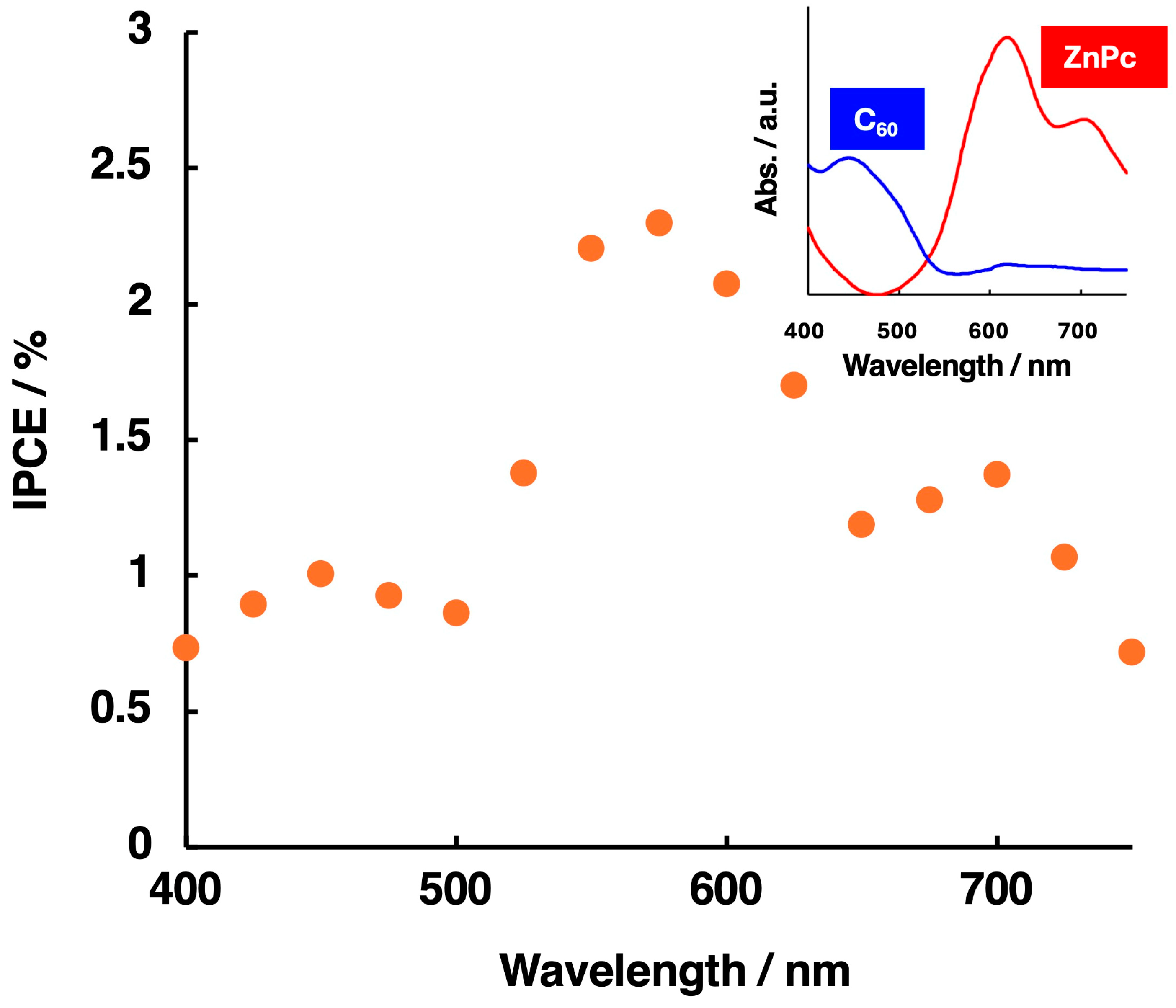
| ZnPc/nm | C60/nm | Amount of H2O2/µmol | |
|---|---|---|---|
| Entry 1 | 75 | 116 | 0.96 |
| Entry 2 | 84 | 2.8 | 2.12 |
| Entry 3 | 11 | 3.5 | 3.22 |
| ZnPc/nm | C60/nm | Rest Potential/V vs. Ag/AgCl (sat.) | |
|---|---|---|---|
| Entry 1 | - | 142 | –0.01 |
| Entry 2 | - | 9.6 | –0.06 |
| Entry 3 | 86 | - | 0.44 |
| Entry 4 | 9.6 | - | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakaguchi, Y.; Ikezoi, K.; Abe, T. Efficient Photocathode of an Ultrathin Organic p-n Bilayer Comprising p-Type Zinc Phthalocyanine and n-Type Fullerene for Hydrogen Peroxide Production. Physchem 2025, 5, 49. https://doi.org/10.3390/physchem5040049
Sakaguchi Y, Ikezoi K, Abe T. Efficient Photocathode of an Ultrathin Organic p-n Bilayer Comprising p-Type Zinc Phthalocyanine and n-Type Fullerene for Hydrogen Peroxide Production. Physchem. 2025; 5(4):49. https://doi.org/10.3390/physchem5040049
Chicago/Turabian StyleSakaguchi, Yuika, Kosuke Ikezoi, and Toshiyuki Abe. 2025. "Efficient Photocathode of an Ultrathin Organic p-n Bilayer Comprising p-Type Zinc Phthalocyanine and n-Type Fullerene for Hydrogen Peroxide Production" Physchem 5, no. 4: 49. https://doi.org/10.3390/physchem5040049
APA StyleSakaguchi, Y., Ikezoi, K., & Abe, T. (2025). Efficient Photocathode of an Ultrathin Organic p-n Bilayer Comprising p-Type Zinc Phthalocyanine and n-Type Fullerene for Hydrogen Peroxide Production. Physchem, 5(4), 49. https://doi.org/10.3390/physchem5040049







