Abstract
Bio-inspired superhydrophobic coatings have garnered significant attention in recent years due to their potential in creating self-cleaning and anti-icing surfaces. Drawing inspiration from natural systems such as lotus leaves and insect wings, these coatings leverage hierarchical nanostructures to achieve extreme water repellency and low surface adhesion. This review explores recent advances in the design, fabrication, and functional performance of bio-inspired superhydrophobic materials, with a focus on hierarchical micro/nanostructured surfaces. We discuss the underlying mechanisms of wettability, the role of surface chemistry, and the integration of durable nanostructures for enhanced durability. Additionally, the paper discusses the latest progress in scalable manufacturing techniques, environmental adaptability, and multifunctional performance, particularly in self-cleaning and anti-icing applications. Emerging trends, such as stimuli-responsive surfaces and smart coatings, are also examined to provide a comprehensive overview of the field. This review discusses the challenges and future directions for translating laboratory-scale innovations into real-world applications, particularly in aerospace, automotive, energy, and infrastructure sectors.
1. Introduction
1.1. Background and Motivation
Bio-inspired superhydrophobic coatings represent a significant advancement in materials science, inspired by natural surfaces such as lotus leaves that exhibit extreme water repellency due to hierarchical micro- and nanostructures coupled with low surface energy materials [1]. These natural phenomena have catalyzed the development of synthetic surfaces capable of self-cleaning, ice resistance, and contaminant rejection, with broad implications for industrial, environmental, and aerospace applications. Advances in nanofabrication and surface functionalization now enable scalable production of coatings tailored for enhanced durability, energy efficiency, and minimal chemical use [2], aligning with sustainability goals in modern engineering. Emerging multifunctional systems also integrate responsive behaviors, allowing coatings to adapt to environmental stimuli paving the way for use in smart buildings, renewable energy systems, and safety-critical aerospace structures
1.2. Significance of Superhydrophobicity in Nature
Nature offers diverse examples of superhydrophobic adaptations, such as the lotus leaf (Nelumbo nucifera), whose dual-scale surface topography enables self-cleaning by minimizing contact area between water droplets and the surface [3]. Similarly, the peristome of Nepenthes pitcher plants uses a wettable anisotropic surface to ensnare insects by leveraging water-triggered slipperiness, combining microstructural ridges and directional wettability to enhance prey capture [4]. These biological systems illustrate how evolutionary pressures have fine-tuned surface wettability for functional gains [5]. Studying these mechanisms provides crucial insights into designing synthetic coatings that replicate or even surpass nature’s efficiency in water repellency, contaminant resistance, and structural adaptability [6,7].
1.3. Objectives and Scope of the Review
This review aims to explore recent advancements in bio-inspired superhydrophobic coatings that employ hierarchical nanostructures, emphasizing their roles in self-cleaning, anti-icing, and multifunctional applications. Drawing from the principles of natural wettability, the paper outlines how biological templates have informed the design of synthetic materials with enhanced hydrophobic performance. The scope includes an in-depth analysis of fabrication techniques, surface chemistry, and functional performance across sectors such as biomedical engineering, energy systems, and corrosion-resistant infrastructure.
Recent developments in the field have yielded materials with exceptional non-adhesive and drag-reducing properties, mimicking natural surfaces to resist biofouling and extend surface longevity. Central to these breakthroughs is the strategic integration of micro- and nanoscale roughness, enabling persistent superhydrophobic behavior under diverse environmental conditions. This review synthesizes key methodologies, evaluates real-world deployment outcomes, and highlights current limitations, such as mechanical fragility and material cost barriers. By consolidating these findings, the paper provides a forward-looking roadmap for advancing next-generation surface coatings inspired by nature’s own engineering [8].
2. Fundamental Concepts and Mechanisms
2.1. Definition and Characteristics of Superhydrophobic Surfaces
Superhydrophobic surfaces are defined by their exceptional ability to repel water, characterized by static water contact angles exceeding 150° and contact angle hysteresis less than 10° using the Drop Shape Analyzer (DSA100-KRUSS GmbH, Hamburg, Germany) [9]. This pronounced water repellency results in water droplets forming nearly spherical shapes upon contact, minimizing adhesion and promoting easy removal from the surface [10] as represented in Figure 1. The remarkable properties of superhydrophobic surfaces stem from a combination of low surface energy materials and hierarchical surface roughness [11]. This dual-scale structure, often inspired by natural examples like lotus leaves, creates a composite interface where air pockets become trapped beneath the liquid, reducing the solid–liquid contact area and facilitating the rolling off water droplets. This mechanism not only imparts water repellency but also endows the surface with self-cleaning capabilities, as dirt particles are picked up and carried away by the moving droplets [12]. Understanding these characteristics is important for the development of advanced materials aimed at applications such as anti-icing, corrosion resistance, and drag reduction. By mimicking the structural and chemical attributes found in nature, researchers can engineer surfaces that exhibit superhydrophobic behavior, leading to innovative solutions across various technological domains.
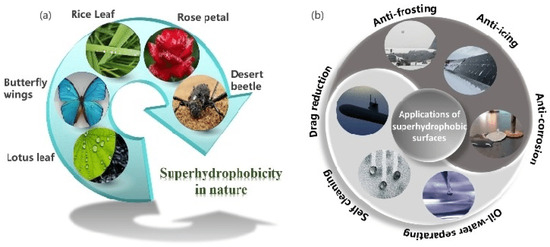
Figure 1.
(a) Natural inspirations of superhydrophobicity. (b) Engineering applications of superhydrophobic surfaces. Reproduced with permission from [13].
Figure 1 illustrates a dual-panel representation supporting Section 2.1 by connecting naturally occurring wetting strategies with their engineered counterparts. Figure 1a displays biological examples such as lotus [14], rice leaves [15], butterfly wings [16], rose petals [17], and desert beetles [18], which are known for their ability to repel water through structural adaptations. These surfaces utilize hierarchical micro- and nanoscale architectures that limit fluid-solid contact, promoting rapid droplet roll-off and self-cleaning functionality [19]. In contrast, Figure 1b highlights the translation of these principles into industrial settings, including aerospace, energy, marine, and construction applications [11]. These engineered surfaces are designed to mitigate issues like icing, corrosion, and fouling while enabling separation processes and reducing drag [11,20]. Collectively, the figure highlights how the defining characteristics of superhydrophobicity are harnessed to enhance performance and durability in modern technological systems [8].
2.2. Wetting Theories: Wenzel and Cassie–Baxter Models
The wettability of rough surfaces is predominantly described by two foundational models: the Wenzel model and the Cassie–Baxter model. These models describe the relationship between surface roughness, texture, and the resulting contact angle of a liquid droplet, which in turn determines whether the surface exhibits hydrophobic or hydrophilic behavior as presented in Table 1 [21]. The Wenzel model posits that when a liquid droplet completely conforms to the micro- and nanoscale asperities of a rough surface, the actual contact area increases compared to a flat surface [22]. This increased contact area amplifies the inherent wettability of the material; hydrophilic surfaces become more hydrophilic, and hydrophobic surfaces become more hydrophobic. Mathematically, the Wenzel equation is expressed as:
where θ* is the apparent contact angle on the rough surface, θ is the contact angle on a smooth surface, and r is the roughness ratio, defined as the ratio of the actual surface area to the projected area. A roughness ratio greater than one indicates an increased surface area due to roughness [23].
cos θ* = r cos θ
In contrast, the Cassie–Baxter model describes a scenario where the liquid droplet rests atop the asperities of a rough surface, trapping air pockets beneath and forming a composite interface of solid and air. This state reduces the contact area between the liquid and the solid surface, leading to higher contact angles and promoting superhydrophobic behavior. The Cassie–Baxter equation is given by:
where φs represents the fraction of the solid surface in contact with the liquid. A lower φs indicates a greater proportion of air pockets, contributing to enhanced hydrophobi-city [24].
cos θ* = φs (cos θ + 1) − 1
Understanding these models is important for the design and optimization of superhydrophobic surfaces, as they elucidate the interplay between surface texture and wettability, guiding the engineering of materials with desired wetting properties. Scheff et al. further demonstrated that the Cassie–Baxter model accurately predicts receding angles, although advancing angles often deviate due to complex contact line dynamic [25].

Table 1.
Summary of wetting theories—Wenzel and Cassie–Baxter models.
Table 1.
Summary of wetting theories—Wenzel and Cassie–Baxter models.
| Model | Key Assumption | Equation | Implication on Surface Wettability |
|---|---|---|---|
| Wenzel Model | Liquid completely penetrates surface roughness | cos θ* = r cos θ | Enhances inherent surface wettability: hydrophilic surfaces become more hydrophilic, and vice versa [23]. |
| Cassie–Baxter Model | Liquid sits atop surface asperities with trapped air underneath | cos θ* = φs (cos θ + 1) − 1 | Produces high contact angles and low adhesion, enabling superhydrophobicity and self-cleaning behavior [26]. |
2.3. Hierarchical Micro/Nanostructures and Surface Chemistry
The exceptional water-repellent properties of superhydrophobic surfaces are primarily attributed to the synergistic interplay between hierarchical micro/nanostructures and tailored surface chemistry [11]. In nature, this phenomenon is exemplified by the lotus leaf, which exhibits a dual-scale architecture comprising microscale papillae adorned with nanoscale epicuticular waxes. This hierarchical structuring minimizes the contact area between water droplets and the leaf surface, enhancing the rolling off of droplets and the removal of contaminants, which is a self-cleaning effect commonly referred to as the “lotus effect” [14]. Mimicking these natural designs, artificial superhydrophobic surfaces are engineered by creating multi-scale roughness through various fabrication techniques, such as lithography [11], etching, and templating [27]. These methods produce surfaces with microstructures (e.g., pillars or grooves) superimposed with nanostructures (e.g., nanoparticles or nanowires), effectively trapping air pockets and reducing the solid–liquid contact fraction [28]. This structural configuration promotes the Cassie–Baxter wetting regime, wherein water droplets rest atop the asperities, leading to high contact angles and low adhesion [29]. Complementing the physical topography, the chemical composition of the surface helps to achieve superhydrophobicity. The application of low surface energy materials, such as fluorinated compounds [30] or silanes [31] is essential for achieving superhydrophobicity in synthetic coatings and further decreases the adhesive interactions between the surface and water molecules [32]. This combination of hierarchical structuring and surface chemistry modification results in surfaces that not only exhibit remarkable water repellency but also possess functionalities like self-cleaning, anti-icing, and corrosion resistance, which are highly desirable in various industrial and environmental applications.
2.4. Contact Angle Hysteresis and Sliding Behavior
Contact angle hysteresis (CAH) is a parameter used to characterize the wetting behavior of superhydrophobic surfaces. It is defined as the difference between the advancing contact angle (θa) and the receding contact angle (θr) of a liquid droplet on a surface [33]. This hysteresis arises due to surface heterogeneities, such as chemical inhomogeneities and physical roughness, which cause the contact line to pin and resist movement [34]. The magnitude of CAH directly influences the sliding behavior of droplets. On surfaces exhibiting low CAH, droplets can easily roll off, which is desirable for self-cleaning applications. Conversely, high CAH indicates strong pinning forces, causing droplets to adhere to the surface even when tilted, thus hindering the self-cleaning effect. Gao and McCarthy (2006) demonstrated that the presence of microscopic defects and variations in surface energy contribute significantly to CAH, affecting the ease with which droplets can slide [35]. Understanding and controlling CAH is essential for designing superhydrophobic surfaces for specific applications, such as anti-icing, where low CAH facilitates the removal of ice-forming droplets, or in microfluidic devices, where precise droplet manipulation is required [18]. By engineering surface topography and chemistry to minimize hysteresis, materials can achieve optimal performance in these contexts.
3. Bio-Inspired Design Strategies
3.1. Inspiration from Lotus Leaf, Butterfly Wings, and Gecko Feet
Nature provides inspiration for the design of superhydrophobic surfaces through organisms such as the lotus leaf, butterfly wings, and gecko feet. The lotus leaf (Nelumbo nucifera) exemplifies self-cleaning capabilities attributed to its hierarchical surface structure [21]. Microscopic papillae combined with nanoscopic wax crystals create a dual-scale roughness that significantly reduces the contact area for water droplets, leading to high contact angles and promoting the rolling off water, which effectively removes contaminants from the surface [3] represented in Figure 2. Butterfly wings exhibit remarkable superhydrophobic properties, owing to their hierarchical micro- and nanostructures that enable water repellency and facilitate self-cleaning. These features ensure that water droplets bead and roll off, preserving the wing’s delicate structure and functionality [36]. Additionally, the same structural complexity contributes to vivid structural coloration through light interference effects [37]. In contrast, gecko feet represent a distinct example where superhydrophobicity coexists with high adhesion; their toe pads are densely covered with microscopic setae that branch into nanoscale spatulae, allowing both water repellency and strong surface grip. This hierarchical structuring enables geckos to adhere to various surfaces through van der Waals forces. Additionally, the hydrophobic nature of their skin facilitates self-cleaning, allowing geckos to maintain their adhesive capabilities by removing dirt particles effectively [38].
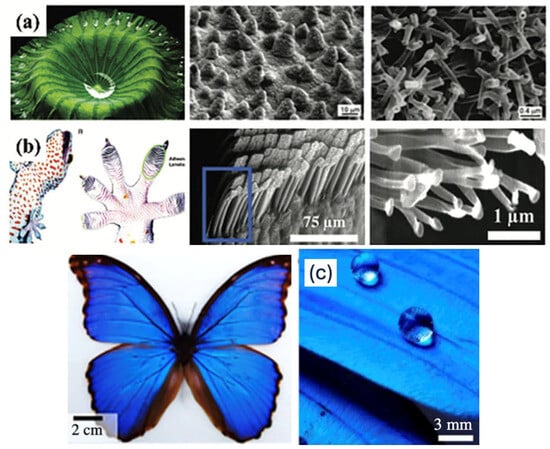
Figure 2.
Natural bio-inspirations for superhydrophobic surface engineering. (a,b) Reprinted with permission from [39]. (c) Adapted with permission from [40].
Figure 2 visually illustrates a diverse array of biological surfaces that have inspired the development of engineered superhydrophobic and multifunctional coatings. Among them, the lotus leaf, butterfly wings, and gecko feet exemplify key natural templates.
The lotus leaf exhibits dual-scale roughness formed by microscale papillae topped with nanoscale wax crystals, producing a self-cleaning effect known as the “lotus effect.” Water droplets resting on this surface maintain a high contact angle and roll off effortlessly, capturing contaminants in the process [3].
The butterfly wings achieve superhydrophobicity through a combination of ridged microstructures and nanostructures aligned along the wing scales. This not only enhances water repellency but also contributes to structural coloration, ensuring lightweight self-cleaning surfaces that preserve aerodynamic efficiency.
The gecko feet utilize hierarchical structuring with micron-scale setae that branch into nanoscale spatulae. These structures enhance dry adhesion via van der Waals forces while maintaining hydrophobicity to prevent dirt buildup. This dual property supports both surface gripping and self-cleaning functionality [38].
Additional natural surfaces in the image such as water strider legs, spider webs, and moth eyes demonstrate varied adaptations for water repellency, light manipulation, and surface adhesion. Together, these organisms offer robust design principles that inform the fabrication of advanced functional coatings for applications ranging from biomedical devices to anti-icing systems [12].
3.2. Biomimetic Fabrication Techniques
The development of artificial superhydrophobic surfaces draws significant inspiration from natural systems, employing biomimetic fabrication techniques to replicate the hierarchical micro- and nanostructures observed in organisms like the lotus leaf and butterfly wings. These techniques aim to create surfaces with high water contact angles and low adhesion, enabling self-cleaning and anti-icing functionalities [40], as presented in Table 2 and shown in Figure 3. One prevalent approach involves chemical vapor deposition (CVD), where thin hydrophobic films are deposited onto substrates to achieve the desired surface chemistry. For instance, [41]. demonstrated that CVD techniques enable the formation of uniform fluorinated silane coatings with enhanced durability and controlled wettability, making them ideal for superhydrophobic textile and glass applications. When combined with surface roughening methods, such as etching or templating, and laser processing, CVD can produce the dual-scale roughness characteristic of superhydrophobicity [29]. Electrospinning is another versatile technique, enabling the fabrication of fibrous mats with nanoscale diameters. By incorporating nanoparticles or adjusting polymer concentrations, electrospun fibers can mimic the complex textures found in natural superhydrophobic surfaces, resulting in enhanced water repellency and self-cleaning properties [42]. Laser ablation offers precise control over surface topography by using laser pulses to generate micro- and nanostructured patterns. This method can directly modify metallic and polymeric substrates to exhibit superhydrophobic behavior without requiring chemical coatings, making it highly suitable for harsh environmental conditions [43]. Additionally, templating techniques utilize molds derived from natural sources, such as leaves or insect wings, to replicate hierarchical architectures on synthetic materials. These approaches enable the durable and scalable fabrication of bio-inspired superhydrophobic surfaces [44]. This approach effectively transfers the hierarchical structures responsible for superhydrophobicity in nature to engineered surfaces.

Table 2.
Summary of biomimetic fabrication techniques.
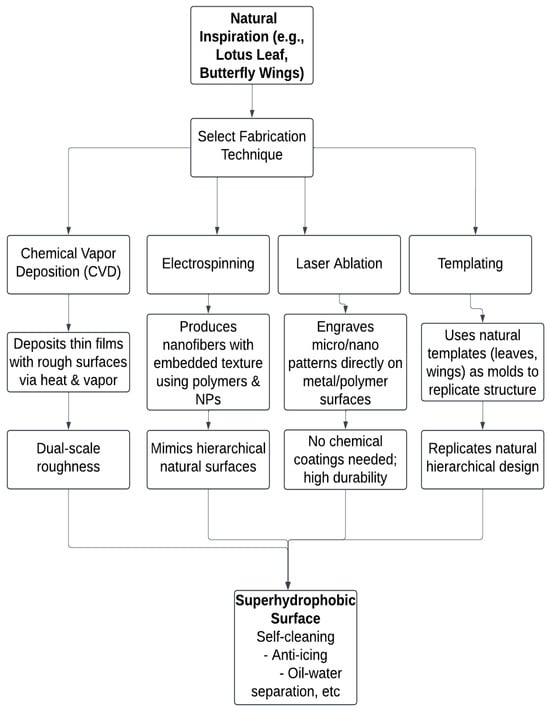
Figure 3.
Process flowchart of biomimetic fabrication techniques for superhydrophobic surfaces.
Figure 3 illustrates the key biomimetic fabrication methods including chemical vapor deposition (CVD), electrospinning, laser ablation, and templating, which are used to replicate natural micro/nanostructures for engineering superhydrophobic surfaces. Each technique follows a unique process pathway, yet they all converge to produce surfaces exhibiting properties such as self-cleaning, anti-icing, and oil–water separation.
3.3. Role of Surface Roughness and Dual-Scale Structuring
The superhydrophobicity observed in natural surfaces, such as lotus leaves, is largely attributed to their hierarchical surface structures, which combines micro- and nanoscale structures. This dual-scale architecture plays an important role in stabilizing the Cassie–Baxter state, where air pockets trapped between surface asperities minimize the solid–liquid contact area, resulting in high water contact angles and low adhesion [51] as represented in Figure 4. Recent studies have investigated the necessity of multiscale roughness for achieving superhydrophobicity. Bittoun and Marmur (2012) examined the role of hierarchical structures and concluded that while multiscale roughness enhances mechanical stability, it is not strictly essential for attaining superhydrophobicity [52]. Their findings suggest that a single-scale roughness can suffice, provided it effectively reduces the contact area between the liquid and the solid surface. In practical applications, engineering surfaces with dual-scale structuring has proven beneficial for creating robust superhydrophobic coatings. Techniques such as laser ablation [53] and chemical etching are employed to fabricate these hierarchical textures, resulting in surfaces that exhibit remarkable water repellency and self-cleaning properties.

Figure 4.
(a) Lotus plant, (b) lotus leaf, and (c) schematic of dual-scale surface structuring. Reprinted with permission from [54].
Figure 4 visually illustrates the Role of Surface Roughness and Dual-Scale Structuring, aligning with Section 3.3. In Figure 4a,b, the lotus leaf is shown as a natural benchmark for superhydrophobicity, where water droplets bead up and roll off effortlessly, carrying away contaminants—a phenomenon known as the “lotus effect.” This exceptional water repellency arises from its dual-scale surface architecture, combining microscale papillae and nanoscale wax crystals. Figure 4c presents a schematic illustrating various surface wetting states. It begins with a smooth surface characterized by high liquid-solid contact area and a low contact angle, representative of the Wenzel regime. As surface roughness increases with the introduction of single-scale microstructures, wettability is slightly enhanced but still favors spreading. Further structuring leads to the Cassie–Baxter regime, where water droplets rest atop micro-protrusions with air pockets trapped beneath, significantly reducing adhesion. The addition of nanoscale features to create dual-scale roughness further stabilizes the Cassie state by amplifying surface heterogeneity. Finally, re-entrant or overhang geometries are depicted, which enable extreme superhydrophobicity even for liquids with lower surface tension, showcasing the most advanced level of wetting resistance.
3.4. Challenges in Mimicking Natural Surfaces
Replicating the superhydrophobic properties observed in natural surfaces presents significant challenges due to the complexity of their hierarchical micro- and nanostructures. The lotus leaf, for instance, exhibits a dual-scale architecture that combines microscale papillae with nanoscale epicuticular waxes, resulting in exceptional water repellency and self-cleaning abilities. Achieving similar structures synthetically requires control over surface morphology at multiple scales, which remains a formidable task in materials science [55]. Traditional fabrication techniques, such as photolithography and chemical vapor deposition, often involve complex procedures and high costs, limiting their scalability for large-area applications. Emerging methods like inkjet printing have been explored for direct micropatterning of superhydrophobic surfaces, offering a more straightforward and cost-effective approach. However, challenges persist in achieving the necessary resolution and durability of the printed patterns to effectively mimic natural superhydrophobicity [56]. Furthermore, the durability of synthetic superhydrophobic surfaces are of concern. Natural surfaces have evolved to maintain their functionality under various environmental conditions, whereas artificial counterparts often suffer from degradation due to wear and environmental exposure. Addressing these challenges necessitates the development of advanced materials and fabrication techniques that can produce durable, large-scale superhydrophobic surfaces with hierarchical structures akin to those found in nature.
4. Fabrication Techniques for Hierarchical Nanostructures
4.1. Chemical Vapor Deposition (CVD) and Sol–Gel Processes
The development of superhydrophobic coatings has been significantly advanced through techniques such as Chemical Vapor Deposition (CVD) and sol–gel processes, both of which enable the fabrication of surfaces with exceptional water-repellent properties. Chemical Vapor Deposition (CVD) is a process wherein volatile precursors are transported in the vapor phase to react or decompose on a substrate, forming a solid thin film [57] as shown in Figure 5. This method allows for precise control over the chemical composition and microstructure of the deposited coatings. For instance, Zhao et al. (2022) demonstrated a facile one-step CVD method to create transparent superhydrophobic coatings with tailored nanocone array structures [57]. These nanocone arrays effectively reduce surface energy and increase roughness, resulting in coatings that exhibit high water contact angles and excellent anti-reflective properties. While conventional techniques such as CVD and sol–gel processing offer fine control of surface chemistry, recent studies have shown that high-volume methods such as injection molding of filled resins can achieve enhanced mechanical and functional performance at industrial scales [58]. In contrast, the sol–gel process involves the transition of a system from a liquid “sol” into a solid “gel” phase. This technique is versatile and can be employed to fabricate coatings with adjusted porosity and surface chemistry. Basu and Saha (2022) introduced a scalable, and cost-effective sol–gel route to obtain durable superhydrophobic coatings [59]. By carefully tuning the sol–gel parameters, they achieved coatings with enhanced mechanical stability and water repellency, suitable for various industrial applications. Both CVD and sol–gel processes offer some advantages in the fabrication of superhydrophobic coatings. CVD provides excellent uniformity and adhesion to complex geometries, making it ideal for applications requiring conformal coatings. Conversely, the sol–gel process is advantageous for its low processing temperatures and ability to incorporate various functional groups, allowing for the design of coatings with specific properties. The selection between these methods depends on the desired application, substrate material, and required coating characteristics.
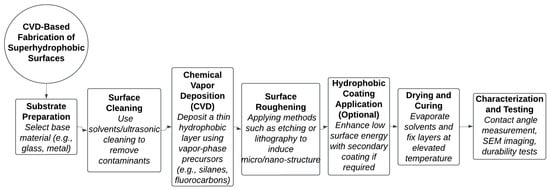
Figure 5.
Fabrication process flowchart for CVD-based superhydrophobic surface synthesis.
Figure 5 shows the workflow outlining sequential steps including substrate preparation, chemical vapor deposition, surface roughening, hydrophobic coating application, and final surface characterization, demonstrating an integrated approach to achieving dual-scale roughness and low surface energy.
4.2. Laser Ablation and Lithographic Methods
The fabrication of superhydrophobic surfaces has been significantly advanced through techniques such as laser surface processing in the femtosecond or picosecond regimes and lithographic methods, both of which enable precise control over surface topography to achieve desired wettability characteristics [60] as shown in Figure 6. Laser surface processing involves the use of high-intensity laser pulses to remove material from a substrate, creating micro- and nanoscale surface textures that mimic the hierarchical structures found in nature. Jagdheesh and Marczak (2019) demonstrated that laser surface texturing can effectively produce superhydrophobic surfaces by generating periodic patterns that reduce surface energy and promote water repellency [61]. This method offers flexibility in pattern design and can be applied to various materials, including metals and polymers. In a study by Liu et al. (2023), laser ablation was utilized to fabricate biomimetic superhydrophobic surfaces on polymer substrates. The resulting textures exhibited high water contact angles and low sliding angles, indicative of enhanced water repellency and self-cleaning properties [62]. This approach changes the potential of laser ablation in creating functional surfaces inspired by natural phenomena. Lithographic methods, such as photolithography and interference lithography, involve the use of light to pattern substrates with high precision. These techniques can produce well-defined micro- and nanostructures that contribute to superhydrophobic behavior. While lithographic methods offer high resolution and reproducibility, they often require complex processes and specialized equipment, which may limit scalability [63]. Integrating laser surface functionalization and lithographic techniques provides a versatile toolkit for engineering superhydrophobic surfaces with tailored properties, suitable for applications ranging from self-cleaning coatings to anti-icing systems.
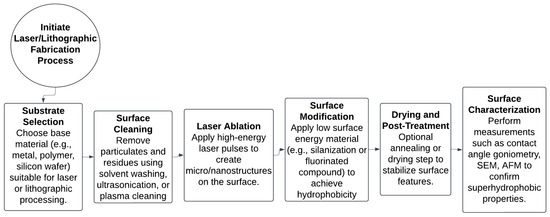
Figure 6.
Process flow diagram for fabricating superhydrophobic surfaces using laser ablation and lithographic methods.
Figure 6 illustrates the workflow which includes substrate preparation, structural patterning via laser, chemical modification for hydrophobicity, and final surface characterization to validate functional properties.
4.3. Electrospinning and Nanoparticle Assembly
The fabrication of superhydrophobic surfaces has been significantly advanced through techniques such as electrospinning and nanoparticle assembly, both of which enable the creation of hierarchical structures that mimic natural water-repellent surfaces.
Electrospinning is a versatile technique that produces continuous nanofibers from polymer solutions or melts under a high-voltage electric field [64] as shown in Figure 7. This process allows for the generation of micro- and nanoscale fibrous structures with high surface area and porosity, essential characteristics for superhydrophobicity [65]. By carefully selecting polymer types and adjusting processing parameters, researchers can fabricate nanofibrous mats that exhibit water contact angles exceeding 150°, indicative of superhydrophobic behavior. These electrospun nanofiber-based surfaces have been applied in areas such as self-cleaning coatings, water-oil separation membranes, and protective textiles due to their remarkable water-repellent properties [66]. Nanoparticle assembly involves the organization of nanoparticles into structured coatings that impart superhydrophobic characteristics to surfaces. This method typically employs the self-assembly of hydrophobic nanoparticles onto substrates, forming a roughened surface topology that reduces contact area with water droplets, thereby enhancing water repellency. For instance, surfaces engineered through the assembly of silica or polymer nanoparticles have demonstrated exceptional anti-icing and self-cleaning capabilities, making them suitable for applications in harsh environmental conditions [67]. Integrating electrospinning with nanoparticle assembly offers a synergistic approach to designing superhydrophobic surfaces. By combining the fibrous architecture of electrospun mats with the hierarchical roughness provided by nanoparticle coatings, it is possible to achieve enhanced durability and functionality in superhydrophobic applications. Granite quarry dust, when used at 60–80 wt.% in alkyd paints, significantly improved the mechanical durability and chemical resistance of coatings, suggesting its viability for protective applications on even steel substrates [68].
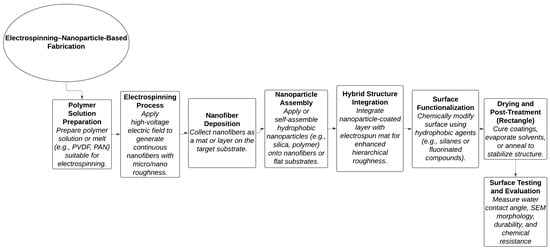
Figure 7.
Process flow diagram for fabricating superhydrophobic surfaces using electrospinning and nanoparticle assembly.
Figure 7 shows the combined approach which creates fibrous networks with hierarchical roughness and enhanced surface energy control, leading to applications in self-cleaning, oil–water separation, anti-icing, and corrosion-resistant coatings.
4.4. Spray Coating and Dip Coating for Scalable Production
The scalable production of superhydrophobic surfaces is important for their widespread application across various industries. Among the numerous fabrication techniques, spray coating and dip coating have emerged as prominent methods due to their simplicity, cost-effectiveness, and adaptability to complex geometries [69] as shown in Figure 8.
Spray coating involves the atomization of a coating solution into fine droplets, which are then uniformly deposited onto a substrate. This technique allows for the rapid application of superhydrophobic layers over large areas and on substrates with intricate shapes. The versatility of spray coating has facilitated its use in creating surfaces that exhibit remarkable water repellency and self-cleaning properties, making it suitable for applications ranging from protective clothing to anti-icing systems [70].
Dip coating, on the other hand, entails immersing a substrate into a coating solution and withdrawing it at a controlled speed, resulting in the formation of a thin, uniform film. This method is particularly advantageous for coating substrates with complex geometries, ensuring comprehensive coverage. The dip-coated surfaces have demonstrated superhydrophobic characteristics, including high water contact angles and low sliding angles, indicative of enhanced water repellency and self-cleaning capabilities [11].
Both spray and dip coating methods offer pathways to the scalable production of superhydrophobic surfaces. However, challenges such as ensuring long-term durability, mechanical robustness, and environmental stability of the coatings remain. Addressing these challenges is essential for the successful commercialization and practical application of superhydrophobic technologies.
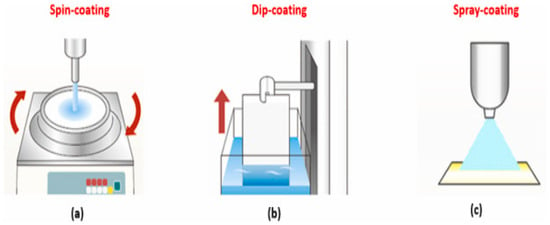
Figure 8.
Picture of Scalable Fabrication Techniques for Superhydrophobic Coatings with arrows showing coating motion directions, Spin-Coating (a), Dip-Coating (b), and Spray-Coating (c) Reprinted with permission from [71].
Figure 8 illustrates three scalable thin-film deposition techniques, spin-coating (a), dip-coating (b), and spray-coating (c) with relevance to Section 4.4: Spray Coating and Dip Coating for Scalable Production of superhydrophobic surfaces. Among these, dip-coating and spray-coating stand out for their industrial viability due to simplicity, substrate versatility, and cost-effectiveness. In dip-coating (b), the substrate is vertically immersed and withdrawn from a coating solution at a controlled speed, allowing a uniform film to form through solvent evaporation and gravitational drainage. This technique is especially suitable for coating complex 3D geometries, and the resulting film thickness depends on withdrawal speed, viscosity, and surface tension of the liquid. Spray-coating (c), by contrast, involves atomizing the coating solution into fine droplets that are uniformly deposited onto a substrate using pneumatic or ultrasonic nozzles. It supports large-area deposition and is highly adaptable to curved or irregular surfaces, making it ideal for mass production of superhydrophobic coatings on construction materials, textiles, or automotive parts. Spin-coating (a), though effective for uniform films on flat substrates, is less favored for industrial scaling due to material wastage and geometric limitations. Together, dip- and spray-coating enable scalable, efficient, and flexible fabrication of functional superhydrophobic films for diverse applications.
Figure 9 shows both techniques, which enable efficient coating of substrates with low-surface-energy materials. Spray coating supports complex geometries via atomized deposition, while dip coating ensures uniform coverage through immersion and withdrawal. Post-treatment and characterization validate the coatings’ hydrophobic performance for commercial deployment.
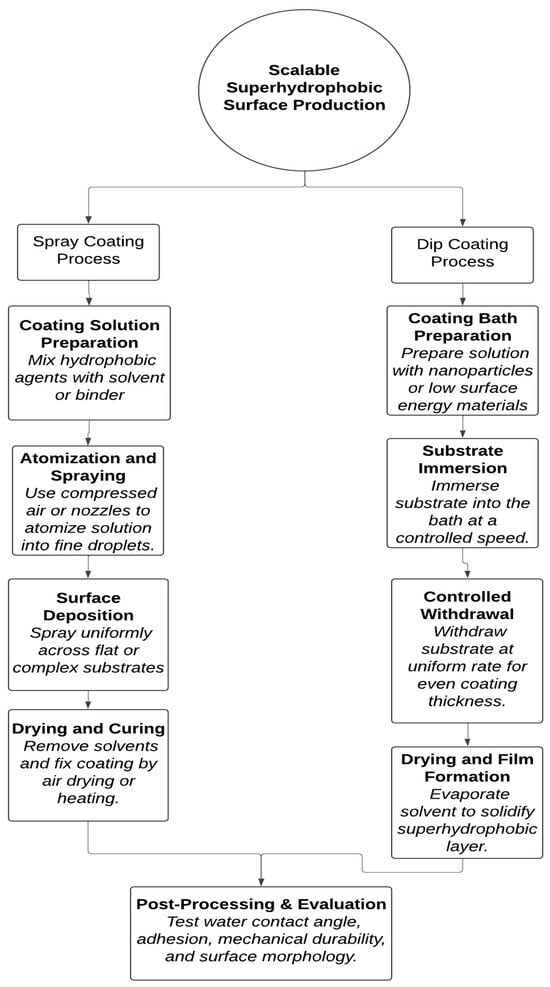
Figure 9.
Process flow diagram for scalable fabrication of superhydrophobic surfaces using spray and dip coating methods.
5. Functional Applications
5.1. Self-Cleaning Surfaces: Mechanisms and Use Cases
The self-cleaning properties of superhydrophobic surfaces are predominantly attributed to their ability to repel water, facilitating the removal of contaminants through mechanisms inspired by natural phenomena. The “lotus effect,” observed in lotus leaves, exemplifies this behavior; water droplets bead up and roll off the leaf’s surface, carrying away dirt particles and maintaining cleanliness. This effect is achieved through a combination of high water contact angles (typically exceeding 150°) and low sliding angles, resulting from the surface’s hierarchical micro- and nanostructures that trap air and minimize adhesion [71]. The underlying mechanism involves the formation of a composite interface where air pockets are trapped beneath the water droplet, reducing the contact area and adhesion between the droplet and the surface. As a result, even minimal external forces, such as gravity or wind, can cause the water droplets to roll off, effectively removing surface contaminants in the process [71] (Geyer et al., 2020). Applications of self-cleaning superhydrophobic surfaces span various industries [72]. In the textile sector, fabrics treated to exhibit superhydrophobicity resist staining and maintain cleanliness with minimal washing. Architectural applications include coatings for building exteriors and windows, reducing maintenance costs and enhancing esthetic longevity [73]. In the energy sector, superhydrophobic coatings on solar panels prevent dust accumulation, thereby maintaining optimal efficiency. Additionally, these surfaces are utilized in medical devices to inhibit biofouling and in automotive industries to enhance visibility and reduce cleaning frequency [74]. The development and implementation of self-cleaning superhydrophobic surfaces continue to evolve, offering promising solutions for reducing maintenance efforts and enhancing the longevity and performance of various products and infrastructures.
5.2. Anti-Icing and De-Icing Capabilities
The application of superhydrophobic surfaces has emerged as a promising strategy for mitigating ice formation and facilitating ice removal, addressing critical challenges in sectors such as aviation, energy, and infrastructure. These surfaces leverage their unique wettability characteristics to impede ice nucleation and adhesion, thereby enhancing safety and operational efficiency in cold environments.
Passive anti-icing mechanisms exploit the inherent water-repellent properties of superhydrophobic coatings to prevent water accumulation and subsequent freezing. By creating a surface that minimizes contact with water droplets, these coatings effectively reduce the likelihood of ice formation. For instance, Wang et al. (2019) developed a nanocomposite film composed of single-walled carbon nanotubes (SWCNT) and poly(dimethylsiloxane) (PDMS) that exhibited low surface energy and high electrical conductivity [74]. This film demonstrated significant resistance to ice formation under subzero conditions, highlighting the efficacy of superhydrophobic surfaces in passive anti-icing applications [75].
Active de-icing approaches involve the integration of functional materials capable of generating localized heat to facilitate ice removal. Deng et al. (2024) introduced a superhydrophobic coating incorporating carbon nanotubes (CNTs) that possessed photothermal properties [76]. Upon exposure to light, the CNTs efficiently converted solar energy into thermal energy, raising the surface temperature and promoting the melting of adhered ice. This photothermal effect, combined with the coating’s superhydrophobic nature, resulted in a dual-action system that effectively prevented ice accumulation and enabled rapid de-icing when necessary [77]. The integration of superhydrophobicity with active heating elements offers an encompassing solution to ice management. By combining passive water repellency with active thermal responses, these hybrid systems can adapt to varying environmental conditions, providing reliable anti-icing and de-icing performance. Such advancements are particularly beneficial in applications where ice accumulation poses significant risks, such as on aircraft surfaces, wind turbine blades, and power transmission lines [41].
In summary, the development of superhydrophobic surfaces with anti-icing and de-icing capabilities represents a significant advancement in materials science. Through the strategic design of surface structures and the incorporation of functional nanomaterials, these surfaces offer effective solutions for ice mitigation, enhancing safety and efficiency across multiple industries.
5.3. Durability, Wear Resistance, and Environmental Stability
The practical application of superhydrophobic surfaces is often constrained by challenges related to their durability, wear resistance, and environmental stability. Addressing these issues is essential for the development of coatings that maintain their functionality under mechanical stress and environmental exposure.
Durability and Wear Resistance: Superhydrophobic coatings frequently suffer from mechanical fragility, leading to the degradation of their water-repellent properties upon abrasion or impact. To enhance mechanical durability, researchers have explored various strategies, including the incorporation of hard nanoparticles and the development of hierarchical structures that mimic natural robust surfaces. For instance, Zhang et al. (2015) demonstrated that electrostatic assembly of silica nanoparticles onto cotton fabrics, followed by hydrophobization, resulted in a durable superhydrophobic surface [77]. This approach not only imparted high water repellency but also maintained the fabric’s flexibility and breathability, showcasing a balance between mechanical robustness and functional performance.
Environmental Stability: Superhydrophobic surfaces must withstand various environmental factors, such as UV radiation, temperature fluctuations, and chemical exposures. Milionis et al. (2016) reviewed advancements in enhancing the environmental stability of superhydrophobic materials, highlighting the importance of selecting appropriate base materials and surface treatments [78]. The study emphasized that combining chemical modifications with physical structuring can lead to surfaces that resist environmental degradation while retaining their superhydrophobic characteristics [79].
In summary, improving the durability, wear resistance, and environmental stability of superhydrophobic surfaces is critical for their widespread adoption. Innovative fabrication techniques and material selections are pivotal in developing coatings that can endure mechanical and environmental challenges while maintaining their desired properties.
5.4. Integration in Aerospace, Automotive, and Civil Engineering
The integration of superhydrophobic surfaces into aerospace, automotive, and civil engineering sectors has garnered significant attention due to their potential to enhance performance, safety, and longevity of materials and structures. These surfaces, characterized by their exceptional water repellency, offer solutions to challenges such as icing, corrosion, and contamination across various engineering applications. In the aerospace industry, superhydrophobic coatings are employed to mitigate ice accretion on aircraft surfaces, which can adversely affect aerodynamics and safety. By preventing water droplet adhesion and subsequent freezing, these coatings reduce the need for chemical de-icing agents and mechanical removal methods, leading to more efficient and environmentally friendly operations. Kumar and Prakash (2022) provide a comprehensive review of superhydrophobic coatings tailored for aerospace applications, emphasizing their role in enhancing aircraft performance and safety [80]. Within the automotive sector, superhydrophobic surfaces contribute to improved visibility and maintenance by preventing water and dirt accumulation on windshields, mirrors, and body panels. This not only enhances driver safety during adverse weather conditions but also reduces the frequency of vehicle cleaning. Additionally, these coatings can protect underlying materials from corrosion, thereby extending the lifespan of automotive components. Zhang et al. (2008) discuss the structural control and functional applications of superhydrophobic surfaces, highlighting their utility in automotive engineering [81]. In civil engineering, the application of superhydrophobic coatings to building materials such as concrete, glass, and metal surfaces offers protection against water infiltration, staining, and microbial growth. This leads to structures that are more durable and require less maintenance over time. For instance, treated concrete surfaces exhibit reduced water absorption, minimizing freeze–thaw damage in colder climates. The self-cleaning properties of these coatings also contribute to the esthetic preservation of buildings and infrastructure [82]. The successful integration of superhydrophobic surfaces in these engineering fields necessitates advancements in coating durability and environmental stability to withstand mechanical wear and exposure to harsh conditions. Ongoing research focuses on developing robust coatings that maintain their functionality over extended periods, ensuring their practical applicability in demanding environments.
6. Emerging Trends
6.1. Smart and Stimuli-Responsive Superhydrophobic Coatings
The advancement of superhydrophobic coatings has led to the development of smart and stimuli-responsive surfaces capable of adapting their wettability in response to external stimuli such as temperature, pH, light, or electrical fields. These dynamic surfaces offer enhanced functionality and versatility across various applications, including controlled drug delivery, self-cleaning materials, and anti-icing systems as represented in Figure 10. Temperature-responsive superhydrophobic coatings have been engineered using polymers that undergo conformational changes at specific temperatures. For instance, Li et al. (2016) synthesized a stimuli-responsive polysiloxane through a catalyst-free aza-Michael addition, which demonstrated the ability to load and release ibuprofen in response to temperature variations [83]. This characteristic is particularly advantageous in biomedical applications where controlled drug release is essential. pH-responsive coatings adjust their wettability based on the acidity or alkalinity of the environment [84]. Such coatings are beneficial in scenarios where surface properties need to change in response to chemical stimuli, such as in sensors or smart textiles. The incorporation of functional groups that ionize or deionize at specific pH levels enables this responsive behavior, allowing for applications in areas like controlled release systems and adaptive filtration membranes. Light-responsive superhydrophobic surfaces utilize photochromic materials that alter their surface energy upon exposure to light of certain wavelengths. This property can be harnessed to create coatings that switch between hydrophobic and hydrophilic states, facilitating applications in microfluidics and optical devices. The integration of such materials enables the development of surfaces with tunable wettability, providing control over liquid adhesion and movement [85]. Electrically responsive coatings change their wettability when subjected to an electric field. By embedding conductive materials within the coating matrix, it is possible to modulate surface properties dynamically. This approach has been explored for applications in droplet manipulation, lab-on-a-chip devices, and adaptive lenses. The ability to control surface wettability electrically offers precise and rapid responsiveness, essential for advanced technological applications. The development of these smart superhydrophobic coatings involves the integration of responsive polymers, nanoparticles, and other functional materials to achieve the desired adaptive behavior. Qing et al. (2019) demonstrated the fabrication of robust superhydrophobic surfaces through the self-assembly of nanoparticles, which exhibited applications in anti-icing and self-cleaning [67]. Such methodologies underscore the potential of combining hierarchical structures with stimuli-responsive components to create surfaces capable of dynamic wettability control.
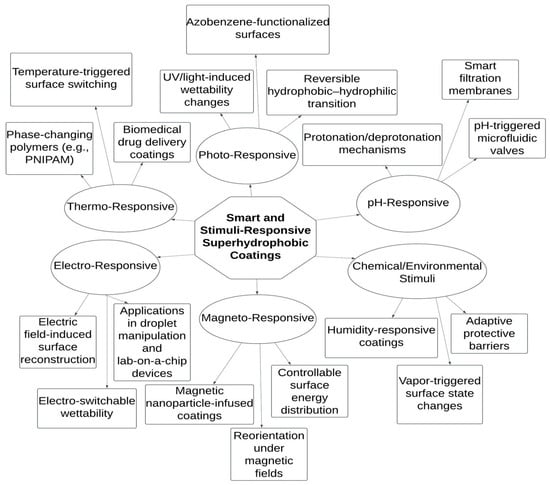
Figure 10.
Diagram illustration of classification of smart and stimuli-responsive superhydrophobic coatings based on external activation mechanisms.
In summary, smart and stimuli-responsive superhydrophobic coatings represent a significant advancement in materials science, offering dynamic control over surface properties in response to external stimuli. These coatings hold promise for a wide range of applications, from biomedical devices to environmental protection, by providing adaptable and multifunctional surface characteristics. The application of mixed-dimensional nanomaterials, as reported by Fesojaye et al. (2023), discusses the transformative potential of engineered surfaces in achieving multifunctionality and adaptive performance [86].
Figure 10 presents a hierarchical classification of intelligent surface systems based on the type of external stimuli they respond to, branching from a central concept. At the core are superhydrophobic coatings engineered to dynamically alter their surface properties in response to environmental cues. The first branch, thermo-responsive, includes materials like PNIPAM that undergo phase transitions near body temperature, enabling applications such as temperature-triggered drug delivery and wettability switching. Photo-responsive coatings, on the second branch, utilize light-sensitive molecules such as azobenzene to toggle between hydrophobic and hydrophilic states under UV or visible light, useful in reversible self-cleaning surfaces. The pH-responsive category involves surfaces with ionizable functional groups that modulate surface energy in acidic or alkaline conditions, allowing integration into smart membranes and chemical sensors. Electro-responsive surfaces incorporate conductive polymers or embedded electrodes, enabling wettability control through applied electric fields, which is ideal for microfluidic systems and droplet manipulation. Magneto-responsive coatings utilize magnetic nanoparticles that realign under a magnetic field to alter surface morphology, providing precise remote control. Lastly, chemical and environmental stimuli-responsive surfaces adapt their wettability based on humidity, vapor, or chemical exposure, making them suitable for protective coatings and adaptive packaging. This structured approach highlights the versatility and multifunctionality of responsive superhydrophobic systems.
6.2. Multifunctional Surfaces: Antibacterial, UV-Resistant, Etc.
The development of multifunctional superhydrophobic surfaces that exhibit properties such as antibacterial activity and UV resistance has garnered significant attention due to their potential applications in various fields, including healthcare, textiles, and environmental protection [87]. These surfaces combine the water-repellent characteristics of superhydrophobicity with additional functionalities, enhancing their utility and performance.
Antibacterial Properties: Integrating antibacterial agents into superhydrophobic coatings can effectively inhibit microbial adhesion and proliferation. For instance, functionalizing surfaces with nanoparticles like titanium dioxide (TiO2) imparts antibacterial activity [88]. In their study, a two-step functionalization process was employed to create superhydrophobic surfaces with embedded TiO2 nanoparticles, resulting in coatings that exhibited both antibacterial properties and UV-blocking capabilities. The incorporation of TiO2 nanoparticles not only provided antibacterial effects but also contributed to the UV resistance of the surface.
UV Resistance: Exposure to ultraviolet (UV) radiation can degrade materials, leading to diminished performance and longevity. To address this, UV-resistant superhydrophobic surfaces have been developed. Li et al. (2023) reported the construction of a robust, UV-resistant multifunctional surface suitable for self-cleaning applications [89]. The study illustrated the use of specific materials and surface structures that confer resistance to UV-induced degradation, thereby enhancing the durability and functionality of the coatings.
Fabrication Techniques: The creation of these multifunctional surfaces often involves the deposition of functional nanoparticles onto substrates, followed by chemical treatments to achieve the desired properties. Techniques such as sol–gel processing, layer-by-layer assembly, and chemical vapor deposition are commonly employed to fabricate coatings that are both superhydrophobic and possess additional functionalities like antibacterial activity and UV resistance.
In summary, the integration of antibacterial and UV-resistant properties into superhydrophobic surfaces represents a significant advancement in material science. These multifunctional coatings offer enhanced performance and durability, making them suitable for a wide range of applications where both water repellency and additional protective features are desired.
6.3. Sustainable and Eco-Friendly Coating Materials
The development of sustainable and eco-friendly superhydrophobic coatings is paramount in reducing environmental impact while maintaining high-performance characteristics. Traditional coatings often rely on fluorinated compounds, which pose ecological and health risks due to their persistence and bioaccumulation [90] as shown in Table 3. Consequently, research has shifted towards utilizing biodegradable and non-toxic materials to achieve superhydrophobicity [91].
Natural fatty acids have emerged as promising candidates for eco-friendly coatings. Prudnikov et al. (2022) demonstrated that coatings derived from saturated fatty acids, such as stearic acid, can self-assemble into crystalline structures exhibiting superhydrophobic properties with water contact angles approaching 165° [92]. These coatings not only repel water effectively but also display antimicrobial activity against bacteria like Escherichia coli and Listeria innocua, making them suitable for applications requiring hygienic surfaces.
Biomimicry offers another sustainable approach by emulating natural surfaces known for their water-repellent properties. The “Lotus Effect,” characterized by the self-cleaning and superhydrophobic nature of lotus leaves, has inspired the design of synthetic surfaces that mimic this behavior. Lai (2003) explored the physical principles underlying the Lotus Effect and discussed methodologies for artificially replicating these structures [93]. By adopting such bioinspired designs, it is possible to create coatings that achieve superhydrophobicity without resorting to environmentally harmful substances.
Incorporating biodegradable polymers and natural fillers into coating formulations further enhances their environmental compatibility. Materials such as polylactic acid (PLA) and chitosan, derived from renewable resources, can be engineered to exhibit superhydrophobic characteristics while degrading harmlessly in the environment. Additionally, the use of bio-based solvents during the fabrication process minimizes the release of volatile organic compounds (VOCs), contributing to safer manufacturing practices.
The transition to sustainable superhydrophobic coatings necessitates a holistic approach that considers the entire lifecycle of the materials, from sourcing and production to application and disposal. By leveraging natural compounds, biomimetic designs, and green chemistry principles, it is feasible to develop coatings that meet performance requirements while safeguarding environmental and human health.

Table 3.
Summary of Commercialization Challenges and Opportunities.
Table 3.
Summary of Commercialization Challenges and Opportunities.
| Challenge/Opportunity | Description | Impact on Commercialization | Strategic Response/Opportunity |
|---|---|---|---|
| Mechanical Durability | Fragile micro/nanostructures degrade under abrasion and wear | Limits lifespan and applicability in real-world environments | Develop self-healing coatings or embed nanostructures in robust matrices [94] |
| Environmental Stability | UV light, temperature, and chemicals deteriorate coating performance | Reduces long-term reliability in outdoor and industrial settings | Use UV-stable, chemically inert, and thermally resistant materials [95,96] |
| Scalability and Cost | Advanced fabrication methods are complex and expensive | Hinders mass production and cost-effectiveness | Shift to scalable methods like spray/dip coating and roll-to-roll manufacturing [82,97] |
| Eco-Regulatory Compliance | Use of fluorinated or toxic compounds raises environmental and health concerns | Restricts product approval and public acceptance | Explore fluorine-free, biodegradable, and non-toxic alternatives for sustainable applications [98,99] |
6.4. Commercialization Challenges and Opportunities
The transition of superhydrophobic surfaces from laboratory research to commercial products presents a myriad of challenges and opportunities. Despite extensive academic interest, the practical application of these materials remains limited due to several critical factors as presented in Table 3.
Mechanical Durability: A primary obstacle is the inherent fragility of superhydrophobic coatings. The micro- and nano-structured surfaces essential for achieving superhydrophobicity are susceptible to mechanical wear, leading to a rapid decline in performance. Liu et al. (2023) emphasizes that enhancing mechanical stability is crucial for practical applications, suggesting strategies such as designing self-healing surfaces and constructing protective micro-skeletons to shield delicate nanostructures as presented in Table 3 [62].
Environmental Stability: Superhydrophobic coatings often degrade under environmental stressors like ultraviolet (UV) radiation, temperature fluctuations, and chemical exposure. This degradation compromises their hydrophobic properties and limits their lifespan. Erbil (2020) highlights the necessity for coatings that can withstand harsh environmental conditions, advocating for the development of materials with improved chemical and thermal stability to ensure long-term functionality [64].
Scalability and Cost: The fabrication of superhydrophobic surfaces typically involves complex and costly processes, hindering large-scale production. Techniques such as lithography and chemical vapor deposition, while effective, are not economically viable for mass production. Liu et al. (2023) proposes adopting scalable methods like spray coating and dip coating, which offer a balance between performance and cost-effectiveness, facilitating broader commercial adoption [62].
Regulatory and Safety Concerns: Many superhydrophobic coatings rely on fluorinated compounds, which pose environmental and health risks due to their persistence and bioaccumulation. Erbil (2020) discusses the importance of developing eco-friendly alternatives that comply with regulatory standards, suggesting the use of biodegradable polymers and non-toxic materials to mitigate potential hazards [64].
Market Opportunities: Despite these challenges, the potential applications for superhydrophobic surfaces are vast. Industries such as aerospace, automotive, textiles, and consumer electronics stand to benefit from coatings that offer water repellency, self-cleaning properties, and corrosion resistance. Liu et al. (2023) predicted that with concerted efforts in addressing current limitations, widespread commercialization of superhydrophobic surfaces could be realized by 2035, unlocking new functionalities and enhancing product performance across various sectors [62].
In summary, while significant hurdles impede the commercialization of superhydrophobic surfaces, targeted research and innovation present viable pathways to overcome these obstacles, paving the way for their integration into diverse industrial applications.
7. Summary, Challenges, and Future Direction
7.1. Summary of Key Advances
Recent advancements in bio-inspired superhydrophobic coatings have demonstrated remarkable progress in material design, fabrication techniques, and multifunctionality. A significant breakthrough lies in the engineering of hierarchical micro- and nanostructures that mimic natural surfaces such as lotus leaves and butterfly wings [93]. These biomimetic structures, when combined with low surface energy materials, have enabled the creation of coatings with water contact angles exceeding 150° and sliding angles below 10°, parameters for achieving self-cleaning and anti-icing behavior. Various fabrication methods, including chemical vapor deposition, laser processing, electrospinning, and nanoparticle self-assembly, have evolved to support large-scale, cost-effective production while retaining functional integrity [93]. The integration of stimuli-responsive features has further advanced the field, allowing surfaces to adapt dynamically to environmental cues such as temperature, pH, or light. This adaptability is particularly valuable in smart textiles, microfluidic devices, and biomedical implants [94]. Multifunctional coatings with added antibacterial, UV-resistant, and photothermal properties have expanded the utility of superhydrophobic surfaces across sectors like aerospace, automotive, civil engineering, and healthcare. Moreover, the shift toward sustainable, eco-friendly materials has addressed environmental concerns, pushing the field toward regulatory compliance and long-term viability. These innovations collectively represent a robust foundation for the continued development and real-world application of superhydrophobic technologies [93].
7.2. Critical Challenges
Despite the notable progress in bio-inspired superhydrophobic coatings, several critical challenges persist that hinder their full-scale commercialization and long-term performance. One of the foremost issues is mechanical durability. The micro/nanostructures responsible for water repellency are inherently fragile and susceptible to abrasion, peeling, and delamination under mechanical stress [95]. Even minor surface damage can lead to the collapse of the air pockets that maintain the Cassie–Baxter state, resulting in a sharp decline in superhydrophobic performance. Environmental stability poses another major concern. Prolonged exposure to UV radiation, high humidity, temperature fluctuations, and aggressive chemical environments can degrade surface chemistries and disrupt the hierarchical roughness. This limits the applicability of such coatings in outdoor and industrial settings, where consistent performance over time is crucial. Scalability and cost-effectiveness remain significant bottlenecks. Advanced fabrication techniques such as lithography or chemical vapor deposition, while effective at the lab scale, are often expensive and not easily adaptable for large-area or three-dimensional surfaces. Additionally, many high-performance formulations rely on fluorinated compounds, which raise concerns about environmental persistence and regulatory restrictions [95]. To address these challenges, research must focus on developing robust, self-healing structures, environmentally benign chemistries, and scalable, economically viable fabrication methods that retain high-performance characteristics under real-world conditions.
7.3. Future Research and Industrial Adoption
The future of bio-inspired superhydrophobic coatings lies in developing multifunctional, scalable, and durable solutions tailored to meet industrial demands across aerospace, automotive, construction, healthcare, and energy sectors [93]. A key direction for research is the integration of adaptive functionalities, such as stimuli-responsive wetting behavior and real-time self-healing, into robust surface architectures. Future coatings should autonomously restore their superhydrophobicity after physical damage or environmental degradation, thereby extending operational lifespans and reducing maintenance costs [95]. Material scientists are expected to explore greener, fluorine-free alternatives using biodegradable polymers, natural waxes, and plant-based oils to address environmental and regulatory constraints. These alternatives must match or exceed the performance of traditional fluorinated materials in terms of hydrophobicity, UV resistance, and thermal stability. Industrially, future adoption hinges on the development of cost-effective fabrication techniques like roll-to-roll printing, dip coating, and spray deposition compatible with high-throughput manufacturing. Ensuring compatibility with diverse substrates, metals, plastics, glass, and textiles will be essential for widespread integration [95]. Moreover, digital simulation tools, such as computational fluid dynamics and machine learning-driven surface optimization, will likely play a central role in the rapid prototyping and prediction of long-term performance [95,100]. A multidisciplinary approach combining materials engineering, environmental science, and manufacturing technology is vital to bridging the gap between academic innovation and real-world implementation.
8. Conclusions
This review consolidates recent progress in the design and development of bio-inspired superhydrophobic coatings that utilize hierarchical micro- and nanostructures to achieve exceptional water repellency, self-cleaning, and anti-icing functionalities. These effects are governed by fundamental wetting mechanisms, notably the Cassie–Baxter state, supported by multiscale roughness and low-surface-energy materials. The integration of micro/nano hierarchical structures allows for enhanced contact and sliding angles, enabling self-cleaning and low adhesion properties. Surface chemistry further supports these effects by stabilizing the wetting state and providing chemical resilience.
Various fabrication methods, such as chemical vapor deposition (CVD), laser ablation, electrospinning, and nanoparticle self-assembly, offer scalable solutions with unique trade-offs in cost, durability, and complexity. While these methods are promising, they must overcome challenges in achieving mechanical robustness and environmental stability. Strategies like combining electrospinning with nanoparticle coatings and incorporating self-healing mechanisms are actively being explored.
Stimuli-responsive coatings that adapt to external prompts such as temperature, pH, and light expand application domains, alongside multifunctional enhancements like antibacterial and UV resistance through nanomaterial integration. However, industrial viability depends on eco-regulatory compliance, reduced reliance on fluorinated compounds, and affordability. Meeting these criteria will require cross-disciplinary innovations. With continued material development and sustainable engineering, a new generation of intelligent, durable, and environmentally responsible superhydrophobic surfaces is on the horizon.
Author Contributions
F.A., D.E. and T.E., methodology; S.A., validation; S.A. and A.O., formal analysis; F.A., investigation; F.A., resources; F.A. and D.E., data curation; F.A., writing—original draft preparation; D.E., S.A. and A.O., writing—review and editing; T.E., visualization; F.A. and D.E., supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The authors would like to express their gratitude to all contributors for their valuable input and support throughout this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barthwal, S.; Kim, Y.-K.; Lim, S.-H.; Park, J.; Kim, H.J. Bioinspired superhydrophobic surfaces: Recent advances in fabrication methods and applications. Prog. Mater. Sci. 2022, 129, 100973. [Google Scholar] [CrossRef]
- Su, B.; Tian, Y.; Jiang, L. Bioinspired interfaces with superwettability: From materials to chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Bohn, H.F.; Federle, W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc. Natl. Acad. Sci. USA 2004, 101, 14138–14143. [Google Scholar] [CrossRef]
- Zheng, Y. Bio-Inspired Wettability Surfaces: Developments in Micro- and Nanostructures. MRS Bull. 2016, 41, 572. [Google Scholar] [CrossRef]
- Joghee, S.H.; Sunil, N.; Selvaraj, G.; Uthandi, K.M.; Pullithadathil, B. Bio-Inspired Multifunctional Superhydrophobic Coatings for Corrosion Resistance. In A Treatise on Corrosion Science, Engineering and Technology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 559–575. [Google Scholar] [CrossRef]
- Wen, J.; Li, P.; Zhu, F. Nature-Inspired Fluorine-Free Robust Superhydrophobic Fabrics. Fibers Polym. 2024, 25, 1243–1251. [Google Scholar] [CrossRef]
- Nature Research Intelligence. Superhydrophobic Surfaces and Drag Reduction in Fluid Flow. Nat. Res. Intell. 2025, 9, 112–130. [Google Scholar]
- Backholm, M.; Molpeceres, D.; Vuckovac, M.; Nurmi, H.; Hokkanen, M.J.; Jokinen, V.; Timonen, J.V.I.; Ras, R.H.A. Water droplet friction and rolling dynamics on superhydrophobic surfaces. Nat. Commun. Mater. 2020, 1, 65–75. [Google Scholar] [CrossRef]
- Quéré, D. Wetting and roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M.; Joseph, G.B.; Durairaj, R.B.; Mageshwaran, G. Superhydrophobic surfaces: A review on fundamentals, applications, and challenges. J. Coat. Technol. Res. 2018, 15, 231–250. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef]
- Fei, L.; He, Z.; LaCoste, J.D.; Sun, Y. A Mini Review on Superhydrophobic and Transparent Surfaces. Available online: https://www.researchgate.net/figure/a-Examples-of-superhydrophobic-surfaces-in-nature-b-the-properties-and-corresponding_fig1_344357380 (accessed on 16 May 2025).
- Bhushan, B.; Jung, Y.C.; Nosonovsky, M. Lotus Effect: Surfaces with Roughness-Induced Superhydrophobicity, Self-Cleaning, and Low Adhesion. In Springer Handbook of Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1437–1524. [Google Scholar] [CrossRef]
- Gou, X.; Guo, Z. Superhydrophobic Plant Leaves with Micro-line Structures: An Optimal Biomimetic Objective in Bionic Engineering. J. Bionic Eng. 2018, 15, 851–858. [Google Scholar] [CrossRef]
- Gorb, S.N. Functional Surfaces in Biology: Little Structures with Big Effects. In Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2009; Volume 1, pp. 1–25. [Google Scholar] [CrossRef]
- Bhushan, B. Characterization of Rose Petals and Fabrication and Characterization of Superhydrophobic Surfaces with High and Low Adhesion. In Biomimetics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 189–206. [Google Scholar] [CrossRef]
- Parker, A.R.; Lawrence, C.R. Water capture by a desert beetle. Nature 2001, 414, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liao, T.; Liu, K.; Jiang, L.; Kim, J.H.; Dou, S.X. Robust superhydrophobicity of hierarchical ZnO hollow microspheres fabricated by two-step self-assembly. Nano Res. 2013, 6, 726–735. [Google Scholar] [CrossRef]
- Li, Y.; Sha, A.; Tian, Z.; Cao, Y.; Li, X.; Liu, Z. Review on superhydrophobic anti-icing coating for pavement. J. Mater. Sci. 2023, 58, 3377–3400. [Google Scholar] [CrossRef]
- Qiao, Y.; Tao, X.; Li, L.; Ruan, M.; Lu, L. Robust α-Fe2O3/Epoxy Resin Superhydrophobic Coatings for Anti-icing Property. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2024, 39, 621–626. [Google Scholar] [CrossRef]
- Barthlott, W.; Mail, M.; Neinhuis, C. Superhydrophobic hierarchically structured surfaces in biology: Evolution, structural principles and biomimetic applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 374, 20160191. [Google Scholar] [CrossRef]
- Bormashenko, E. Progress in understanding wetting transitions on rough surfaces. Adv. Colloid Interface Sci. 2015, 222, 92–103. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, Y.; Yan, M.; Qian, B.; Jiang, A.; Zhang, X.; Bi, J.; Tong, Y.; Yu, L.; Li, W. Recent advancements in fabrication strategies and applications of superhydrophobic coatings. J. Mater. Sci. 2025, 60, 7826–7858. [Google Scholar] [CrossRef]
- Liu, K.; Feng, L.; Zhai, J.; Song, Y.; Jiang, L. Patterned superhydrophobic surfaces: Toward a synthetic mimic of the Namib desert beetle. Adv. Mater. 2010, 22, 999–1002. [Google Scholar] [CrossRef]
- Scheff, T.; Acha, F.; Diaz Armas, N.; Mead, J.L.; Zhang, J. Tuning Wetting Properties Through Surface Geometry in the Cassie–Baxter State. Biomimetics 2025, 10, 20. [Google Scholar] [CrossRef]
- Liu, K.; Yao, X.; Jiang, L. Recent developments in bio-inspired special wettability. Chem. Soc. Rev. 2010, 39, 3240–3255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; He, D.; Yu, Y.; Liu, J. Design and application of fluorinated materials for superhydrophobic surfaces: A review. J. Fluor. Chem. 2020, 236, 109558. [Google Scholar] [CrossRef]
- Lin, X.; Chen, X.; Zhao, S.; Dou, B.; Peng, Q.; Deng, Z.; Emori, W.; Gao, X.; Fang, Z. Effect of silane-modified fluorinated graphene on the anticorrosion property of epoxy coating. J. Appl. Polym. Sci. 2023, 140, e53637. [Google Scholar] [CrossRef]
- Ihimoyan, M.K.; Enyejo, J.O.; Ali, E.O. Monetary Policy and Inflation Dynamics in Nigeria, Evaluating the Role of Interest Rates and Fiscal Coordination for Economic Stability. Int. J. Sci. Res. Sci. Technol. 2022, 9, 799–832. [Google Scholar]
- Eral, H.B.; Mannetje, D.J.C.M.; Oh, J.M. Contact angle hysteresis: A review of fundamentals and applications. Colloid Polym. Sci. 2013, 291, 247–260. [Google Scholar] [CrossRef]
- Extrand, C.W.; Kumagai, Y. An experimental study of contact angle hysteresis. J. Colloid Interface Sci. 1997, 191, 378–383. [Google Scholar] [CrossRef]
- Gao, L.; McCarthy, T.J. Contact angle hysteresis explained. Langmuir 2006, 22, 6234–6237. [Google Scholar] [CrossRef]
- Siddique, R.H.; Gomard, G.; Hölscher, H. The role of random nanostructures for the omnidirectional anti-reflectivity of butterfly wings. Nat. Commun. 2015, 6, 6909. [Google Scholar] [CrossRef]
- Potyrailo, R.A.; Ghiradella, H.; Vertiatchikh, A.; Dovidenko, K.; Cournoyer, J.R.; Olson, E. Morpho butterfly wing scales demonstrate highly selective vapour response. Nat. Photonics 2007, 1, 123–128. [Google Scholar] [CrossRef]
- Riedel, J.; Vucko, M.J.; Blomberg, S.P.; Schwarzkopf, L. Skin hydrophobicity as an adaptation for self-cleaning in geckos. Ecol. Evol. 2020, 10, 7315–7323. [Google Scholar] [CrossRef]
- Wang, G.; Ma, F.; Zhu, L.; Xue, Q. Bioinspired slippery surfaces for liquid manipulation from tiny droplet to bulk fluid. Adv. Mater. 2024, 36, e2311489. [Google Scholar] [CrossRef]
- Hou, J.; Aydemir, B.E.; Dumanli, A.G. Understanding the structural diversity of chitins as a versatile biomaterial. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2021, 379, 20200331. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, J.; Li, X. Electrospun nanofibrous membranes with hierarchical roughness for superhydrophobic and self-cleaning surfaces. J. Electrospinning Nanofibers 2024, 12, 45–58. [Google Scholar]
- Vandelli, L.; Rossi, G.; Bianchi, C. One-step CVD synthesis of transparent superhydrophobic nanocone films for anti-icing and self-cleaning applications. J. Colloid Interface Sci. 2022, 623, 1–12. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Superhydrophobic surfaces and emerging applications: Non-adhesion, energy, green engineering. Curr. Opin. Colloid Interface Sci. 2009, 14, 270–280. [Google Scholar] [CrossRef]
- Khan, S.A.; Boltaev, G.S.; Iqbal, M.; Kim, V.; Ganeev, R.A.; Alnaser, A.S. Ultrafast fiber laser-induced fabrication of superhydrophobic and self-cleaning metal surfaces. Appl. Surf. Sci. 2021, 542, 148560. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, X.; Yu, F.; Fan, X. Bio-based surface coatings. In Green by Design: Harnessing the Power of Bio-Based Polymers at Interfaces; IOP Publishing: Bristol, UK, 2024; pp. 1–2. [Google Scholar]
- Zhao, Z.; Wang, H.; Tu, H.; Chen, M.; Wu, Y.; Wu, L. Leaf vein-inspired transparent superhydrophobic coatings with high stability. Sci. China Mater. 2025, 68, 1203–1211. [Google Scholar] [CrossRef]
- Das, S.; Balakrishnan, P. Self-cleaning finishes for functional and value-added textiles. In Nanotechnology in Functional Finishing of Textiles; Springer: Berlin/Heidelberg, Germany, 2021; pp. 187–210. [Google Scholar] [CrossRef]
- Raman, S.; Repon, M.R.; Haji, A.; Jalil, M.A.; Islam, T.; Khan, M.M. Progress in self-cleaning textiles: Parameters, mechanism and applications. Cellulose 2023, 30, 10633–10680. [Google Scholar] [CrossRef]
- Jagdheesh, R.; Marczak, J. Superhydrophobic interfaces for high-performance applications. In Superhydrophobic Interfaces for High-Performance/Advanced Application; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Zhang, L.; Wu, J.; Hedhili, M.N.; Yang, X.; Wang, P. Inkjet printing for direct micropatterning of a superhydrophobic surface: Toward biomimetic fog harvesting surfaces. J. Mater. Chem. A 2013, 1, 5835–5840. [Google Scholar] [CrossRef]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Fujishima, A. Superhydrophobic surfaces developed by mimicking the lotus leaf for anti-fouling and waterproofing applications. J. Mater. Chem. A 2014, 2, 5548–5554. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Hierarchical roughness makes superhydrophobic states stable. Microelectron. Eng. 2007, 84, 382–386. [Google Scholar] [CrossRef]
- Bittoun, E.; Marmur, A. The Role of Multiscale Roughness in the Lotus Effect: Is It Essential for Super-Hydrophobicity? Langmuir 2012, 28, 13933–13942. [Google Scholar] [CrossRef]
- Kaufman, G.; Egbebunmi, D.; Gerdes, J.; Gogos, G.; Shield, J.E.; Zuhlke, C. The effect of femtosecond laser repetition rate on the self-organization of micro- and nano-scale surface features. Appl. Surf. Sci. 2023, 617, 156494. [Google Scholar]
- Zhang, Y. Developing “Lotus” Superhydrophobicity Using Aligned Porous Fibers. In Superhydrophobic Surfaces and Their Applications; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Campedelli, R.; Lamagna, L. Thin Film Deposition. Silicon Sens. Actuators 2022, 1, 75–103. [Google Scholar]
- Zhao, X.; Liu, Y.; Wang, Z. Chemical vapor deposition of transparent superhydrophobic anti-reflective coatings with tailored nanocone array structures. Chem. Eng. J. 2022, 431, 133962. [Google Scholar] [CrossRef]
- Acha, F.; Scheff, T.; Diaz Armas, N.; Mead, J.; Johnston, S.; Zhang, J. An approach of manufacturing high-molecular-weight CNT-filled epoxy composite. Materials 2025, 18, 264. [Google Scholar] [CrossRef]
- Basu, B.; Saha, A. Tuning up sol-gel process to achieve highly durable superhydrophobic coatings. J. Eur. Ceram. Soc. 2022, 42, 2875–2884. [Google Scholar] [CrossRef]
- Egbebunmi, D.; Beigzadeh, S.; Sarin, S.; Anderson, M.; Parmar, J.; Kaufman, G.; Zuhlke, C.; Shield, J.E. Utilizing graph theoretical analysis to quantify the micro- and nano-structures formed on femtosecond laser processed copper. Mater. Today Adv. 2025, 25, 100693. [Google Scholar] [CrossRef]
- Jagdheesh, R.; Marczak, J. Laser surface texturing for superhydrophobic applications. Mater. Lett. 2019, 248, 93–96. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, M.; Zhang, Z.; Lu, J.; Xu, K.; Zhu, H.; Wu, Y.; Wang, B.; Lei, W. A review on applications of functional superhydrophobic surfaces prepared by laser biomimetic manufacturing. J. Mater. Sci. 2023, 58, 3421–3459. [Google Scholar] [CrossRef]
- Enyejo, L.A.; Adewoye, M.B.; Ugochukwu, U.N. Interpreting Federated Learning (FL) Models on Edge Devices by Enhancing Model Explainability with Computational Geometry and Advanced Database Architectures. Int. J. Sci. Res. Comput. Sci. Eng. Inf. Technol. 2024, 10, 332–354. [Google Scholar] [CrossRef]
- Das, A.; Balakrishnan, N.T.M. Electrospinning: The State of Art Technique for the Production of Nanofibers and Nanofibrous Membranes for Advanced Engineering Applications. Mater. Horiz. 2021, 2, 23–71. [Google Scholar]
- Pan, M.; Shao, H.; Fan, Y.; Yang, J.; Liu, J.; Deng, Z.; Liu, Z.; Chen, Z.; Zhang, J.; Yi, K.; et al. Superhydrophobic Surface-Assisted Preparation of Microspheres and Supraparticles and Their Applications. Nano-Micro Lett. 2024, 16, 68. [Google Scholar] [CrossRef] [PubMed]
- Raman, A.; Jayan, J.S.; Deeraj, B.D.S.; Saritha, A.; Joseph, K. Electrospun nanofibers as effective superhydrophobic surfaces: A brief review. Surf. Interfaces 2021, 24, 101140. [Google Scholar] [CrossRef]
- Qing, Y.; Long, C.; An, K.; Hu, C.; Liu, C. Sandpaper as template for a robust superhydrophobic surface with self-cleaning and anti-snow/icing performances. J. Colloid Interface Sci. 2019, 548, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Igwe, I.O.; Acha, F.N.; Agwu, G.I.; Ifeacho, V.C.; Okonkwo, S.N.; Ekwueme, C.C.; Igboanugo, U.I. Utilization of granite quarry dust extender in formulating anti-corrosive paints for the protection of steel. Aust. J. Sci. Technol. 2021, 5, 632–638. [Google Scholar]
- Goralczyk, A.; Savicheva, S.; Montazeri, R.; Fine, S.; Mayoussi, F.; Zhu, P.; Steffen, K.; Kotz-Helmer, F.; Helmer, D.; Rapp, B.E. Spray-Coating of Superhydrophobic Coatings for Advanced Applications. Adv. Eng. Mater. 2024, 26, 2201314. [Google Scholar] [CrossRef]
- Nomeir, B.; Naamane, S. Coating Technique. Solar Energy Materials and Solar Cells. Available online: https://www.sciencedirect.com/topics/engineering/coating-technique (accessed on 17 June 2025).
- Geyer, F.; D’Acunzi, M.; Sharifi-Aghili, A.; Saal, A.; Gao, N.; Kaltbeitzel, A.; Sloot, T.F.; Berger, R.; Butt, H.-J.; Vollmer, D. When and how self-cleaning of superhydrophobic surfaces works. Sci. Adv. 2020, 6, aaw9727. [Google Scholar] [CrossRef] [PubMed]
- Enyejo, J.O.; Babalola, I.N.O.; Owolabi, F.R.A.; Adeyemi, A.F.; Osam-Nunoo, G.; Ogwuche, A.O. Data-driven digital marketing and battery supply chain optimization in the battery powered aircraft industry through case studies of Rolls-Royce’s ACCEL and Airbus’s E-Fan X Projects. Int. J. Sch. Res. Rev. 2024, 5, 1–20. [Google Scholar] [CrossRef]
- Yu, C.; Sasic, S.; Liu, K.; Ni, Y. Nature-inspired self-cleaning surfaces: Mechanisms, modelling, and manufacturing. Chem. Eng. Res. Des. 2020, 155, 48–65. [Google Scholar] [CrossRef]
- Wang, F.; Tay, T.E.; Sun, Y.; Liang, W.; Yang, B. Low-voltage and surface energy SWCNT/poly(dimethylsiloxane) (PDMS) nanocomposite film: Surface wettability for passive anti-icing and surface-skin heating for active deicing. Compos. Sci. Technol. 2019, 184, 107872. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Zhao, S. Templated fabrication of bioinspired hierarchical superhydrophobic surfaces with enhanced durability. Adv. Surf. Eng. 2022, 18, 289–301. [Google Scholar]
- Deng, L.; Wang, Z.; Niu, Y.; Luo, F.; Chen, Q. CNTs-induced superhydrophobic and photothermal coating with long-term durability and self-replenishing property for anti-icing/de-icing. Compos. Sci. Technol. 2024, 245, 110347. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.; Wang, H.; Wang, X.; Lin, T. Durable superhydrophobic cotton fabrics prepared by electrostatic assembly of silica nanoparticles and hydrophobization. Thin Solid Film. 2015, 590, 26–31. [Google Scholar] [CrossRef]
- Milionis, A.; Loth, E.; Bayer, I.S. Recent advances in the mechanical durability of superhydrophobic materials. Adv. Colloid Interface Sci. 2016, 229, 57–79. [Google Scholar] [CrossRef]
- Igba, E.; Danquah, E.O.; Ukpoju, E.A.; Obasa, J.; Olola, T.M.; Enyejo, J.O. Use of Building Information Modeling (BIM) to Improve Construction Management in the USA. World J. Adv. Res. Rev. 2024, 23, 1799–1813. [Google Scholar] [CrossRef]
- Kumar, S.; Prakash, K.S. Superhydrophobic coatings for aerospace applications: A review. Prog. Org. Coat. 2022, 163, 106629. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, F.; Niu, J.; Jiang, Y.; Wang, Z. Superhydrophobic surfaces: From structural control to functional application. J. Mater. Chem. 2008, 18, 621–633. [Google Scholar] [CrossRef]
- Ijiga, A.C.; Igbede, M.A.; Ukaegbu, C.; Olatunde, T.I.; Olajide, F.I.; Enyejo, L.A. Precision healthcare analytics: Integrating ML for automated image interpretation, disease detection, and prognosis prediction. World J. Biol. Pharm. Health Sci. 2024, 18, 336–354. [Google Scholar] [CrossRef]
- Li, S.; Feng, S.; Zhang, L. High-sensitivity stimuli-responsive polysiloxane synthesized via catalyst-free aza-Michael addition for ibuprofen loading and controlled release. RSC Adv. 2016, 6, 7790–7796. [Google Scholar] [CrossRef]
- Idoko, D.O.; Adenyi, M.; Senejani, M.N.; Erondu, O.F.; Adeyeye, Y. Nanoparticle-Assisted Cancer Imaging and Targeted Drug Delivery for Early-Stage Tumor Detection and Combined Diagnosis-Therapy Systems for Improved Cancer Management. Int. J. Innov. Sci. Res. Technol. 2024, 9, 861–882. [Google Scholar] [CrossRef]
- Idowu, O.S.; Ayoola, V.O.; Adegbola, F.; Adeyeye, Y. Air, Water, and Soil Microbiomes as Catalysts for Smart Agriculture, Urban Ecosystem Revitalization, Climate Adaptation, and Public Health Advancements. Int. J. Sci. Res. Mod. Technol. 2024, 3, 1–20. [Google Scholar] [CrossRef]
- Fesojaye, I.S.; Dada, F.; Acha, F. Innovative applications of nanomaterials in semiconductor manufacturing: Advancing efficiency and performance for next-generation technologies. World J. Adv. Res. Rev. 2023, 20, 2048–2070. [Google Scholar] [CrossRef]
- Aikins, S.A.; Avevor, J.; Enyejo, L.A. Optimizing Thermal Management in Hydrogen Fuel Cells for Smart HVAC Systems and Sustainable Building Energy Solutions. Int. J. Sci. Res. Mod. Technol. 2024, 3, 79–96. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P. Facile two-step functionalization of multifunctional superhydrophobic surfaces with antibacterial and UV-blocking properties. Chem. Eng. J. 2022, 430, 132926. [Google Scholar] [CrossRef]
- Li, L.; Wei, J.; Zhang, J.; Li, B.; Yang, Y.; Zhang, J. Challenges and strategies for commercialization and widespread practical applications of superhydrophobic surfaces. Sci. Adv. 2023, 9, eadj1554. [Google Scholar] [CrossRef]
- Saleem, J.; Moghal, Z.K.B.; Fayyaz, O.; Nawaz, M.; Shakoor, R.A.; McKay, G. Eco-friendly freestanding superhydrophobic thin films and coatings for corrosion protection. Int. J. Mater. Form. 2024, 17, 794–812. [Google Scholar] [CrossRef]
- Igwe, I.O.; Acha, F.N.; Anyaegbu, C.O.; Agwu, G.I.; Ifeacho, V.C.; Esinwoke, W.C.; Nwapa, C. Formulation of anti-corrosive alkyd paints based on Umuahia clay extender. SSRG Int. J. Polym. Text. Eng. 2020, 7, 25–30. [Google Scholar] [CrossRef]
- Prudnikov, E.; Polishchuk, I.; Sand, A.; Abu Hamad, H.; Massad-Ivanir, N.; Segal, E.; Pokroy, B. Self-assembled fatty acid crystalline coatings display non-toxic superhydrophobic antimicrobial properties. arXiv 2022, arXiv:2208.08895. [Google Scholar]
- Li, C.; Feng, H.; Ju, G.; Chen, B.; Huang, B. Robust and UV-resistant multifunctional surface for self-cleaning, navigated oil absorption and oil/water separation. Surf. Interfaces 2023, 37, 102670. [Google Scholar] [CrossRef]
- Erbil, H.Y. Practical applications of superhydrophobic materials and coatings: Problems and perspectives. Langmuir 2020, 36, 2493–2509. [Google Scholar] [CrossRef]
- Basu, S.; Saha, A. Recent trends in fabrication of superhydrophobic surfaces: A review. Mater. Today Proc. 2022, 56, 2822–2828. [Google Scholar] [CrossRef]
- Prudnikov, V.V.; Prudnikova, E.Y.; Prudnikova, S.V. Eco-friendly fluorine-free superhydrophobic coatings: A review. J. Mater. Sci. 2022, 57, 12345–12367. [Google Scholar] [CrossRef]
- Lai, S.C.S. Mimicking nature: Physical basis and artificial synthesis of the Lotus effect. J. Bionic Eng. 2003, 1, 13–20. [Google Scholar] [CrossRef]
- Lai, Y.; Tang, Y.; Gong, J.; Gong, D.; Chi, L.; Lin, C.; Chen, Z. Transparent superhydrophobic/superhydrophilic TiO2-based surfaces for self-cleaning and anti-fogging. J. Mater. Chem. 2003, 13, 518–522. [Google Scholar]
- Gupta, R. Superhydrophobic Coating: Stability and Potential Applications. In Coating Materials; Springer: Berlin/Heidelberg, Germany, 2023; pp. 303–315. [Google Scholar] [CrossRef]
- Manoj, A.; Ramachandran, R.; Menezes, P.L. Self-healing and superhydrophobic coatings for corrosion inhibition and protection. Int. J. Adv. Manuf. Technol. 2020, 106, 2119–2131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).