Abstract
The partition coefficients of seven low molecular weight compounds were tested in different aqueous two-phase systems. The ionic composition of each system included specific salt additives, and it was found that there is a linear relationship between the solute partition coefficients and the presence of different salt additives. The study suggests that the solute structure and the type of ions influence the solute response to the ionic environment. Additionally, it was observed that the solutes’ polar surface area and the solvent-accessible surface area are the essential structural features governing partitioning in aqueous two-phase systems.
1. Introduction
Aqueous two-phase systems (ATPSs) are created when mixtures of two (or more) water-soluble polymers, such as dextran and polyethene glycol (PEG), or a single polymer and a specific salt, or a single polymer and a particular small water-soluble organic molecule, reach specific threshold concentrations in water. The two separate aqueous phases have different polymer compositions and solvent properties [1,2,3,4,5,6,7,8], providing a safe and distinct solvent environment compatible with biological products [1,2,3,4,5,6,7,8,9,10,11,12]. Differences in solute–solvent interactions in the two phases often result in uneven solute distribution, measured by the partition coefficient, K. This coefficient is defined as the ratio between the solute concentrations in the phases. It can detect changes in the solute structure [1,13,14].
Partitioning in aqueous two-phase systems (ATPSs) has proven beneficial for separating and concentrating various substances and a wide range of analytical applications. It has been demonstrated [15] that differences in the 3D structures of closely related proteins can be measured by studying how these proteins partition in four or more ATPSs consisting of the same polymer but different ionic compositions. Researchers have reported that specific protein–ion interactions [16] and protein–protein interactions [17] can be identified by observing changes in partition behavior. Additionally, it has been shown that solute-specific coefficients for biological molecules, which represent their interactions with aqueous environments, can be determined by studying the partitioning of these biomolecules in multiple ATPSs [18,19] with well-characterized solvent properties in the coexisting phases.
Our recent study examined how a group of polar organic compounds interacted with different ATPSs [20]. Our findings [20] revealed that the compounds’ partition behavior is influenced not only by dipole–dipole and hydrogen-bond interactions with the aqueous environment but also, in many cases, by dipole–ion interactions.
One of the exciting aspects of solute behavior in ATPSs is that relatively small amounts of salt additives may significantly affect the solute partitioning [9,10,11]. However, the effects of salt additives on the partitioning of organic compounds in polymer–polymer ATPSs have never been systematically studied.
To better understand how salt affects the distribution of solutes in ATPS (aqueous two-phase systems), we investigated the impact of three different salt additives, Na2SO4, NaCl, and NaClO4−, in the presence of 0.01 M sodium phosphate buffer (NaPB) at pH 7.4. We examined how these salts influenced the behavior of seven different organic compounds in various polymer–polymer ATPSs.
2. Materials and Methods
2.1. Materials
2.1.1. Polymers
Dextran 75 (lot 124339), with a weight-average molecular weight (Mw) of 75,000, was purchased from USB (Cleveland, OH, USA).
Polyethylene glycol 8000 (lot BCBJ3787V), with a Mw of 8000, and polyethylene glycol 4000 (lot BCBD2874), with a Mw of 4000, were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Ucon 50-HB-5100 (lot SJ1955S3D2), with a Mw of 3930, was purchased from Dow-Chemical (Midland, MI, USA).
Ficoll 70 (lot 10085600), with a Mw of 70,000, was purchased from GE Healthcare Biosciences AB (Uppsala, Sweden).
All polymers were used without further purification.
2.1.2. Other Chemicals
Phenol; benzyl alcohol; 2-Phenylethanol; vanillin; 4-nitrophenyl-α-D-glucopyranoside; coumarin (2H-chromen-2-one); and methyl anthranilate were purchased from Sigma-Aldrich (St. Louis, MO, USA). All salts and other chemicals used were of analytical-reagent grade.
2.2. Methods
Partitioning
The solutions of each compound were meticulously prepared in water at concentrations ranging from 1 to 5 mg/mL. Various volumes (e.g., 0, 10, 20, 30, 40, and 50 µL) of a specific compound solution, in conjunction with complementary volumes (e.g., 100, 90, 80, 70, 60, and 50 µL) of water, were meticulously added to a set of identical polymer/buffer/salt mixtures using a Multipette Xstream pipette (Eppendorf, Hamburg, Germany) to achieve a final volume of 1200 μL. The systems underwent a rigorous process of vortexing and centrifugation for 30–60 min at 10,000 rpm in a Minispin centrifuge (Eppendorf) to accelerate phase settling. Subsequently, duplicate aliquots of 20 to 70 µL from both the upper and lower phases were carefully withdrawn using a Multipette Xstream pipette for analysis. These aliquots from both phases were then meticulously diluted with water up to 250 µL in microplate wells. Following moderate shaking at room temperature (23 °C), an optical absorbance measurement at the maximum wavelength of each compound was meticulously taken using a Synergy-2 UV-VIS plate reader (Bio-Tek Instruments, Winooski, VT, USA). The phases of blank systems at corresponding dilutions were meticulously calculated to facilitate comparison.
The partition ratio, denoted as K, represents the ratio of the concentration of a compound in the upper phase to its concentration in the lower phase. To determine the partition ratio for each solute, we calculated the slope of the plot depicting the solute concentration in the upper phase against the solute concentration in the lower phase as a function of different solute concentrations and the fixed composition of the system. Through six independent experiments conducted in duplicate, we consistently obtained an average K value with a deviation below 5% and, in most cases, below 3%.
3. Results and Discussion
Partition coefficients for all compounds examined in all the ATPSs (see Table 1) are presented in Table 2. Analysis of the data in Table 2 shows that all the solutes in all the ATPSs distribute preferentially into the top phase (K > 1).

Table 1.
Polymer compositions a of the phases in the aqueous two-phase systems used for partitioning.

Table 2.
Partition ratios of selected compounds in aqueous two-phase systems (for ATPS compositions see Table 1).
The data in Table 2 indicate that the impact of salt additives on the solute partitioning in a polymer–polymer ATPS depends on the specific solute and salt used and the ATPS system. When 0.1 M Na2SO4 is present in 0.01 M NaPB, the K values for all solutes in each ATPS generally exceed the corresponding K values in the presence of 0.01 M NaPB alone, except for 2-phenylethanol in S2 (dextran-PEG-4000) ATPS. In the presence of NaCl or NaClO4 (in 0.01 M NaPB), the K values for most compounds with the salt additives exceed those observed with 0.01 M NaPB alone, but there are multiple exceptions. For instance, K values for phenol in the presence of 0.15 M NaCl in S3, S5, and S6 ATPSs exceed those in the presence of 0.01 M NaPB, while the opposite trend is observed in S1, S2, and S4 ATPSs. Likewise, for coumarin, K values in the presence of 0.15 M NaClO4 exceed those in the presence of 0.01 M NaPB in almost all ATPS systems except S1 ATPS. Analysis of the data in Table 2 demonstrates differences in the effects of salt on different compounds in the ATPS used.
To examine the general trends in the effects of salt additives on solute partition behavior, we looked at the relationship between the partition coefficients (K values) for all the solutes studied in different ATPSs with and without a specific salt additive. The relationships for the K values in the presence of 0.15 M NaCl and 0.10 M Na2SO4 compared to the K values in 0.01 M NaPb are shown in Figure 1.
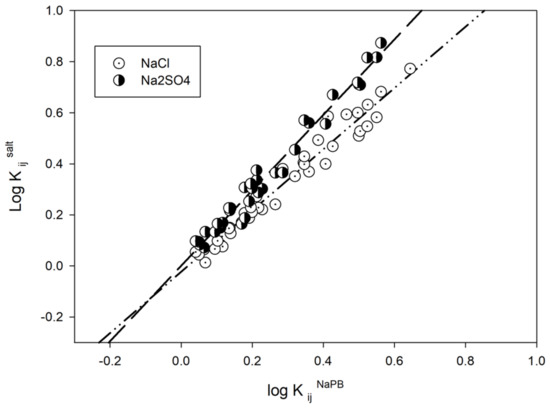
Figure 1.
Partition coefficient K values for all the solutes studied in all ATPSs in logarithmic scale in the presence of 0.15 M NaCl, 0.1 M Na2SO4, both in 0.01 M sodium phosphate buffer (NaPB), pH 7.4, versus the corresponding K values in logarithmic scale for the same solutes in the same ATPSs without salt additive (in 0.01 M sodium phosphate buffer (NaPB), pH 7.4).
For the three salt additives used, the observed relationships may be described as:
where Kijsalt and KijNaPB are partition coefficients for the ith solute in the jth ATPS in the presence and absence of the indicated salt additive, respectively; N is the number of experimental points; r is the correlation coefficient; F is the ratio of variance; and SD is the standard deviation.
Log KijNa2SO4 = 0.00±0.01 + 1.47±0.04log KijNaPB
N = 35, r2 = 0.9760, F = 1343, SD = 0.04
logKijNaCl = −0.02±0.01 + 1.20±0.04logKijNaPB
N = 42, r2 = 0.9642, F = 1076, SD = 0.04
logKijNaClO4 = 0.01±0.01 + 1.09±0.04logKijNaPB
N = 42, r2 = 0.9459, F = 700, SD = 0.05
It has been demonstrated [21] that the impact of different salts (Na2SO4, NaCl, NaClO4, and NaSCN) on the solubilities of 17 polar organic compounds in aqueous solutions, as measured by the corresponding Setschenow constant values, show a linear interrelation for all the polar compounds studied. Similar relationships were observed for partition coefficients of nonionic organic compounds in aqueous polyethene glycol–sodium sulfate two-phase systems in the presence of various salt additives [22] and for the effects of different salts on the optical rotation of amino acids and glucose [23,24]. It was suggested [21] that all these observed effects result from the compounds’ responses to a specific ionic environment and its interaction with the compounds through the formation of direct or solvent-separated ionic pairs. The response is specific to each compound, and its strength is determined by the compound’s structure and the type (and concentration) of the ions causing the response.
We analyzed the data presented in Table 2 to explore if the partition coefficients of compounds in all ATPSs used in the presence of different salt additives are interrelated. The results obtained are illustrated graphically in Figure 2 and Figure 3 and may be described as:
where KijNaPB, Kij0.15M NaCl, and Kij0.1M Na2SO4 are partition coefficients for the same jth compound in the ith ATPS in the presence of 0.01 M NaPB, 0.15 M NaCl in 0.01 M NaPB, and 0.1 M Na2SO4 in 0.01 M NaPB, respectively; all the other parameters are as defined above, and:
where Kij0.15M NaClO4 is the partition coefficient for the same jth compound in the ith ATPS in the presence of 0.15 M NaClO4 in 0.01 M NaPB; all the other parameters are as defined above.
logKijNaPB = 0.09±0.07 + 0.38±0.09logKij0.15M NaCl + 0.38±0.07logKij0.1M Na2SO4
N = 35; R2 = 0.9840; SD = 0.02; F = 981
logKijNaPB = 0.011±0.007 + 0.48±0.05logKij0.15M NaCl + 0.37±0.06logKij0.15M NaClO4
N = 42; R2 = 0.9826; SD = 0.02; F = 1102
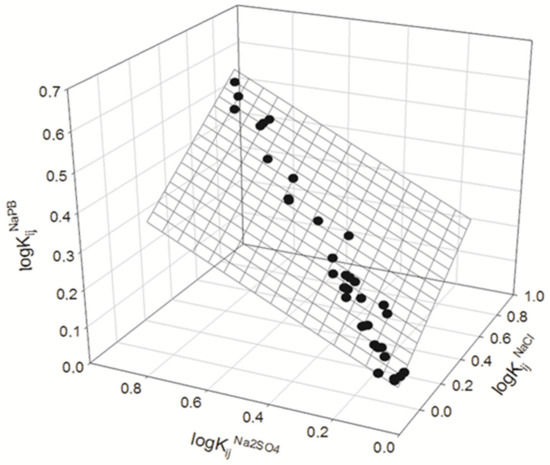
Figure 2.
Interrelationship between the logarithms of partition coefficients for organic compounds in ATPSs in the presence of NaPB, logarithms of partition coefficients of the same compounds in the same ATPS in the presence of 0.10 M Na2SO4-NaPB, and logarithms of partition coefficients for the same compounds in the same ATPS in the presence of 0.15 M NaCl-NaPB. NaPB—0.01 M sodium phosphate buffer, pH 7.4.
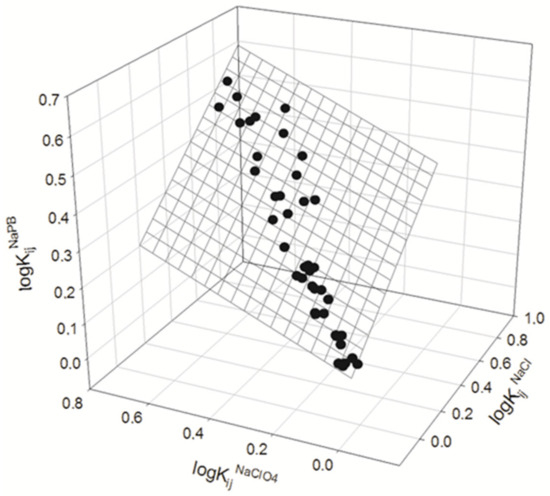
Figure 3.
Depicts the relationship between the logarithms of partition coefficients for organic compounds in ATPSs. The figure also shows the logarithms of partition coefficients for the same compounds in the presence of 0.15 M NaClO4-NaPB and 0.15 M NaCl-NaPB, along with the presence of NaPB (0.01 M sodium phosphate buffer, pH 7.4).
The linear relationships described above can be understood in the context of the salting-out and salting-in effects observed for various salts on polar organic compounds. This can be explained by considering the changes in the partitioning coefficients of solutes in the presence of different salt additives as specific responses of the solutes to variations in their ionic environment in the solution. These responses are influenced by the interactions between the solute, ions, and water, with the strength of these interactions determined by the properties of the ions and the unique characteristics of the solute structures.
It is important to note that the examined compounds exhibit specific responses to the salt additives used. To assess the response of each compound to a particular salt additive, we analyzed the relationships between the logarithms of the compound partition coefficients in all ATPSs (aqueous two-phase systems) in the presence of a given salt additive and those for the same compound in salt additive-free ATPSs. Figure 4 provides illustrative examples of these relationships, which can be described by the following equation:
where Kijsalt is partition coefficients for the jth compound in the ith ATPS in the presence of a given salt additive; KijNaPB is defined above; aj and bj are coefficients.
logKijsalt = aj + bjlogKijNaPB
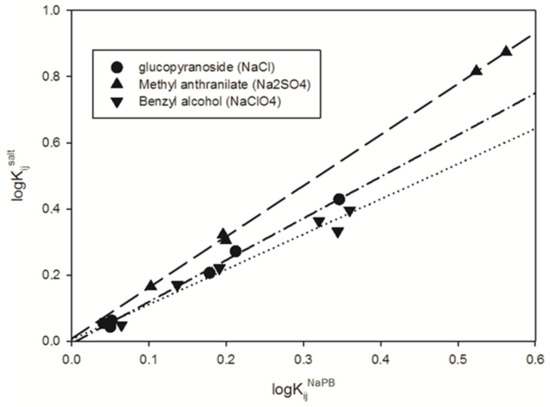
Figure 4.
Partition coefficient K values for methyl anthranilate, 4-nitrophenyl-α-D-glucopyranoside, and benzyl alcohol in logarithmic scale in the presence of 0.1 M Na2SO4-NaPB, 0.15 M NaCl-NaPB, and 0.15 M NaClO4-NaPB, in all the ATPS studied, versus the corresponding K values, in logarithmic scale, for the same solutes in the same ATPSs in the presence of NaPB. NaPB—0.01 M sodium phosphate buffer, pH 7.4.
The aj and bj coefficient values calculated from data in Table 2 with Equation (3) are listed in Table 3.

Table 3.
Coefficients ai and bi in Equation (3) with different solutes partitioned in ATPSs with phenol used as a reference solute.
The bj values represent the response of the partition behavior of a given compound to the presence of a particular salt additive averaged over all the different ATPSs employed. These averaged responses are different for the compounds examined. If the assumption [21] that the solute structure governs the solute response to its ionic environment is correct, we may view the set of the bj values characterizing the responses of the jth solute to different salt additives as a representation of the jth compound’s responsiveness to different ionic environments or as that of the compound structure. It should be noted that the responsiveness to three different salt additives used here provides only partial, incomplete information for each compound. This information, however, may be sufficient for the analysis of structural features important for the observed effects, given that essentially all the compounds examined except vanillin are non-ionizable.
To reduce the set of ionic composition responsiveness descriptions to a single numerical value, we used the approach suggested earlier [15,16] for analyzing differences between proteins’ 3D structures. The approach [15,16] is based on considering the set of bj values determined for the jth compound in the presence of different salt additives as a signature of ionic responsiveness for the compound. Phenol was selected as a reference compound.
The differences between the ionic responsiveness of the compounds studied and phenol may be calculated. The bj values for all the ionic compositions examined are normalized against the bo values for the reference compound (phenol), and the normalized Euclidian distances between the normalized ionic responsiveness signatures for each compound and phenol can be evaluated. The distance, d, is calculated as:
where di,o is the distance between the ionic responsiveness signature of jth compound to that of phenol, and bji and boi are the b coefficients for compound j and the reference.
The distances between the ionic responsiveness for each compound and phenol were calculated by Equation (4) using data in Table 3. The computed distance values, dj,o, are presented in Table 4 together with various structural features of the compounds calculated using ChemAxon software at http://www.chemspider.com (accessed on 23 January 2024).

Table 4.
Distances, di,0 in Equation (4) and structural features for the studied compounds, taking phenol as a reference. (PSA—polar surface area; SASA—solvent-accessible surface area; MP—molecular polarizability; MR—molecular refractivity). Data calculated using ChemAxon 23.16.0 software at http://www.chemspider.com (accessed on 23 January 2024).
Analysis of the structural descriptors listed in Table 4 shows that there is a linear regression illustrated graphically in Figure 5 and described as:
where PSAj is the polar surface area (in Å2) and SASA is the solvent-accessible surface area (in Å2) for the jth compound; all the other parameters are as defined above. Statistical significance for all the parameters is high, p < 0.002.
di,o = −0.88±0.07 − 0.0102±0.0008PSAj + 0.0065±0.0005SASAj
N = 6; R2 = 0.9865; SD = 0.014; F = 109.3
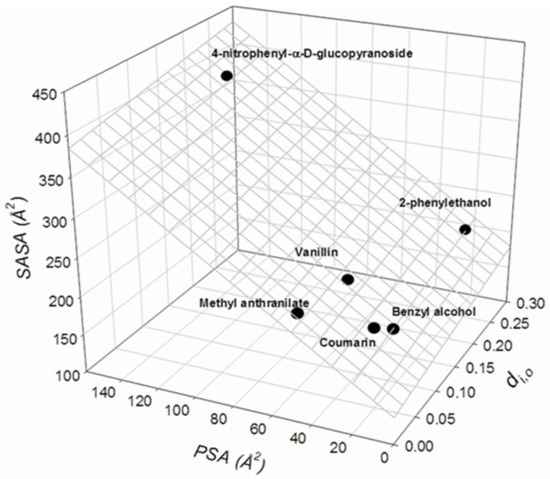
Figure 5.
Interrelationship between the calculated distance values, dj,o, from Equation (4) for the studied compounds using phenol as a reference and the structural features of the compounds, PSA (polar surface area) and SASA (solvent-accessible surface area).
Given the limited number of compounds examined, the linear regression described by Equation (5) should be considered a trend and not a reliable relationship. It indicates that the differences between the ionic responsiveness of mostly non-ionizable compounds are governed by the areas of the total and polar surface of the molecule.
The number of structural descriptors analyzed here is admittedly limited. Additional studies are necessary to understand better the factors that influence the response of nonionic organic compounds to their ionic environment. These studies are currently underway in our laboratories.
4. Conclusions
In this study, the impact of three salt additives, Na2SO4, NaCl, and NaClO4, in sodium phosphate buffer (NaPB) at pH 7.4 on the distribution of seven different organic compounds in various polymer–polymer ATPSs was investigated. The findings suggest that the salt additives significantly influence the solute partition behavior within ATPSs, indicating that the ionic environment influences the solute’s response. Interestingly, a linear relationship was observed between the responses of all the compounds to different salt additives. Furthermore, the study estimated the ionic responsiveness of the compounds and calculated the differences between each compound’s responsiveness and that of phenol. These differences were found to be associated with the polar surface area and solvent-accessible surface area of the solute molecules examined in this study.
Author Contributions
Conceptualization, P.P.M. and B.Y.Z.; methodology, M.C.; software, B.Y.Z.; validation, P.P.M., V.N.U. and B.Y.Z.; formal analysis, P.P.M., V.N.U. and B.Y.Z.; investigation, M.C. and P.P.M.; resources, P.P.M.; data curation, P.P.M., V.N.U. and B.Y.Z.; writing—original draft preparation, P.P.M., V.N.U. and B.Y.Z.; writing—review and editing, M.C., P.P.M., V.N.U. and B.Y.Z.; visualization, P.P.M., V.N.U. and B.Y.Z.; supervision, P.P.M. and B.Y.Z.; project administration, P.P.M. and B.Y.Z.; funding acquisition, P.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FCT—Fundação para a Ciência e a Tecnologia, developed within the scope of the CICECO-Aveiro Institute of Materials project, UIDB/50011/2020, UIDP/50011/2020, and LA/P/0006/2020. It is funded by national funds (O.E.) through FCT—Fundação para a Ciência e a Tecnologia, I.P., in the scope of the framework contract foreseen in the numbers 4, 5, and 6 of article 23 of the Decree-Law 57/2016, of 29 August, changed by Law 57/2017, of 19 July.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
This work was developed within the scope of the CICECO-Aveiro Institute of Materials project, UIDB/50011/2020, UIDP/50011/2020, and LA/P/0006/2020. It is funded by national funds (O.E.) through FCT—Fundação para a Ciência e a Tecnologia, I.P., in the scope of the framework contract foreseen in the numbers 4, 5, and 6 of article 23 of the Decree-Law 57/2016, of 29 August, changed by Law 57/2017, of 19 July.
Conflicts of Interest
Author Boris Y. Zaslavsky was employed by the company Cleveland Diagnostics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zaslavsky, B.Y. Aqueous Two-Phase Partitioning: Physical Chemistry and Bioanalytical Applications; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Titus, A.R.; Herron, P.; Streletzky, K.A.; Madeira, P.P.; Uversky, V.N.; Zaslavsky, B.Y. Effect of trimethylamine-N-oxide on the phase separation of aqueous polyethylene glycol-600-Dextran-75 two-phase systems. Phys. Chem. Chem. Phys. 2024, 26, 10546–10556. [Google Scholar] [CrossRef] [PubMed]
- Titus, A.R.; Madeira, P.P.; Ferreira, L.A.; Chernyak, V.Y.; Uversky, V.N.; Zaslavsky, B.Y. Mechanism of Phase Separation in Aqueous Two-Phase Systems. Int. J. Mol. Sci. 2022, 23, 14366. [Google Scholar] [CrossRef] [PubMed]
- Moody, M.L.; Willauer, H.D.; Griffin, S.T.; Huddleston, J.G.; Rogers, R.D. Solvent Property Characterization of Poly(ethylene glycol)/Dextran Aqueous Biphasic Systems Using the Free Energy of Transfer of a Methylene Group and a Linear Solvation Energy Relationship. Ind. Eng. Chem. Res. 2005, 44, 3749–3760. [Google Scholar] [CrossRef]
- Willauer, H.D.; Huddleston, J.G.; Rogers, R.D. Solvent Properties of Aqueous Biphasic Systems Composed of Polyethylene Glycol and Salt Characterized by the Free Energy of Transfer of a Methylene Group between the Phases and by a Linear Solvation Energy Relationship. Ind. Eng. Chem. Res. 2002, 41, 2591–2601. [Google Scholar] [CrossRef]
- Albertsson, P.A. Partition of Cell Particles and Macromolecules, 3rd ed.; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Walter, H.; Brooks, D.E.; Fisher, D. Partitioning in Aqueous Two-Phase Systems: Theory, Methods, Use, and Applications to Biotechnology; Academic Press: Orlando, FL, USA, 1985. [Google Scholar]
- Rosa, P.A.J.; Azevedo, A.M.; Sommerfeld, S.; Bäcker, W.; Aires-Barros, M.R. Aqueous two-phase extraction as a platform in the biomanufacturing industry: Economical and environmental sustainability. Biotechnol. Adv. 2011, 29, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Andrews, B.A.; Nielsen, S.; Asenjo, J.A. Partitioning and purification of monoclonal antibodies in aqueous two-phase systems. Bioseparation 1996, 6, 303–313. [Google Scholar] [PubMed]
- Rosa, P.A.J.; Azevedo, A.M.; Sommerfeld, S.; Mutter, M.; Aires-Barros, M.R.; Bäcker, W. Application of aqueous two-phase systems to antibody purification: A multi-stage approach. J. Biotechnol. 2009, 139, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Oelmeier, S.A.; Dismer, F.; Hubbuch, J. Application of an aqueous two-phase systems high-throughput screening method to evaluate mAb HCP separation. Biotechnol. Bioeng. 2011, 108, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Rito-Palomares, M. Practical application of aqueous two-phase partition to process development for the recovery of biological products. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 807, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zaslavsky, B.Y.; Uversky, V.N.; Chait, A. Analytical applications of partitioning in aqueous two-phase systems: Exploring protein structural changes and protein–partner interactions in vitro and in vivo by solvent interaction analysis method. Biochim. Biophys. Acta 2016, 1864, 622–644. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; Zaslavsky, B.Y. Characterization of Molecules. U.S. Patent US-8211714-B2, 3 July 2012. [Google Scholar]
- Zaslavsky, A.; Madeira, P.; Breydo, L.; Uversky, V.N.; Chait, A.; Zaslavsky, B. High throughput characterization of structural differences between closely related proteins in solution. Biochim. Biophys. Acta 2013, 1834, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; Madeira, P.P.; Mikheeva, L.; Uversky, V.N.; Zaslavsky, B. Effect of salt additives on protein partition in polyethylene glycol–sodium sulfate aqueous two-phase systems. Biochim. Et Biophys. Acta Proteins Proteom. 2013, 1834, 2859–2866. [Google Scholar] [CrossRef] [PubMed]
- Stovsky, M.; Ponsky, L.; Vourganti, S.; Stuhldreher, P.; Siroky, M.B.; Kipnis, V.; Fedotoff, O.; Mikheeva, L.; Zaslavsky, B.; Chait, A.; et al. Prostate-specific Antigen/Solvent Interaction Analysis: A Preliminary Evaluation of a New Assay Concept for Detecting Prostate Cancer Using Urinary Samples. Urology 2011, 78, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Madeira, P.P.; Reis, C.A.; Rodrigues, A.E.; Mikheeva, L.M.; Chait, A.; Zaslavsky, B.Y. Solvent properties governing protein partitioning in polymer/polymer aqueous two-phase systems. J. Chromatogr. A 2011, 1218, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Madeira, P.P.; Reis, C.A.; Rodrigues, A.E.; Mikheeva, L.M.; Zaslavsky, B.Y. Solvent Properties Governing Solute Partitioning in Polymer/Polymer Aqueous Two-Phase Systems: Nonionic Compounds. J. Phys. Chem. B 2010, 114, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Madeira, P.P.; Bessa, A.; Teixeira, M.A.; Álvares-Ribeiro, L.; Aires-Barros, M.R.; Rodrigues, A.E.; Zaslavsky, B.Y. Study of organic compounds–water interactions by partition in aqueous two-phase systems. J. Chromatogr. A 2013, 1322, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.A.; Chervenak, A.; Placko, S.; Kestranek, A.; Madeira, P.P.; Zaslavsky, B.Y. Responses of polar organic compounds to different ionic environments in aqueous media are interrelated. Phys. Chem. Chem. Phys. 2014, 16, 23347–23354. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.A.; Teixeira, J.A.; Mikheeva, L.M.; Chait, A.; Zaslavsky, B.Y. Effect of salt additives on partition of nonionic solutes in aqueous PEG–sodium sulfate two-phase system. J. Chromatogr. A 2011, 1218, 5031–5039. [Google Scholar] [CrossRef] [PubMed]
- Lo Nostro, P.; Ninham, B.W.; Milani, S.; Fratoni, L.; Baglioni, P. Specific anion effects on the optical rotation of glucoseand serine. Biopolymers 2006, 81, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Lo Nostro, P.; Lagi, M.; Ninham, B.W.; Baglioni, P. Specific Anion Effects on the Optical Rotation of α-Amino Acids. J. Phys. Chem. B 2007, 111, 10510–10519. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).