Abstract
Maize is one of the globally most important cereal crops used for food, feed and fuel. It requires optimum soil nutrients such as Nitrogen (N), Phosphorus (P), and Potassium (K) for proper growth and development as well as for tolerance to biotic and other abiotic stresses. Yield potentials are not met under suboptimal soil fertility. One of the innovations that can reduce environmental impacts of continuous fertilization and lower the cost of maize production under low soil nutrient conditions is the development and use of tolerant cultivars. This paper provides spotlights on the following: (1) morphology and physiology of root and shoot systems; (2) genetics and genomics; and (3) transcriptome, proteome, and metabolome profiles, to elucidate maize tolerance to low amounts of soil nutrients, N, P, and K. Maize cultivars having deeper rooting structure, more lateral roots, dense roots, and high root exudates are more tolerant to N, P, and K limited conditions. Cultivars that are tolerant to N, P, and K stress (low) have high nutrient use efficiency, good photosynthetic and translocation activity that support high aboveground shoot weight under suboptimal N, P, and K conditions. Maize tolerance to N, P, and K stress (low) is quantitative, and mainly controlled by additive genes. Maize cultivar development and dissemination programs can exploit the mechanisms highlighted in this review.
1. Introduction
Maize (Zea mays L.) is an economically important crop that can contribute to eradication of poverty and hunger in most developing countries. It is grown worldwide. Soil nutrients such as nitrogen (N), phosphorus (P), and potassium (K) are major mineral elements required by maize for effective growth and development [1,2,3]. Nitrogen is required for protein synthesis and forms an essential component of chlorophyll, which plays an important role in photosynthesis [4]. Many plants, including maize, are very sensitive to phosphorus availability since they require it in considerable amounts for their physiological activities [5,6,7]. Indeed, P is the second-largest nutrient demanded by maize plants with an immense impact on the growth and yield of the crop [8,9]. P is considered as one of the crucial nutrients contributing largely to crop yield and quality. It plays a key role in many physiological processes, vital components in osmoregulation, enzyme activation in various metabolic pathways, photosynthesis, and the transport of assimilates [10]. K is the most abundant cation in plant tissues [11]. Since these mineral elements are essential for plant growth and development, crop growers always ensure to supply plants with these additional mineral elements through fertilizer application because the soil is not able to provide all the required amounts plants need to grow well [12,13]. Overfertilization can result in excess soil nutrients which have negative impacts on the ecosystem. Sustainable food production at a reduced cost and environmental safety have necessitated the development and dissemination of maize cultivars that are tolerant to low amounts of soil N, P, and K.
In recent years, the advent of molecular markers such as single-sequence repeats (SSRs) and single-nucleotide polymorphisms (SNPs) and next-generation sequencing technologies have revolutionized the study of molecular mechanisms of many agronomically important traits in maize and other crops. Genetic markers, RNA, proteome and metabolome technologies are used to dissect the genetic architecture and molecular pathways of traits related to maize tolerance to N, P, and K deficiency [14,15,16].
To improve and select maize genotypes that are resilient to low N, P, and K availability, there is the need to understand the physiological and molecular mechanisms for the traits associated with this phenomenon. This review provides a spotlight on maize morphology, physiology, genetics and molecular mechanisms for tolerance to low N, P, and K stress in maize.
2. Maize Morphology and Physiology for Tolerance to Low Nitrogen (N), Phosphorus (P), Potassium (K)
2.1. Maize Shoot Morphology and Physiology for Tolerance to Low Nitrogen (N)
Under low N conditions, maize plants induce N translocation mainly from the older to the younger leaves and sometimes from vegetative to generative organs. This phenomenon of N retranslocation may result in chlorosis in older leaves [17].
Maize which is a C4 plant can increase the amount of CO2 at the site where Rubisco activity occurs, consequently investing much less N to produce Rubisco protein, which renders the crop very efficient for carbon assimilation [18,19]. This phenomenon could be one of the possible reasons why the crop is able to generate some yield in situations where the optimal N availability for crop growth and development is not met [20]. Moreover, maize plants have an adaptation mechanism under field conditions to ensure a homeostatic N concentration in the leaves, which contributes to a reduced leaf expansion during the seedling stage of growth. However, at the anthesis and grain filling stages, the plant is able to retranslocate N in the vegetative organs into the ears [17].
It has been established that an N-efficient cultivar has the potential to produce a higher yield under low N conditions compared to cultivars which are less efficient in N use [17]. Fluorescence measurements associated with photosystems have been found to be intact under low N conditions; however, a low photosynthetic rate has been reported under low N maize growing conditions [21].
It is important to mention that most of the N amassed in the leaf is associated with Rubisco content which, in return, contributes to the photosynthetic activity by plant mesophyll tissues. In wheat, for instance, studies have shown that almost 75% of N in leaves is used for photosynthetic processes which is controlled by the Rubisco enzyme [1].
In N-deficient conditions, a decrease in the quantum photosystem II could possibly be a mechanism in maize plants to downregulate the photosynthetic electron transport to bring the production of ATP and NADPH into equilibrium with a decreased CO2 assimilation ability for N-deficient maize plants [21]. Their study further suggested that the mechanism adopted was facilitated by a reduction in the absorption of light energy associated with a decrease in chlorophyll content.
In some cereals such as barley, maize, and wheat, the flag leaf senescence has a direct influence on the grain N content. The delay in leaf senescence is found to be associated with a high yield since it ensures prolonged photosynthesis and continuous grain filling [22,23]. Despite the positive outcome of delayed senescence of the flag leaf on yield performance in maize and other cereals, Masclaux-Daubresse et al. [24] have demonstrated that a delayed leaf senescence has the potential to decrease grain protein content due to N remobilization efficiency.
2.2. Morphological and Physiological Mechanisms Employed by the Maize Shoot System during Phosphorus (P) Deficiency
In agricultural cropping systems, P is considered the second most limiting nutrient after N for crop growth and development, which has a huge impact on global food security [6]. In essence, the maize plant has some forms of adaptation mechanisms including morphological and physiological means to thrive under low P conditions. In P-deficient conditions, the maize plant shows purple colorations, especially around the leaf edges and tips, increased fine root growth, with reduced growth and yield performance. Yao et al. [25] observed that leaf area and aboveground shoot weight decline significantly under P-deficient conditions for low P-tolerant maize plants. Conversely, this growth performance was different for the root growth of the crop. This could possibly mean that in P-deficient conditions, photo assimilates are partitioned more to the root zone of the plant. Nonetheless, Rodriguez et al. [26] have revealed that the supply of carbohydrate to growing tissues of P-deficient plants does not limit growth. Similar morphological effects have been observed with white clover (Trifolium repens L.), where leaf area per plant was smaller under P-deprived conditions, resulting further in a reduction in whole-plant photosynthesis [27].
A study has shown that under P-deficient conditions, some maize landraces generally have low protein content, however, with an increase in proline content [25]. Various studies have demonstrated that under certain stress conditions such as drought, soil salinity, nutrient deficiency such as P deficiency, plants trigger the production of proline serving as an adaptor molecule in order to inure to the effect of the stress [28,29,30].
Wen et al. [7] made a remarkable observation in maize where an increased shoot P concentration resulted in a decreased root morphological response, which subsequently contributed to an improvement in root exudation. Their research findings further explained that maize plants may trigger large root morphological plasticity partly due to varying P supply in the soils, which may have a direct influence on shoot P amount which, in turn, impact on the various morphological responses by the crop.
In an attempt to ascertain the influence of various P concentrations applied on growth of maize, Plenet et al. [31] observed no influence of P deficiency on the final number of leaves developed despite the effect of a reduced leaf elongation rate found with maize crops growing under P-deficient conditions. Significantly longer cells characterized maize leaves for all positions under low P conditions except at the end of the growth zone when compared to maize plant with adequate P [32]. However, their study further indicated that cell division at the shorter zone of the epidermal layer coupled with lower cell production rates along the length of most leaves was the regulatory mechanism that led to the reduction in cell production and subsequently leaf elongation rates in P-deficient maize plants. In contrast to these findings, Plenet et al. [31] pointed out in their study that it is unclear by which mechanism P deficiency affects leaf growth in maize.
2.3. Morphological and Physiological Mechanisms of Maize Growth under Low Potassium (K) Conditions
Plants in diverse ways have elicited both morphological and physiological responses to K deficiency. Thornburg et al. [33] reported the effect of K+ deficiency on the growth and development of wheat seedlings. In fact, their research demonstrated the influence of K+ deficiency on microRNAs (miRNAs) alteration. Based on the outcome of this significant finding, they envisage that manipulating miRNAs and their targets could be a bastion to enhance fertilizer efficiency and subsequently increase biomass production and crop yield. Maize plants subjected to K deficiency were able to mobilize higher concentrations of Ca, Mg, and Na ions into the plant tissue than maize plants growing under optimal K conditions [11]. Interestingly, Jordan-Meille and Pellerin [11] further revealed that, despite a higher concentration of Ca, Mg, and Na uptake by K-deprived maize plants, this did not completely compensate for the reduced molarity due to the lower K content. Field experiments revealed no differences between physiological maturity of maize subjected to foliar K spray and that of the control [34].
Luxurious growth in maize has been attributed to an increase in N and P uptake, which is because of the application of K, and further delaying the physiological maturity [35]. Various morphological responses among various varieties of maize to K amount has been reported with some varieties showing a tolerance to low K [36]. Essentially, there have been findings where the application of K has contributed to the alleviation of drought susceptibility among various maize hybrids [37]. It is imperative to state that the morphological and physiological functions of maize in response to K deficiency are more centred on the root system [7,38], rather than the aboveground plant tissues. This is obvious since the root anchors the plant in any growth substrate and serves as the primary point of K uptake.
2.4. Root Morphology and Physiology for Tolerance to Low Nitrogen (N), Phosphorus (P), Potassium (K)
Roots play a vital role in resources such as mineral and water mobilization for plant physiological and morphological development from seedling development to harvest in both stress and optimal growing seasons and conditions. These functions of the root are accomplished through traits such as root architecture (vertical, horizontal, and cluster), root length (long or short), root hairs (fine and dense), and root exudates, which are influenced by crop species, genetic make-up of the plant, soil nutrients and properties, and environmental stress [39,40]. This section focuses on the role of maize root morphology and physiology on N, P, and K uptake with a focus on their properties and how the maize root traits could maximize their accessibility and utilization, especially under stress (low N, P, and K levels).
N is the number one nutrient required by maize but it is a very mobile mineral and easily leached into deeper soil profiles depending on the N-form. Nitrate forms of N are leached easily due to the negative charge, especially in sandy soils, while ammonium forms tend to leach less due to the positive charge. Maize varieties with a rapid root growth capability have the potential to reduce nutrients leaching from applied N at planting. Also, a deeper rooting structure (Figure 1c) would maximize and benefit from the leached nitrate from previous crop and season residues more than maize with more lateral roots (Figure 1d), which would depend mostly on the in-season N applications. To enhance N use efficiencies, breeding should target development of early rooting with both lateral and deep root systems that would help the plants to effectively take up N and increase its sustainable use.
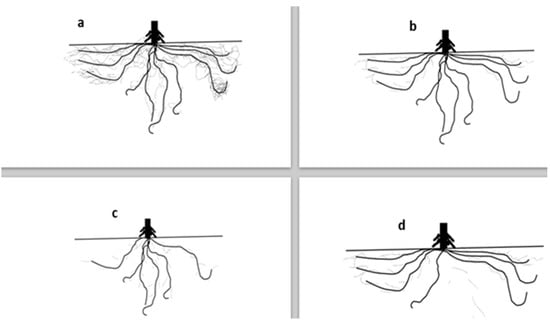
Figure 1.
Maize roots with different morphological and physiological architecture; (a) maize roots with good root hair, vertical and horizontal architecture; (b) maize roots with good vertical and horizontal root architecture; (c) maize roots with good vertical root architecture; (d) maize roots with good horizontal root architecture (image prepared by Dr. Isaac K. Mpanga).
P is the second most important mineral for plant growth and development; however, its availability is mostly limited to the topsoil and in a pH range of 6 to 7 with less mobility in the soil. The implication is that maize plants with more lateral and dense rooting systems (Figure 1a,b) and exudates would optimize P access and uptake by growing into P spots with its lateral roots and fine root hairs compared to deep roots and less root hairs (Figure 1c) with less exudates [7]. According to Neumann and Martinoia [41], dense and cluster roots help in P availability to plants by increasing the exploration surface area, intense root exudations, and microbial attractions. Both the ammonium form of N and plant growth promoting microbial inoculations were shown to induce fine root growth, organic acids release that could help reduce rhizosphere pH, and nutrient uptake in maize [42,43,44,45,46,47]. Breeding programs could help increase P uptake and use efficiencies in maize if focused on traits such as good lateral and fine roots hairs. It must be noted that breeding for only lateral roots could negatively affect yield in drought locations and lodging in areas with heavy winds, hence, deep rooting traits should be added.
K is also more abundant in the soil than N and P, especially in the topsoil and as in P and both lateral and deep rooting traits of maize would help access it more efficiently. Root exudates from the plants are signals used by maize plants to recruit beneficial soil microbes that support mineral availability, especially sparingly soluble P in alkaline soils [41,42,46], disease and other stress factor defense mechanisms. Maize plants with high mucilage and other low molecular compounds as a trait enhance their adaptability to low mineral soils and support their tolerance to other stress factors [7,40].
To enhance nutrient uptake under low nutrients availability stress conditions in the soil, breeding programs should target maize with good rooting architecture (Figure 1a) that would maximize both deep and topsoil layers available nutrients and other resources, such as water.
3. Genetics and Genomic Components of Maize for Tolerance to Low Soil Nitrogen (N), Phosphorus (P), and Potassium (K)
Various traits relating to tolerance to low N, P, and K in maize have been studied over the past years at the genetic and genomic levels (Table 1). Classical and molecular genetics show that the inheritance of tolerance to low N, P, and K in maize can be additive or non-additive (dominance, and epistasis interaction). Some traits (e.g., grain yield and plant height) associated with N, P, and K stress tolerance may be influenced by genotype-environment interaction [48,49]. By classical genetic analyses such as general combining ability (GCA) and specific combining ability (SCA), both additive and non-additive genetic control for maize grain yield and other agronomic traits under both low N and optimum N conditions were reported [48,50,51]. Similarly, Liu et al. [52] found additive and non-additive genetic effects for traits associated with tolerance to low P. In previous studies, heritability of traits analyzed for tolerance to low soil nutrient (stress) ranged from 0.12 to 0.95, depending on the trait and type of soil nutrient (Table 1). This suggests that there are genetic components for tolerance to N, P, and K deficiency that can be harnessed through selection. Wang et al. [53] found high correlation among traits analyzed for tolerance to low P at the seedling stage of maize.

Table 1.
Genetic and genomic parameters for traits associated with maize tolerance to suboptimal soil nitrogen, phosphorus and potassium conditions.
Tolerance of maize to suboptimal N, P, and K stress is highly polygenic, controlled by several loci (Table 1). By genomic analysis such as linkage or quantitative trait loci (QTL) mapping and genome-wide association studies (GWAS), a multitude of QTLs or SNPs have been identified to decipher the mechanisms of tolerance to low N, K, and P (Table 1). Because of the quantitative nature of inheritance of tolerance to the low soil nutrient-related traits, genomic prediction (GP), which involves predicting phenotypes of untested but genotyped lines, based on a model trained on genotyped and phenotyped materials, is applied to exploit additive genes to improve maize for resilience to low N, P, and K. For example, Ertiro et al. [15] used GP to estimate breeding values for maize traits relating to low-N stress tolerance and found prediction accuracies ranging from 0.24 to 0.67. Xu et al. [54] also used five genomic selection models to predict accuracy of selecting maize lines for low-P stress tolerance in maize natural populations from temperate, tropical and subtropical regions.
4. Genes, Proteins, and Metabolites Associated with Maize Response to Low Nitrogen (N), Phosphorus (P), and Potassium (K)
Genes, proteins, and metabolites that regulate the response of maize to low soil nutrients are elucidated through transcriptional, proteomic, and metabolomic profiling. By GWAS, candidate genes (CGs) associated with low N, P, and K tolerance can be identified. Ertiro et al. [15] reported more than 100 CGs for grain yield and other agronomic traits under low-N and optimal-N conditions in tropical maize. A proteomic analysis of two contrasting maize hybrids at the 12 leaf stage showed that lignin biosynthesis, ubiquitin-mediated proteolysis, and stress defense proteins were important for N-stress (low) tolerance [57]. Gene ZmTGA was found to respond to low-N stress [57]. Singh et al. [16] reported key genes involved in maize adaptation to N stress using comparative transcriptome analysis of root and shoot tissues. In a tolerant genotype, key genes such as high-affinity nitrate transporter 2.2 and 2.5 (involved in N uptake); glutamine synthetase and asparagine synthetase (involved in N assimilation and metabolism); SOD and POX (involved in redox homeostasis); MYB36 and AP2-EREBP (involved in transcription factors) were highly expressed. Most of the deferentially expressed genes reported were placed into metabolic pathways, biosynthesis of secondary metabolites, signal transduction, amino acid metabolism, N-assimilation and metabolism, and starch metabolism [16].
For low P tolerance in maize, over 200 CGs have been reported in previous GWAS studies [14,53,54]. These CGs reported for low P tolerance were involved in major pathways such as transcriptional regulation, reactive oxygen scavenging, hormone regulation, and remodelling of cell walls [14,53,54]. In a comparative transcriptomic profiling, about 300 and 600 genes were upregulated in maize leaves and roots, respectively, while 147 and 297 genes were downregulated in the leaves and roots, respectively, under low P conditions [58]. Recently, Xiong et al. [59] analyzed the transcriptional and metabolic responses of maize shoots to long-term low-K stress. About 1000 genes were differentially expressed in two maize genotypes under low-K and sufficient-K conditions. For DH605 genotype, 676 and 246 genes were upregulated and downregulated, respectively, while 922 and 184 genes were upregulated and downregulated, respectively, in Z58 genotype (see [59] for list of genes). Many of the stress-induced genes were involved in transport, primary and secondary metabolism, regulation, and other processes, which play roles in K acquisition and homeostasis. Under low K, metabolic profiles revealed a high accumulation of amino acids, phenolic acids, organic acids, and alkaloids in shoots. They also found increased levels of sugars and sugar alcohols in the shoot. Accumulation of putrescine and putrescine derivatives play a role in maize shoot growth under low-K conditions [59]. Integrative omics analyses are vital in understanding the regulatory mechanisms of tolerance to low soil nutrients in maize at the molecular level. Future studies can explore this approach.
5. Conclusions and the Way Forward
Modifications in root and shoot architecture as well as physiological and agronomic traits help to decipher the maize response to suboptimal N, P, and K conditions. Traits related to tolerance to low levels of the three soil macronutrients in maize are highly quantitative, mainly controlled by additive genes, though non-additive genes may be important in some instances [48,50,51]. The focus should be on how to accumulate the additive genetic effects in maize populations. The dominance genetic effects can be exploited in hybrid maize breeding. Genetic control analysis for tolerance to low-K conditions should be investigated in future studies, though K is not as limiting as N, and P in the soil. A meta-QTL analysis and validation of the numerous loci reported in literature for tolerance to low N, P, and K levels will be worthwhile. The training set size and composition are critical to successful implementation of genomic selection to improve maize for low N, P, and K stress. Multi-environment analysis is necessary in selecting stable maize genotypes for tolerance to soil conditions having low amounts of N, P, and K, because of the importance of genotype-environment interaction. This review provides insights into maize adaptation strategies such as root architecture and genetic mechanisms under N, P, and K limited conditions. This can guide breeding and dissemination of maize cultivars for low N, P, and K inputs for sustainable food production against food insecurity, especially in developing countries.
Author Contributions
D.S.G. conceived the topic and wrote the section on genetic and genomic components as well as the omics profiles for N, P, and K stress (low) tolerance. J.O. and I.K.M. wrote on the maize shoot and root morpho-physiological responses to low N, P, and K, respectively. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sandhu, N.; Sethi, M.; Kumar, A.; Dang, D.; Singh, J.; Chhuneja, P. Biochemical and genetic approaches improving nitrogen use efficiency in cereal crops: A review. Front. Plant Sci. 2021, 12, 657629. [Google Scholar] [CrossRef]
- Torres-Rodríguez, J.; Salazar-vidal, M.; Montes, R.; Massange-Sánchez, J.A.; Gillmor, C.; Sawers, R. Low nitrogen availability inhibits the phosphorus starvation response in maize (Zea mays ssp. mays L.). BMC Plant Biol. 2021, 5, 259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yan, L.; Zhang, H. Morphological and physiological responses of winter wheat seedlings to nitrogen and phosphorus deficiency. J. Plant Nutr. 2013, 36, 1234–1246. [Google Scholar] [CrossRef]
- Yang, W.; Yoon, J.; Choi, H.; Fan, Y.; Chen, R.; An, G. Transcriptome analysis of nitrogen-starvation- responsive genes in rice. BMC Plant Biol. 2015, 15, 31. [Google Scholar] [CrossRef]
- Ao, X.; Guo, X.H.; Zhu, Q.; Zhang, H.J.; Wang, H.Y.; Ma, Z.H.; Han, X.R.; Zhao, M.H.; Xie, F.T. Effect of phosphorus fertilization to P uptake and dry matter accumulation in soybean with different P efficiencies. J. Integr. Agric. 2014, 13, 326–334. [Google Scholar] [CrossRef]
- Roberts, T.L.; Johnston, A.E. Phosphorus use efficiency and management in agriculture. Resour. Conserv. Recycl. 2015, 105, 275–281. [Google Scholar] [CrossRef]
- Wen, Z.; Li, H.; Shen, J.; Rengel, Z. Maize responds to low shoot P concentration by altering root morphology rather than increasing root exudation. Plant Soil 2017, 416, 377–389. [Google Scholar] [CrossRef]
- Dhillon, J.; Torres, G.; Driver, E.; Figueiredo, B.; Raun, W.R. World phosphorus use efficiency in cereal crops. Agron. J. 2017, 109, 1670–1677. [Google Scholar] [CrossRef]
- Pereira, N.C.M.; Galindo, F.S.; Gazola, R.P.D.; Dupas, E.; Rosa, P.A.L.; Mortinho, E.S.; Filho, M.C.M.T. Corn yield and phosphorus use efficiency response to phosphorus rates associated with plant growth promoting bacteria. Front. Environ. Sci. 2020, 8, 40. [Google Scholar] [CrossRef]
- Pettigrew, W.T. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant 2008, 133, 670–681. [Google Scholar] [CrossRef]
- Jordan-Meille, L.; Pellerin, S. Shoot and root growth of hydroponic maize (Zea mays L.) as influenced by K deficiency. Plant Soil 2008, 304, 157–168. [Google Scholar] [CrossRef]
- Demari, G.; Carvalho, I.; Nardino, M.; JSzareski, V.; Dellagostin, S.; da Rosa, T.; Follmann, N.; Monteiro, M.; Basso, C.; Pedó, T.; et al. Importance of nitrogen in maize production. Int. J. Curr. Res. 2016, 8, 36629–36634. [Google Scholar]
- Zhu, X.-K.; Li, C.-Y.; Jiang, Z.-Q.; Huang, L.-L.; Feng, C.-N.; Guo, W.-S.; Peng, Y.-X. Responses of phosphorus use efficiency, grain yield, and quality to phosphorus application amount of weak-gluten wheat. J. Integr. Agric. 2012, 11, 1103–1110. [Google Scholar] [CrossRef]
- Li, D.; Wang, H.; Wang, M.; Li, G.; Chen, Z.; Leiser, W.L.; Wei, T.M.; Lu, X.; Wang, M.; Chen, S.; et al. Genetic dissection of phosphorus use efficiency in a maize association population under two p levels in the field. Int. J. Mol. Sci. 2021, 22, 9311. [Google Scholar] [CrossRef]
- Ertiro, B.T.; Labuschagne, M.; Olsen, M.; Das, B.; Prasanna, B.M.; Gowda, M. Genetic dissection of nitrogen use efficiency in tropical maize through genome-wide association and genomic prediction. Front. Plant Sci. 2020, 11, 474. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, K.; Jha, A.K.; Yadava, P.; Pal, M.; Rakshit, S.; Singh, I. Global gene expression profiling under nitrogen stress identifies key genes involved in nitrogen stress adaptation in maize (Zea mays L.). Sci. Rep. 2022, 12, 4211. [Google Scholar] [CrossRef]
- Mi, G.; Chen, F.; Zhang, F. Physiological and genetic mechanisms for nitrogen-use efficiency in maize. J. Crop Sci. Biotech. 2005, 10, 57–63. [Google Scholar]
- Hirel, B.; Bertin, P.; Quilleré, I.; Bourdoncle, W.; Attagnant, C.; Dellay, C.; Gouy, A.; Cadiou, S.; Retailliau, C.; Falque, M.; et al. Towards a better understanding of the genetic and physiological basis for nitrogen use efficiency in maize. Plant Physiol. 2001, 125, 1258–1270. [Google Scholar] [CrossRef]
- Schlüter, U.; Mascher, M.; Colmsee, C.; Scholz, U.; Bräutigam, A.; Holger, F.; Sonnewald, U. Maize source leaf adaptation to nitrogen deficiency affects not only nitrogen and carbon metabolism but also control of phosphate homeostasis. Plant Physiol. 2012, 160, 1384–1406. [Google Scholar] [CrossRef]
- Guo, T.; Wang, D.; Fang, J.; Zhao, J.; Yuan, S.; Xiao, L.; Li, X. Mutations in the rice OsCHR4 gene, encoding a CHD3 family chromatin remodeler, induce narrow and rolled leaves with increased cuticular wax. Int. J. Mol. Sci. 2019, 20, 2567. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, J. Photosynthetic CO2 assimilation, chlorophyll fluorescence and photoinhibition as affected by nitrogen deficiency in maize plants. Plant Sci. 2000, 151, 135–143. [Google Scholar] [CrossRef]
- Martin, A.; Lee, J.; Kichey, T.; Gerentes, D.; Zivy, M.; Tatout, C.; Dubois, F.; Balliau, T.; Valot, B.; Davanture, M.; et al. Two cytosolic glutamine synthetase isoforms of maize are specifically involved in the control of grain production. Plant Cell 2006, 18, 3252–3274. [Google Scholar] [CrossRef] [PubMed]
- Uauy, C.; Brevis, J.C.; Dubcovsky, J. The high grain protein content gene Gpc-B1 accelerates senescence and has pleiotropic effects on protein content in wheat. J. Exp. Bot. 2006, 57, 2785–2794. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Yao, Q.; Yang, K.; Pan, G.; Rong, T. The effects of low phosphorus stress on morphological and physiological characteristics of maize (Zea mays L.) landraces. Agric. Sci. China 2007, 6, 559–566. [Google Scholar] [CrossRef]
- Rodriguez, D.; Andrade, F.; Goudriaan, J. Does assimilate supply limit leaf expansion in wheat grown in the field under low phosphorus availability? Field Crops Res. 2000, 67, 227–238. [Google Scholar] [CrossRef]
- Høgh-Jensen, H.; Schjoerring, J.K.; Soussana, J.F. The influence of phosphorus deficiency on growth and nitrogen fixation of white clover plants. Ann. Bot. 2002, 90, 745–753. [Google Scholar] [CrossRef]
- Chun, S.C.; Paramasivan, M.; Chandrasekaran, M. Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front. Microbiol. 2019, 9, 2525. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef]
- Siddique, A.; Kandpal, G.; Kumar, P. Proline accumulation and its defensive role under diverse stress condition in plants: An overview. J. Pure Appl. Microbiol. 2018, 12, 1655–1659. [Google Scholar] [CrossRef]
- Plenet, D.; Etchebest, S.; Mollier, A.; Pellerin, S. Growth analysis of maize field crops under phosphorus deficiency. Plant Soil 2000, 223, 117–130. [Google Scholar] [CrossRef]
- Assuero, S.G.; Mollier, A.; Pellerin, S. The decrease in growth of phosphorus-deficient maize leaves is related to a lower cell production. Plant Cell Environ. 2004, 27, 887–895. [Google Scholar] [CrossRef]
- Thornburg, T.E.; Liu, J.; Li, Q.; Xue, H.; Wang, G.; Li, L.; Julia Elise, F.; Kyele, E.D.; Wanying, L.; Baohong, Z.; et al. Potassium deficiency significantly affected plant growth and development as well as microRNA- mediated mechanism in wheat (Triticum aestivum L.). Front. Plant Sci. 2020, 11, 1219. [Google Scholar] [CrossRef]
- Amanullah; Iqbal, A.; Irfanullah; Hidayat, Z. Potassium management for improving growth and grain yield of maize (Zea mays L.) under moisture stress condition. Sci. Rep. 2016, 6, 34627. [Google Scholar] [CrossRef] [PubMed]
- Bukhsh, M.; Ahmad, R.; Ishaque, M.; Malik, A.U. Response of maize hybrids to varying potassium application in Pakistan. Pak. J. Agri. Sci. 2009, 46, 179–184. [Google Scholar]
- Jan, M.F.; Khan, A.A.; Liaqat, W.; Ahmad, H.; Ahmadzai, M.D.; Rehan, W. Response of phenology, growth and productivity of maize hybrids to integrated potassium management. Pak. J. Agric. Res. 2018, 31, 306–312. [Google Scholar] [CrossRef]
- Ul-Allah, S.; Ijaz, M.; Nawaz, A.; Sattar, A.; Sher, A.; Naeem, M.; Shahzad, U.; Farooq, U.; Nawaz, F.; Mahmood, K. Potassium application improves grain yield and alleviates drought susceptibility in diverse maize hybrids. Plants 2020, 9, 75. [Google Scholar] [CrossRef]
- Sustr, M.; Soukup, A.; Tylova, E. Potassium in root growth and development. Plants 2019, 8, 435. [Google Scholar] [CrossRef]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Ryan, P.R.; Delhaize, E.; Watt, M.; Richardson, A.E. Plant roots: Understanding structure and function in an ocean of complexity. Ann. Bot. 2016, 118, 555–559. [Google Scholar] [CrossRef]
- Neumann, G.; Martinoia, E. Cluster roots—An underground adaptation for survival in extreme environments. Trends Plant Sci. 2002, 7, 162–167. [Google Scholar] [CrossRef]
- Mpanga, I.K.; Ludewig, U.; Dapaah, H.K.; Neumann, G. Acquisition of rock phosphate by combined application of ammonium fertilizers and Bacillus amyloliquefaciens FZB42 in maize as affected by soil pH. J. Appl. Microbiol. 2020, 129, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Mpanga, I.K.; Nkebiwe, P.M.; Kuhlmann, M.; Cozzolino, V.; Piccolo, A.; Geistlinger, J.; Berger, N.; Ludewig, U.; Neumann, G. The form of N supply determines plant growth promotion by P-solubilizing microorganisms in maize. Microorganisms 2019, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Mpanga, I.K. Fertilization Strategies to Improve the Plant Growth-Promoting Potential of Microbial Bio-Effectors. Ph.D. Dissertation, University of Hohenheim, Stuttgart, Germany, 2020. [Google Scholar]
- Mpanga, I.K.; Dapaah, H.K.; Geistlinger, J.; Ludewig, U.; Neumann, G. Soil type-dependent interactions of P-solubilizing microorganisms with organic and inorganic fertilizers mediate plant growth promotion in tomato. Agronomy 2018, 8, 213. [Google Scholar] [CrossRef]
- Mpanga, I.K.; Gomez-Genao, N.; Moradtalab, N.; Wanke, D.; Chrobaczek, V.; Ahmed, A.; Windisch, S.; Geistlinger, J.; Hafiz, B.F.; Walker, F.; et al. The role of N form supply for PGPM-host plant interactions in maize. J. Plant Nut. Soil Sci. 2019, 182, 908–920. [Google Scholar] [CrossRef]
- Nkebiwe, P.M.; Weinmann, M.; Bar-Tal, A.; Müller, T. Fertilizer placement to improve crop nutrient acquisition and yield: A review and meta-analysis. Field Crops Res. 2016, 196, 389–401. [Google Scholar] [CrossRef]
- Amegbor, I.K.; Abe, A.; Adjebeng-Danquah, J.; Adu, G.B. Genetic analysis and yield assessment of maize hybrids under low and optimal nitrogen environments. Heliyon 2022, 8, e09052. [Google Scholar] [CrossRef]
- Das, B.; Atlin, G.N.; Olsen, M.; Burgueño, J.; Tarekegne, A.; Babu, R.; Ndou, E.N.; Mashingaidze, K.; Moremoholo, L.; Ligeyo, D.; et al. Identification of donors for low-nitrogen stress with maize lethal necrosis (MLN) tolerance for maize breeding in Sub-Saharan Africa. Euphytica 2019, 215, 80. [Google Scholar] [CrossRef]
- Sunday, I.; Babatunde, A.; Stephen, A.; Charity, A.; Kayode, O. Gene action in low nitrogen tolerance and implication on maize grain yield and associated traits of some tropical maize populations. Open Agric. 2020, 5, 801–805. [Google Scholar] [CrossRef]
- Mastrodomenico, A.T.; Bohn, M.O.; Lipka, A.E.; Below, F.E. Genomic selection using maize ex-plant variety protection germplasm for the prediction of nitrogen-use traits. Crop Sci. 2019, 59, 212–220. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Craft, E.J.; Yuan, L.; Cheng, L.; Mi, G.; Chen, F. Physiological and genetic analysis for maize root characters and yield in response to low phosphorus stress. Breed. Sci. 2018, 68, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yuan, Y.; Liao, Z.; Jiang, Y.; Wang, Q.; Zhang, L.; Gao, S.; Wu, F.; Li, M.; Xie, W.; et al. Genome-wide association study of 13 traits in maize seedlings under low phosphorus stress. Plant Genome 2019, 12, 190039. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, H.; Sun, J.; Guo, Z.; Zou, C.; Li, W.X.; Xie, C.; Huang, C.; Xu, R.; Liao, H.; et al. Genome-wide association study dissects yield components associated with low-phosphorus stress tolerance in maize. Theor. Appl. Genet. 2018, 131, 1699–1714. [Google Scholar] [CrossRef] [PubMed]
- Minjian, C.; Haiqiu, Y.; Hongkui, Y.; Chunji, J. Difference in tolerance to potassium deficiency between two maize inbred lines. Plant Prod. Sci. 2007, 10, 42–46. [Google Scholar] [CrossRef]
- Zhao, X.H.; Yu, H.Q.; Wen, J.; Wang, X.G.; Du, Q.; Wang, J.; Wang, Q. Response of root morphology, physiology and endogenous hormones in maize (Zea mays L.) to potassium deficiency. J. Integr. Agric. 2016, 15, 785–794. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, N.; Liu, S.; Dong, A.; Zenda, T.; Liu, X.; Li, J.; Duan, H. Comparative proteomic analysis of two contrasting maize hybrids’ responses to low nitrogen stress at the twelve leaf stage and function verification of ZmTGA gene. Genes 2022, 13, 670. [Google Scholar] [CrossRef]
- Sun, P.; Mu, C.; Chen, Y.; Kong, X.; Xu, Y.; Zheng, H.; Zhang, H.; Wang, Q.; Xue, Y.; Li, Z.; et al. Comparative transcript profiling of maize inbreds in response to long-term phosphorus deficiency stress. Plant Physiol. Biochem. 2016, 109, 467–481. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, Y.; Guo, Y.; Tang, W.; Zhao, Y.; Yang, G.; Pei, Y.; Chen, J.; Song, X.; Sun, J. Transcriptional and metabolic responses of maize shoots to long-term potassium deficiency. Front. Plant Sci. 2022, 13, 922581. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).