Molecular Identification of Trypanosoma cruzi Isolated from Wild Triatomines and Evaluation of Its Pathogenicity in Experimental Hosts
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains Under Study
2.2. Collection and Storage of Biological Samples
2.3. Histological Sections and Stains
2.4. Obtaining and Quantifying DNA from Biological Samples
2.4.1. Cultures of Epimastigotes
2.4.2. Fecal Feces with Metacyclic Trypomastigotes
2.4.3. Sample with Blood Trypomastigotes
2.4.4. Rodent Muscle with Amastigotes
2.5. Selection of Genetic Regions and Primer Design
2.6. Amplification of satDNA and mtCytB in T. cruzi Samples
2.7. Selected kDNA Sequence and Amplicon Generation Conditions
2.8. Sequencing of the Amplification Product
2.9. Alignment and Percentage of Identity
3. Results
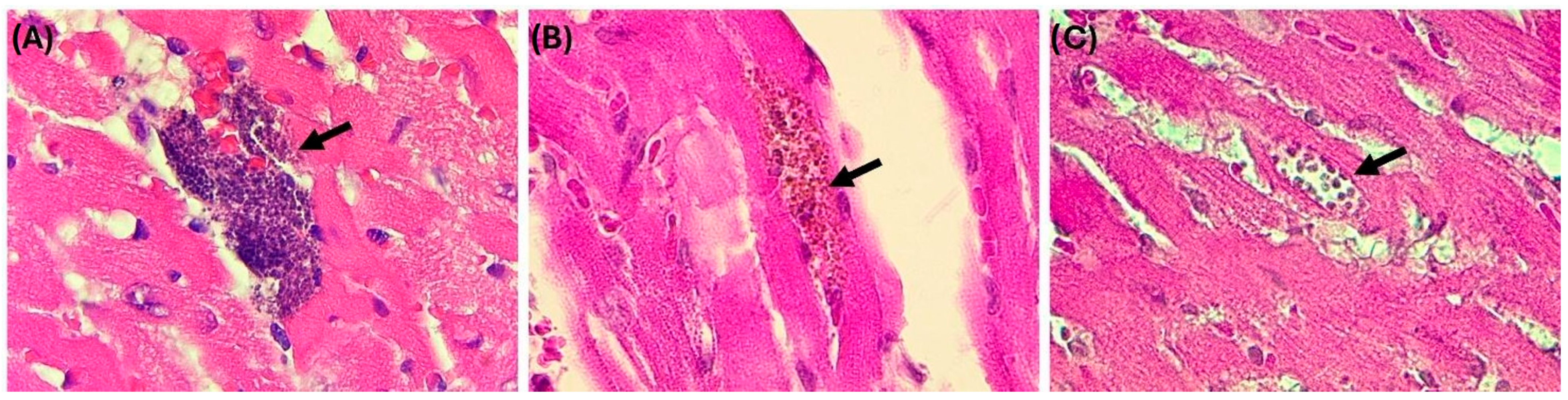
3.1. Collection of Biological Samples
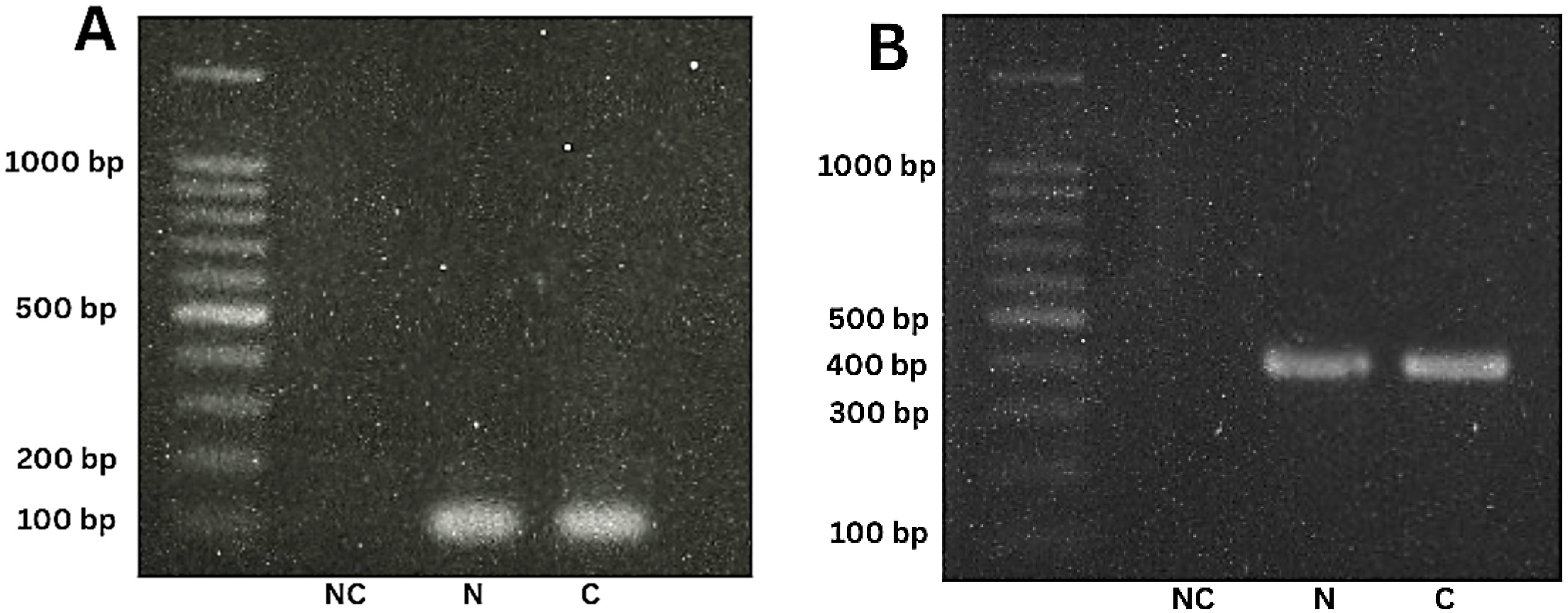
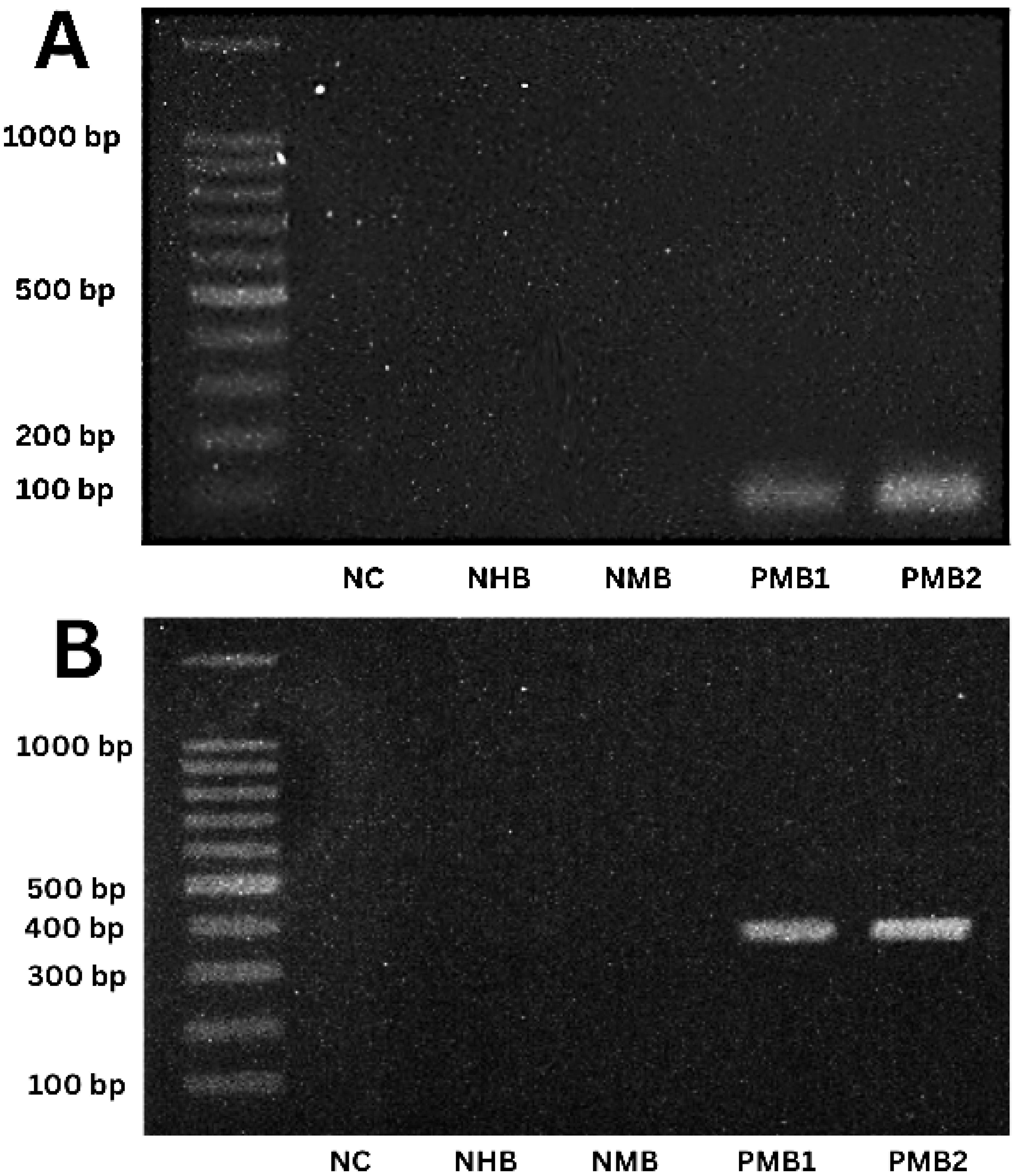
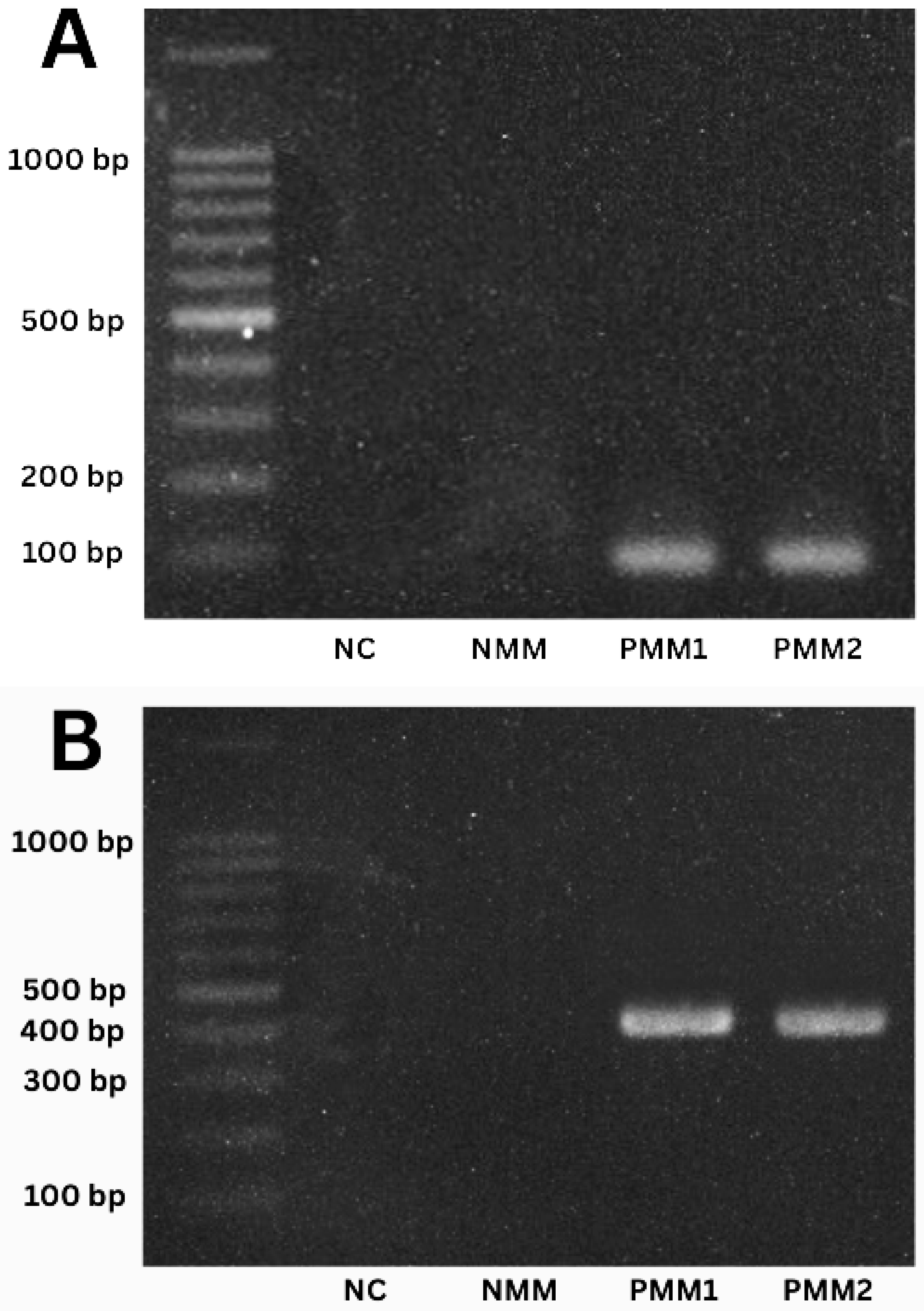
3.2. Quantification of DNA and PCR Analysis of Biological Samples
3.3. Sequencing and Analysis of the Selected kDNA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| satDNA | satellite DNA |
| mtCytB | cytochrome B gene |
| kDNA | Kinetoplast DNA |
References
- Nguyen, T.; Waseem, M.; Winters, R. Chagas Disease. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Parasites—American Trypanosomiasis (Also Known as Chagas Disease). Available online: https://www.cdc.gov/chagas/index.html (accessed on 15 May 2025).
- Álvarez-Hernández, D.A.; Franyuti-Kelly, G.A.; Díaz-López-Silva, R.; González-Chávez, A.M.; González-Hermosillo-Cornejo, D.; Vázquez-López, R. Chagas disease: Current perspectives on a forgotten disease. Rev. Med. Hosp. Gen. Méx. 2018, 81, 154–164. [Google Scholar] [CrossRef]
- Enfermedad de Chagas (Tripanosomiasis Americana). Available online: https://www.who.int/es/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 15 May 2025).
- Morales-Moran, S.; Sánchez-García, E.; Chávez-Gómez, R.; Carrasco-Esparza, N.; Aguayo-Acosta, A.; Hernández-Marín, D. Distribution of Triatoma (Meccus) phyllosoma and Triatoma (Meccus) longipennis as vectors of Trypanosoma cruzi in the state of Aguascalientes, Mexico and surroundings. Rev. Alianzas Tend. BUAP 2021, 6, 1–15. [Google Scholar] [CrossRef]
- Cesaretto, N.R.; de Oliveira, J.; Ravazi, A.; Madeira, F.F.; Dos Reis, Y.V.; de Oliveira, A.B.B.; Vicente, R.D.; Cristal, D.C.; Galvão, C.; de Azeredo-Oliveira, M.T.V.; et al. Trends in taxonomy of Triatomini (Hemiptera, Reduviidae, Triatominae): Reproductive compatibility reinforces the synonymization of Meccus Stål, 1859 with Triatoma Laporte, 1832. Parasites Vectors 2021, 14, 340. [Google Scholar] [CrossRef] [PubMed]
- Rivera, H.N.; McAuliffe, I.; Aderohunmu, T.; Wiegand, R.E.; Montgomery, S.P.; Bradbury, R.S.; Handali, S. Evaluation of the Performance of Ortho T. cruzi ELISA Test System for the Detection of Antibodies to Trypanosoma cruzi. J. Clin. Microbiol. 2022, 60, e0013422. [Google Scholar] [CrossRef]
- Ferrer, E. Técnicas moleculares para el diagnóstico de la enfermedad de Chagas. Saber 2015, 27, 359–371. [Google Scholar]
- Manual de Procedimientos Para la Enfermedad de Chagas en México; Gobierno de México: Ciudad de México, Mexico, 2019; pp. 1–109.
- Domínguez, J.L.; Ligonio, A.R.; Mendoza, A.C.; Díaz, M.C.M.; Cruz, V.A.R.; Monteón, A.L. El diagnóstico para la enfermedad de Chagas: A más de 110 años de su descubrimiento. JETERAPS 2021, 27, 31–39. [Google Scholar] [CrossRef]
- Ferreira Filho, J.C.R.; Braz, L.M.A.; Andrino, M.L.A.; Yamamoto, L.; Kanunfre, K.A.; Okay, T.S. Mitochondrial and satellite real time-PCR for detecting T. cruzi DTU II strain in blood and organs of experimentally infected mice presenting different levels of parasite load. Exp. Parasitol. 2019, 200, 13–15. [Google Scholar] [CrossRef]
- Kann, S.; Concha, G.; Weinreich, F.; Hahn, A.; Rückert, C.; Kalinowski, J.; Landt, O.; Frickmann, H. Comparative Assessment of Two Commercial Real-Time PCR Assays for the Diagnosis of Trypanosoma cruzi DNA in Serum. Microorganisms 2023, 11, 901. [Google Scholar] [CrossRef]
- Gómez-Palacio, A.; Cruz-Saavedra, L.; Van den Broeck, F.; Geerts, M.; Pita, S.; Vallejo, G.; Carranza, J.C.; Ramírez, J.D. High-throughput analysis of the Trypanosoma cruzi minicirculome (mcDNA) unveils structural variation and functional diversity. Sci. Rep. 2024, 14, 5578. [Google Scholar] [CrossRef]
- Silvestrini, M.M.A.; Alessio, G.D.; Frias, B.E.D.; Sales Júnior, P.A.; Araújo, M.S.S.; Silvestrini, C.M.A.; Brito Alvim de Melo, G.E.; Martins, H.R. New insights into Trypanosoma cruzi genetic diversity, and its influence on parasite biology and clinical outcomes. Front. Immunol. 2024, 15, 1342431. [Google Scholar] [CrossRef]
- Kann, S.; Kunz, M.; Hansen, J.; Sievertsen, J.; Crespo, J.J.; Loperena, A.; Arriens, S.; Dandekar, T. Chagas Disease: Detection of Trypanosoma cruzi by a New, High-Specific Real Time PCR. J. Clin. Med. 2020, 9, 1517. [Google Scholar] [CrossRef]
- Torres-Barajas, A.L.; Salas-Baéz, K.D.; Chávez-Gómez, R.I.; Carrasco-Esparza, N.A.; Muñoz-Ortega, M.H.; Sánchez-García, E.; Hernández-Marín, D.A. Actividad tripanocida de cinco plantas latinoamericanas. Rev. Biol. Trop. 2024, 72, e54026. [Google Scholar] [CrossRef]
- Davies, C.; Poma, R.H.; Marino Cardozo, R.; Mora, M.C.; Ramos, F.; Rajal, V.B.; Basombrío, M.A. Detección de Trypanosoma cruzi en tejido y sangre murina por PCR convencional y en tiempo real. Acta Bioquím. Clín. Latinoam. 2014, 48, 421–428. [Google Scholar]
- Ucan-Euan, F.; Hernández-Betancourt, S.; Arjona-Torres, M.; Panti-May, A.; Torres-Castro, M. Estudio histopatológico en tejido cardiaco de roedores infectados con Trypanosoma cruzi, capturados en barrios suburbanos, Mérida, México. Biomédica 2019, 39, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Reboreda-Hernandez, O.A.; Gonzalez-Rodriguez, N.; Cruz-Gonzalez, A.R.; Roman-Cedillo, A.; Ortiz-Butron, R. Influencia de la inoculación oral en la enfermedad de Chagas en modelo murino. Horiz. Sanit. 2021, 20, 198–206. [Google Scholar] [CrossRef]
- Serra-Campos, A.O.; Abreu-Junior, A.N.; Nascimento, A.A.; Abidu-Figueiredo, M.; Lima, M.S.C.S.; Machado-Santos, C. Gastroesophageal tube of the Iguana iguana (Iguanidae): Histological description, histochemical and immunohistochemical analysis of 5-HT and SS cells. Braz. J. Biol. 2023, 83, e242086. [Google Scholar] [CrossRef]
- Braz, L.M.; Raiz-Júnior, R.; Alárcon, R.S.; Gakiya, E.; Amato-Neto, V.; Okay, T.S. Suitability of a rapid DNA isolation and amplification for detection of Trypanosoma cruzi in Triatoma infestans dry fecal spots collected on filter paper. Parasite 2008, 15, 595–598. [Google Scholar] [CrossRef][Green Version]
- López, M.; Herrera, L.; Morocoima, A.; Rivera, M.G.; Viettri, M.; Lares, M.; Ferrer, E. Utility of a Fluid Library with Samples of Humans, Reservoirs and Vectors Collected in Filter Paper, for Retrospective Diagnosis of American Trypanosomiasis in Endemic Areas of Venezuela. Acta Parasitol. 2020, 66, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Bravo, A.; Cirignoli, S.; Wehrendt, D.; Schijman, A.; León, C.M.; Flores-Chaves, M.; Nieto, J.; Kieran, T.J.; Abril, M.; Guhl, F. Zoonotic Cycle of American Trypanosomiasis in an Endemic Region of the Argentine Chaco, Factors That Influenced a Paradigm Shift. Insects 2024, 15, 471. [Google Scholar] [CrossRef]
- Rueda-Concha, K.L.; Payares-Mercado, A.; Guerra-Castillo, J.; Melendrez, J.; Arroyo-Munive, Y.; Martínez-Abad, L.; Cochero, S.; Bejarano, E.E.; Paternina, L.E. Circulación de Leishmania infantum y Trypanosoma cruzi en perros domésticos de áreas urbanas de Sincelejo, región Caribe de Colombia. Biomédica 2022, 42, 633–649. [Google Scholar] [CrossRef]
- Probst, C.M.; Melo, M.F.A.D.; Pavoni, D.P.; Toledo, M.J.O.; Galdino, T.S.; Brandão, A.A.; Britto, C.; Krieger, M.A. A new Trypanosoma cruzi genotyping method enables high resolution evolutionary analyses. Mem. Inst. Oswaldo Cruz 2021, 116, e200538. [Google Scholar] [CrossRef]
- Callejas-Hernández, F.; Herreros-Cabello, A.; Del Moral-Salmoral, J.; Fresno, M.; Gironès, N. The Complete Mitochondrial DNA of Trypanosoma cruzi: Maxicircles and Minicircles. Front. Cell. Infect. Microbiol. 2021, 11, 672448. [Google Scholar] [CrossRef]
- Marcel, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Licón-Trillo, A.; Balsimelli-De La Peña, K.; Acosta-Legarda, M.; Leal-Berumen, I.; Nogueda-Torres, B.; Martínez-Ibarra, J. Infección natural por Trypanosoma cruzi en triatominos del Centro y Norte de México. Bol. Malariol. Sal. Amb. 2010, 50, 311–314. [Google Scholar]
- Hylton, A.; Fitzpatrick, D.M.; Suepaul, R.; Dobson, A.P.; Charles, R.A.; Peterson, J.K. Preliminary characterization of triatomine bug blood meals on the island of Trinidad reveals opportunistic feeding behavior on both human and animal hosts. Trop. Med. Infect. Dis. 2020, 5, 166. [Google Scholar] [CrossRef]
- Hart, M.L.; Meyer, A.; Johnson, P.J.; Ericsson, A.C. Comparative evaluation of DNA extraction methods from feces of multiple host species for downstream next-generation sequencing. PLoS ONE 2015, 10, e0143334. [Google Scholar] [CrossRef]
- Galla, G.; Praeg, N.; Rzehak, T.; Sprecher, E.; Colla, F.; Seeber, J.; Illmer, P.; Hauffe, H.C. Comparison of DNA extraction methods on different sample matrices within the same terrestrial ecosystem. Sci. Rep. 2024, 14, 8715. [Google Scholar] [CrossRef]
- Silva, A.N.B.D.; Souza, R.D.C.M.D.; Honorato, N.R.M.; Martins, R.R.; Câmara, A.C.J.D.; Galvão, L.M.D.C.; Chiari, E. Comparison of phenol-chloroform and a commercial deoxyribonucleic acid extraction kit for identification of bloodmeal sources from triatomines (Hemiptera: Reduviidae). Rev. Soc. Bras. Med. Trop. 2020, 53, e20200189. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, G.V.F.; Oliveira, A.C.S.; Silva, A.S.; Silva, M.C.L.; Lima, J.S.; Roos, T.B.; Moraes, C.M. Protocol optimization for the detection of Trypanosoma cruzi DNA in açai (Euterpe oleraceae) pulp. Acta Amazon. 2021, 51, 79–84. [Google Scholar] [CrossRef]
- Stanzick, K.J.; Simon, J.; Zimmermann, M.E.; Schachtner, M.; Peterhoff, D.; Niller, H.H.; Überla, K.; Wagner, R.; Heid, I.M.; Stark, K.J. DNA extraction from clotted blood in genotyping quality. Biotechnol. Tech. 2023, 74, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Lutz, Í.; Miranda, J.; Santana, P.; Martins, T.; Ferreira, C.; Sampaio, I.; Vallinoto, M.; Gomes, G.E. Quality analysis of genomic DNA and authentication of fisheries products based on distinct methods of DNA extraction. PLoS ONE 2023, 18, e0282369. [Google Scholar] [CrossRef]
- Al-Griw, H.H.; Zraba, Z.A.; Al-Muntaser, S.K.; Draid, M.M.; Zaidi, A.M.; Tabagh, R.M.; Al-Griw, M.A. Effects of storage temperature on the quantity and integrity of genomic DNA extracted from mice tissues: A comparison of recovery methods. Open Vet. J. 2017, 7, 239–243. [Google Scholar] [CrossRef]
- Chacón, F.; Bacigalupo, A.; Álvarez-Duhart, B.; Cattan, P.E.; Solís, R.; Muñoz-San Martín, C. The parasite load of Trypanosoma cruzi modulates feeding and defecation patterns of the Chagas disease vector Triatoma infestans. Microorganisms 2022, 10, 1003. [Google Scholar] [CrossRef]
- de Oliveira, M.T.; Sulleiro, E.; Silgado Gimenez, A.; de Lana, M.; Zingales, B.; Santana da Silva, J.; Marin-Neto, J.A.; Molina, I. Quantification of parasite burden of Trypanosoma cruzi and identification of Discrete Typing Units (DTUs) in blood samples of Latin American immigrants residing in Barcelona, Spain. PLoS Negl. Trop. Dis. 2020, 14, e0008311. [Google Scholar] [CrossRef]
- Herrera, L.; Aguilar, C.M.; Morocoima, A.; Viettri, M.; Lares, M.; Ferrer, E. Detection of Trypanosoma cruzi DNA in false negative samples of collected triatomines, xenodiagnosis material, and biopsies of experimentally infected animals. Int. Microbiol. 2021, 24, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.C.; Torres, C.; Curto, M.L.A.; Schijman, A.G. New insights into Trypanosoma cruzi evolution, genotyping and molecular diagnostics from satellite DNA sequence analysis. PLoS Negl. Trop. Dis. 2017, 11, e0006139. [Google Scholar] [CrossRef]
- Rossi, I.V.; de Souza, D.A.S.; Ramirez, M.I. The end justifies the means: Chagas disease from a perspective of the host–Trypanosoma cruzi interaction. Life 2024, 14, 488. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-San Martín, C.; Solari, A.; Apt, W.; Zulantay, I. Caracterización de las Unidades Discretas de Tipificación de Trypanosoma cruzi según sus marcadores moleculares. Rev. Ibero Latinoam. Parasitol. 2013, 72, 5–21. [Google Scholar]
- Rodrigues, M.S.; Morelli, K.A.; Jansen, A.M. Cytochrome c oxidase subunit 1 gene as a DNA barcode for discriminating Trypanosoma cruzi DTUs and closely related species. Parasit. Vectors 2017, 10, 488. [Google Scholar] [CrossRef]
- de Souza, M.L.; Lapierre, T.J.W.J.D.; de Lima Marques, G.V.; Ferraz, W.R.; Penteado, A.B.; Trossini, H.G.G.; Murta, S.M.F.; de Oliveira, R.B.; de Oliveira Rezende, C., Jr.; Ferreira, R.S. Molecular targets for Chagas disease: Validation, challenges and lead compounds for widely exploited targets. Expert. Opin. Ther. Targets 2023, 27, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Arenas, M.; Campos, R.; Coronado, X.; Ortiz, S.; Solari, A. Trypanosoma cruzi genotypes of insect vectors and patients with Chagas of Chile studied by means of cytochrome b gene sequencing, minicircle hybridization, and nuclear gene polymorphisms. Vector-Borne Zoonotic Dis. 2012, 12, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Roach, S.L.; Barnes, S.W.; Hoepfner, D.; Walker, J.R.; Chatterjee, A.K.; Neitz, R.J.; Arkin, M.R.; McNamara, C.W.; Ballard, J.; et al. Utilizing chemical genomics to identify cytochrome b as a novel drug target for Chagas disease. PLoS Pathog. 2015, 11, e1005058. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Curtis-Robles, R.; Chirra, B.; Auckland, L.D.; Mai, A.; Bocanegra-Garcia, V.; Clark, P.; Clark, W.; Cottingham, M.; Fleurie, G.; et al. Characterization of triatomine bloodmeal sources using direct Sanger sequencing and amplicon deep sequencing methods. Sci. Rep. 2022, 12, 10234. [Google Scholar] [CrossRef]
- Gómez-Palacios, R.L.; Ruiz-Tovar, K.; Huerta, H.; González-Reyes, M.O.; Padilla-Medina, I.; De la Rosa-Arana, J.L.; Fonseca-Coronado, S. Natural infection with Trypanosoma cruzi and feeding habits of Triatominae (Hemiptera: Reduviidae) from the state of Durango, Mexico. Acta Trop. 2025, 251, 107555. [Google Scholar] [CrossRef] [PubMed]
- Thertulien, R.; Simpson-Haidaris, P.J.; Haidaris, C.G. Intracellular localization of a Trypanosoma cruzi kDNA minicircle transcript using RNA:RNA in situ hybridization. J. Eukaryot. Microbiol. 1994, 41, 402–407. [Google Scholar] [CrossRef]
- DeLorenzo, M.; Carias, E.; Mustonen, A.; Gonzalez, O.; Dick, E.J., Jr.; Kumar, S. In situ hybridization assay for the diagnosis of Chagas myocarditis and orchitis in a rhesus macaque (Macaca mulatta): A case report. J. Med. Primatol. 2019, 48, 182–185. [Google Scholar] [CrossRef]
- Kann, S.; Concha, G.; Frickmann, H.; Hagen, R.M.; Warnke, P.; Molitor, E.; Hoerauf, A.; Backhaus, J. Chagas disease: Comparison of therapy with nifurtimox and benznidazole in indigenous communities in Colombia. J. Clin. Med. 2024, 13, 2565. [Google Scholar] [CrossRef]
- Herreros-Cabello, A.; Callejas-Hernández, F.; Gironès, N.; Fresno, M. Trypanosoma cruzi genome: Organization, multi-gene families, transcription, and biological implications. Genes 2020, 11, 1196. [Google Scholar] [CrossRef]
- Telleria, J.; Lafay, B.; Virreira, M.; Barnabé, C.; Tibayrenc, M.; Svoboda, M. Trypanosoma cruzi: Sequence analysis of the variable region of kinetoplast minicircles. Exp. Parasitol. 2006, 114, 279–288. [Google Scholar] [CrossRef]



| Sample Type | Concentration | Absorbance 230/260 | Absorbance 260/280 |
|---|---|---|---|
| Culture media (n = 3) | 160 ± 10 µg/mL | 1.62 ± 0.25 | 1.96 ± 0.10 |
| Triatomine feces (n = 5) | 13.5 ± 4 µg/mL | 0.41 ± 10 | 2.05 ± 0.33 |
| Blood (n = 2) | 34.3 ± 6.5 µg/mL | 0.96 ± 34 | 1.88 ± 0.11 |
| Muscle (n = 2) | 34.5 ± 9.5 µg/mL | 1.37 ± 41 | 2.02 ± 0.21 |
| Access Number | Sequence Information | Percent Identity with the Problem-Sequence |
|---|---|---|
| M15511.1 | T.cruzi kinetoplast minicircle pTckAWP-2, complete | 33.54 |
| M15512.1 | T.cruzi kinetoplast minicircle pTckCA1-73 DNA fragment | 33.71 |
| HG008629.1 | Homo sapiens genomic DNA containing Trypanosoma cruzi kinetoplast minicircle DNA, family B patient 028, clone FH093 | 33.42 |
| M19177.1 | T.cruzi kinetoplast minicircle DNA, clone cl1 cst 4 | 33.21 |
| M18815.1 | T.cruzi kinetoplast minicircle DNA, clone pTc-21 | 33.23 |
| U07846.1 | Trypanosoma cruzi Y kinetoplast minicircle sequence, clone M369r, orf, complete cds | 60.94 |
| U07845.1 | Trypanosoma cruzi Y kinetoplast minicircle sequence, clone M835, orf, complete cds | 60.46 |
| X56188.1 | T. cruzi cDNA52 minicircle transcript (highly homologous to kinetoplast DNA minicircle sequences) | 60.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Barajas, A.L.; Rincón-González, M.P.; Martínez-Hernández, S.L.; Muñoz-Ortega, M.H.; Ibarra-Martínez, D.; Sánchez-García, E.; López-Macías, E.; Aguayo-Acosta, A.; Elizondo-Luevano, J.H.; Hernández-Marín, D.A. Molecular Identification of Trypanosoma cruzi Isolated from Wild Triatomines and Evaluation of Its Pathogenicity in Experimental Hosts. Parasitologia 2025, 5, 46. https://doi.org/10.3390/parasitologia5030046
Torres-Barajas AL, Rincón-González MP, Martínez-Hernández SL, Muñoz-Ortega MH, Ibarra-Martínez D, Sánchez-García E, López-Macías E, Aguayo-Acosta A, Elizondo-Luevano JH, Hernández-Marín DA. Molecular Identification of Trypanosoma cruzi Isolated from Wild Triatomines and Evaluation of Its Pathogenicity in Experimental Hosts. Parasitologia. 2025; 5(3):46. https://doi.org/10.3390/parasitologia5030046
Chicago/Turabian StyleTorres-Barajas, Ana Lucía, Melissa Paola Rincón-González, Sandra Luz Martínez-Hernández, Martín Humberto Muñoz-Ortega, David Ibarra-Martínez, Eduardo Sánchez-García, Erick López-Macías, Alberto Aguayo-Acosta, Joel Horacio Elizondo-Luevano, and David Alejandro Hernández-Marín. 2025. "Molecular Identification of Trypanosoma cruzi Isolated from Wild Triatomines and Evaluation of Its Pathogenicity in Experimental Hosts" Parasitologia 5, no. 3: 46. https://doi.org/10.3390/parasitologia5030046
APA StyleTorres-Barajas, A. L., Rincón-González, M. P., Martínez-Hernández, S. L., Muñoz-Ortega, M. H., Ibarra-Martínez, D., Sánchez-García, E., López-Macías, E., Aguayo-Acosta, A., Elizondo-Luevano, J. H., & Hernández-Marín, D. A. (2025). Molecular Identification of Trypanosoma cruzi Isolated from Wild Triatomines and Evaluation of Its Pathogenicity in Experimental Hosts. Parasitologia, 5(3), 46. https://doi.org/10.3390/parasitologia5030046








