Abstract
This study aimed to explore and identify soil-dwelling nematophagous fungi (NF) from the “El Texcal” Ecological Reserve in Morelos, Mexico, and evaluate their potential as biological control agents against Haemonchus contortus infective larvae (HcL3), a major parasitic threat in livestock systems. The fungi were isolated from soil using the sprinkling of soil on water agar plates. The identification of NF was achieved using morphological identification keys, which was corroborated by molecular procedures using the PCR technique in the ITS4 and ITS5 regions. The nematocidal effects occasioned by these NF were examined through their predatory activity (PA) against HcL3 on water agar plates, and additionally, the larval mortality attributed to their liquid filtrates (LFs) was assessed at three different concentrations (25, 50, and 100 mg/mL) on 96-well microtiter plates. Two NF were identified and classified as two species of Dactylellina genus, namely D. haptospora (Dh) and D. phymatopaga (Dp). The PA exhibited by these NF were 94.79% for Dh and 68.88% for Dp; while their LFs showed 27.83% mortality for Dh and 32.86% for Dp at the highest concentration assessed. While the PA was notably high, the moderate larvicidal effect of the LF suggests that their efficacy may primarily rely on direct physical interaction rather than metabolite-mediated toxicity. The high PA demonstrated by these two isolates of NF indicates that they could be effective candidates for biological control agents against HcL3.
1. Introduction
In the soil, a wide variety of microorganisms of different taxonomic groups including fungi, bacteria, protozoa, viruses, and an enormous group of nematodes coexist, sharing their microhabitat, and as a result, different biological associations are established [1,2]. The Nematophagous fungi (NF) are members of the soil microbiota living as saprophyte organisms, becoming predators or parasites of nematodes, and they are considered one of the main groups of natural nematode antagonistic microorganisms [3]. These organisms have developed different strategies to capture or invade living nematodes, i.e., the transformation of their mycelia in trapping devices specially designed to capture nematodes [4,5], molecules that mimic the sexual hormones of nematodes to attract them and to capture them [6], and enzymes and compounds derived from the secondary metabolites with nematocidal activity [7,8]. The genus Dactylellina comprises nematode-trapping fungi that can adapt their mycelia to form various trapping devices, such as simple rings, adhesive branches, or adhesive knobs, depending on the specific species [9]. However, there are only a few records regarding the biological activity of Dactylellina species against nematodes that are significant in agriculture [10]. Additionally, there is limited information on the impact of this genus of fungi on nematodes that are important in the livestock industry [11].
Haemonchus contortus is a blood-feeding nematode that causes significant harm to animal health and productivity. This nematode resides in the stomachs of small ruminants, i.e., ovine (Ovis aries) and goats (Capra hircus), where it sucks blood and is responsible for a serious condition known as Haemonchosis [12]. The most common strategy for controlling nematode infections and similar conditions involves using chemically synthesized compounds known as anthelmintics. However, this approach has several drawbacks. A major concern is the potential risk to public health due to the possibility of residual contamination in milk, meat, or other products intended for human consumption [13]. Additionally, the frequent and continuous use of anthelmintics can negatively impact non-target organisms, which may alter the biodiversity of species in the ecosystem [14]. Therefore, it is essential to explore sustainable and eco-friendly strategies to effectively control parasites while minimizing harm to ecosystems, such as the use of nematophagous fungi.
Species of nematophagous fungi from the genus Dactylellina may be explored as potential agents for controlling Haemonchosis in small ruminants. However, it is essential to gather information about their predatory activity and the possible nematocidal effects of compounds released into their liquid culture filtrates. This information could be crucial as a preliminary step to evaluate this genus in the control of sheep Haemonchosis.
Despite increasing interest in nematophagous fungi as biocontrol agents, few studies have focused on native fungal isolates from ecologically unique areas such as the El Texcal Ecological Reserve, where biodiversity may provide novel strains with high predatory potential.
The objective of this study was to isolate, identify, and assess the effectiveness of nematophagous fungi and their liquid culture filtrates against the infective larvae of Haemonchus contortus, exploring novel alternative control strategies for sheep haemonchosis.
2. Materials and Methods
2.1. Allocation
This study was performed at the Laboratory of Helminthology in the National Center of Disciplinary Research in Animal Health and Innocuity, which is a part of the National Institute of Research in Forestry, Agriculture and Livestock (INIFAP-Mexico) in Jiutepec, Morelos, Mexico.
2.2. Biological Material
2.2.1. Panagrellus Redivivus
A strain of the free-living nematode P. redivivus was commercially obtained from a local pet store as fish food in Jiutepec, Morelos, Mexico. This strain was used and cultivated on a medium of wet oat flakes on plastic Petri dishes [15]. A large population of juveniles of this nematode was obtained after 2 weeks of incubation at room temperature (18–25 °C). Juveniles were recovered from the plates by filtration using a kitchen drain and then passed through sieves with different pore sizes of 500, 120, and 0.74 μm. Nematodes in suspension were finally cleaned by sedimentation in distilled water and resuspended in sterile water.
2.2.2. Haemonchus Contortus
A strain of the blood-feeding nematode H. contortus, obtained from CENID-SAI-INIFAP, was utilized for this study. A 6-month-old, nematode-free, male-haired lamb served as the parasite egg donor animal. The lamb was orally infected with 350 infective larvae of the parasite per kilogram of body weight. After a pre-patent period of 21 days, fecal samples were collected directly from the lamb’s rectum and used to elaborate fecal cultures in plastic bowls [16]. It is important to mention that the parasite egg donor animal was maintained under indoor conditions according to the rules of total respect and animal welfare, avoiding any unnecessary animal suffering. The animal care and management of this lamb were carried out according to the Good Management Practices policies settled down at INIFAP. The Norma Oficial Mexicana (Official Mexican Standards) with official rule number NOM-052-ZOO-1995 (http://www.senasica.gob.mx accessed on 23 May 2022) and the Ley Federal de Sanidad Animal (Federal Law for Animal Health) DOF 07-06-2012 were strictly adhered to (https://www.gob.mx/cms/uploads/attachment/file/118761/LFSA.pdf accessed on 23 May 2022), following the ethical standards outlined by INIFAP. The animal management procedure was revised and eventually approved by the Bioethics Committee of the National Center for Disciplinary Research in Animal Health and Innocuity of INIFAP.
The feces containing the eggs of the parasite were mixed with polyurethane particles and water to form a soft paste, which provided an oxygen-rich environment to promote better hatching of the parasitic eggs into larvae. To obtain the infective larvae, stumps were prepared with the stool culture and placed in Baermann funnels [17]. Subsequently, the collection tube with the recovered larvae was removed. The liquid containing larvae in the tubes was filtered, and the larvae were washed using the density gradients of sucrose (40%) to eliminate residues [18]. Larvae were unsheathed by exposure to 0.187% sodium hypochlorite [19].
2.2.3. Obtaining Nematophagous Fungi Isolate
Soil samples were collected from the Ecological Reserve “El Texcal” (ERET) in the Municipality of Jiutepec, located in Morelos, Mexico. El Texcal is a protected ecological area located in the northwestern region of Morelos. This place is geographically situated at 18°53′56″ north latitude to the equator and at 99°09′01″ west. This protected natural zone is known for its rich biodiversity and ecological importance [20] (Figure 1).

Figure 1.
Maps showing the Mexican Republic (A), the State of Morelos (B), El Texcal Ecological Reserve (C), and the sampling site (D).
Ten soil samples of 100 g each were randomly collected from different sites of the ERET. The samples were identified with an indelible pen and transported in plastic bags to the laboratory and kept at 4 °C until use. A small amount of soil (0.3 g approximately) was sprinkled on the surface of sterile water agar plates (2%) and incubated at room temperature (18–25 °C) for five days. After that, five drops of 100 µL of an aqueous suspension containing approximately 500 juveniles of the free-living nematode P. redivivus were deposited on the surface of each plate to enhance the development of aerial structures of nematophagous fungi. The plates were examined under a stereomicroscope twice a week to identify structures associated with the presence of nematophagous fungi, including conidiophores, conidia, trapping devices, or trapped nematodes [21,22]. When aerial structures or trapped nematodes were observed, they were transferred to sterile water agar plates using passes [23]. The number of passes to new sterile agar plates for isolating fungi was determined by the purity of our isolates.
2.3. Fungal Morphometric Taxonomical Identification
The fungal isolates were observed under a microscope, and the structures of taxonomical identification, such as conidia, number of conidial septa, conidiophores, the presence or absence of chlamydospores, and the type of trapping devices, among other characteristics, were recorded. The Cotton Blue colorant was used to enhance the visualization of the fungal structures. The fungi were cultured on water agar plates and also using the micro-culture technique (on a slide) to observe their aerial structures [24]. Twenty-five conidia were measured, recording their shapes, length, and width. Likewise, conidiophore lengths were also measured and recorded. The fungal material was photographed using a Leica Zeiss DM6B microscope (Wetzlar, Germany). The images and measurements were obtained by taking photographs using objectives 10×, 20×, and 40× of the microscope camera using the LAS program (version 4.9). Finally, the measurements were compared with those described in different taxonomic keys specially designed to identify nematophagous fungi [25,26].
2.4. Molecular Identification of Nematophagous Fungi
To obtain the genetic material, a DNA genomic purification kit (PROMEGA® Wizard®, Madison, WI, USA) was used. The mycelia of two fungal strains cultured on potato–dextrose broth were obtained after 5 days of incubation at 18–25 °C, following the protocol provided by the manufacturer. The endpoint PCR technique was used to amplify the DNA using a commercial kit (PROMEGA®, Madison, WI, USA) together with two oligonucleotides, namely ITS5 (5′-GGA AGT AAA AGT CGT AAC AAG G-3′) and ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) [27,28]. The PCR conditions were as follows: initial denaturation at 94 °C for 3 min, followed by an amplification phase consisting of 35 cycles with denaturation at 94 °C for 1 min, hybridization at 42 °C for 90 s, and an extension at 72 °C for 90 s. A final extension stage at 72 °C for 5 min was carried out in an endpoint PCR thermocycler (BIORAD C1000 Touch, Hercules, CA, USA). Then, the PCR products were visualized through electrophoresis in 1.5% agarose gel [29]. Finally, the cleaning of the PCR product was performed using the kit Gel and PCR clean-up System (PROMEGA®), following the procedure recommended by the manufacturer. The PCR products were sequenced at the Institute of Biotechnology of the National Autonomous University of Mexico (IBT-UNAM) using an Applied Biosystem sequencer (7700, Foster, CA, USA) [30].
2.5. Phylogenetic Analysis
A phylogenetic analysis was performed using the obtained sequences from the ITS region; sequences from each isolated fungus were aligned in the NCBI-BLAST tool to confirm the morphological taxonomic analysis. All species of Dactylellina reported at Index Fungorum (https://www.indexfungorum.org/NAMES/NAMES.asp) (accessed on 28 November 2024) and with the ITS region reported at NCBI and both sequences from our isolates were included in a database for posterior analysis, including the Vermispora fusarina strain YXJ13-5 (accession code: AY773447). A multiple alignment was carried out using the CLUSTAL algorithm in MEGA software (v.11.0.13), which was also used to edit the alignment.
The obtained alignments were used to construct a phylogenetic tree; the best substitution model was estimated in JModelTest (v.2.1.10) based on the Akaike Information Criterion (AIC); and this model was used to obtain the consensus tree. Maximum likelihood analysis was developed in IQTREE (v.1.6.12) by obtaining the statistical support values by an Ultrafast Bootstrapping analysis with 10 000 replicates. The consensus tree was visualized using FigTree software (v.1.4.4) and edited using Nitro Pro software (13.9.1).
2.6. Assessment of Predatory Activity of Fungal Isolates
One plug (1 cm2) was taken from the surface of a plate containing a 10-day-old fungus cultured in water agar plates and was transferred to a sterile water agar plate. In total, 15 plates were inoculated with the corresponding fungus. Another additional 15 plates (without fungi) were also used as a control group (n = 15). All the plates were cultured at room temperature (18–25 °C) for 7 days. After the incubation period, 100 µL of an aqueous suspension containing 200 H. contortus infective larvae were placed on top of the fungus, where they migrated in different directions across the plate. The plates were incubated for 7 days and maintained under the same experimental conditions, allowing for the interaction between larvae and fungi.
After the incubation, the agar of each plate was removed and individually placed on the Baermann funnel system (in an inverted way) for 24 h to allow the non-trapped larvae to move to remain in the sediment. The whole larvae from the control group also descended to the base of the tubes. Larvae from the sediments were counted using the aliquot drop larvae-counting technique, visualizing the recovered larvae on a slide under a microscope (10×, 40× magnifications) [19]. Finally, the reduction percentage attributed to the predatory effect of the fungi was estimated following the ABBOTT formula:
where
2.7. Nematophagous Fungi Liquid Cultures
A sweet potato–dextrose broth medium (SPDB) was prepared in a 500 mL flask as follows: 200 g of organic sweet potato were weighed and 20 g of glucose were added. The content was divided into twelve 250 mL flasks with 50 mL of the medium per flask. The flasks containing the culture medium were sterilized, and 7.5 μL of levofloxacin was added to each flask in a flue cabinet. Later on, three plugs (1 cm2) were obtained from the surface of a 15-day-old culture of the corresponding fungi, either Dactylellina haptospora or D. phymatopaga growing on water agar and deposited into the flasks. The flasks were incubated for 15 days at (25–30 °C). Three other flasks without fungi were used as negative controls.
2.8. Obtaining Liquid Culture Filtrates
After the incubation period, the mycelia of fungi were separated from the liquid media by filtration using Whatman papers #4 (25 and 11 µm) and Millipore filters (1.6 and 0.22 µm). The filtration was achieved using a filtration unit connected to a vacuum pump. The liquid filtrates were concentrated with a rotavaporator (Buchi R-300, Flawil, Switzerland). The concentrated filtrates were frozen using ultra-freezing (Reach-ins vertical freezer, ATOSA, MBF8501GR model, Brea, CA, USA) at −20 °C, and they were eventually lyophilized using a conventional lyophilizer (LABCONCO, Kanzas city, MO, USA)
2.9. Assessing In Vitro Nematocidal Activity of Fungal Liquid Culture Filtrates Against Haemonchus contortus Infective Larvae
In this experiment, H. contortus unsheathed infective larvae were used. The larvae were exposed to three different concentrations of fungal liquid culture filtrates (100, 50, and 25 mg/mL). The assay was performed in 96-well microtiter plates, and four treatments of fungal liquid culture filtrates were established as follows: two groups containing the corresponding fungi: group 1 with D. haptospora and group 2 with D. phymatopaga; and two additional groups (without fungi) were used as control groups: group 3, with sweet potato–dextrose broth medium and group 4 with phosphate-buffer solution (PBS). Fifty microliters of the corresponding concentration of the fungal filtrates were deposited into each well, followed by the addition of 50 μL of an aqueous suspension containing a mean of 110 H. contortus infective larvae. The same number of larvae was placed on the wells of the control groups. The plates were incubated for 72 h at room temperature (25–30 °C). After this period, lectures were performed as follows: from each well of the corresponding treatments and concentrations, ten aliquot drops were randomly taken, put on a slide, and observed under a compound microscope (objectives 10×, and 20×). The criterion to establish the viability/mortality of the larvae after exposure to liquid filtrates was based on the movement of larvae. Stretched and motionless larvae that remained in the same position after applying a physical stimulus (touching them with a needle) were considered dead [16]. Both alive and dead larvae were counted, and the means per group were recorded and compared with the control groups without the effect of the fungal filtrates.
2.10. Statistical Analysis
Data from the predation assessment of the two fungi against the infective larvae of H. contortus were analyzed using a completely randomized design through an ANOVA (analysis of variance). The mean number of recovered larvae from the plates used in the fungus–nematode interaction served as the dependent variable. Similarly, the results regarding the lethal activity of the fungal liquid culture filtrate were individually analyzed using an ANOVA. In this case, the mean number of dead and live larvae recorded was considered the dependent variable.
3. Results
3.1. Isolation and Morphological Identification of Fungi Isolates
Two fungal isolates were obtained as pure cultures from soil samples collected from the “El Texcal” Ecological Reserve. These isolates were assigned using the key codes D1 and D2 for record-keeping purposes.
3.1.1. Isolate D1
After we carefully revised the isolate D1 under the microscope, we observed hyaline, elongated, and septate conidia with a globose distal cell. Conidiophores appeared lightly branched and elongated, and long and sessile conidia were observed. The main measurements of fungal structures, including conidia length and width, distal cell length and width, conidiophore length and width, and the presence or absence of chlamydospores and the type of trapping devices that are relevant to taxonomy, are presented in Table 1.

Table 1.
Means and ranges of measurements of the main morphological structures observed in the D1 isolate.
After the fungus/nematode interaction took place in the plates, sessile conidia and adhesive knobs attached to the nematode cuticular coat were observed (Figure 2 and Figure 3, as well as a video, published as Supplementary Video S1). After analyzing in detail, the whole morphological characteristics of this isolate and according to the descriptions published in the taxonomic keys, we decided to classify this isolate into the Dactylellina haptospora species.

Figure 2.
Microphotographs showing the development of morphological structures of taxonomic importance produced by the D1 isolate identified as Dactylellina haptospora. (A,B) Conidiophores and sessile conidia, (C) catenulate chlamydospores.

Figure 3.
Microphotographs showing the main taxonomic characteristics observed in the isolate D1. (A) Two Haemonchus contortus infective larvae (L3) trapped with sessile conidia and adhesive knobs of the isolate D1 identified as Dactylellina haptospora. (B) Sessile conidia attached to the cuticle of a larva and (C) aspect of adhesive knobs.
3.1.2. Isolate D2
This isolate showed the vegetative hyphae formation that appeared hyaline, branched, and septate. Likewise, conidiophores appeared erect and septate, measuring from 122.89 to 375.35 μm in length, and spindle-shaped conidia formed with three to five cells separated by septa. Conidia length ranged between 37.99 and 50.22 μm, with a widening in the middle cell and thinning in both extremes (Figure 4D). The presence of chlamydospores was also observed (Figure 4B). After adding nematodes, the fungus triggered the formation of an adhesive knob system used as a trapping device where nematodes were captured and invaded by the fungus (Figure 4A and Figure 5). After we analyzed the morphological information and compared the characteristics of this fungus with those described in the taxonomic guidelines, we decided to classify it into the genus/species D. phymatopaga.

Figure 4.
A set of microphotographs showing the aspect of the D2 fungal isolate, identified as Dactylellina phymatopaga. (A) Adhesive knobs, (B) chlamydospores, (C) (1) conidium, (2) conidiophore, (D) conidia, (E,F) adhesive knobs.
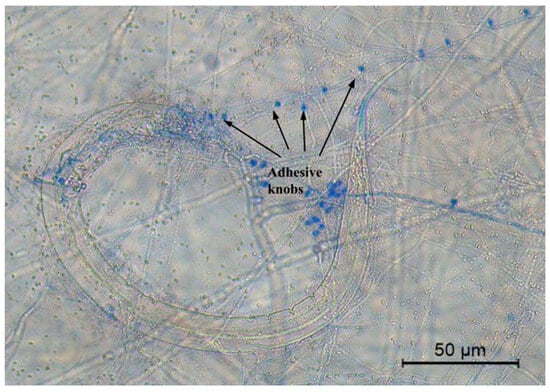
Figure 5.
A microphotograph showing the formation of adhesive knobs (stained with cotton blue) produced by Dactylellina phymatopaga and a Haemonchus contortus infective larvae (L3) trapped by this fungus.
3.2. Molecular Identification of Fungi Isolates
After we obtained the DNA sequences of our isolates and completed the alignment, we compared these sequences with those previously reported in the NCBI database. We analyzed the highest coverage and identity percentages of various isolates recorded in the NCBI, and the information regarding the closest phylogenetic isolates is presented in Table 2 and Table 3.

Table 2.
Results about the alignment of the DNA sequences obtained from the ITS regions of the D1 isolate concerning sequences previously reported in the NCBI database.

Table 3.
Results about the alignment of the sequences obtained from the ITS regions of the D2 isolate concerning sequences previously reported in the NCBI database.
After performing a multiple alignment of the sequences obtained from the D1 and D2 isolates, along with nineteen sequences of the ITS regions from Dactylellina species, we used this alignment to construct a phylogenetic tree with IQTREE software (v.2.3.6). Our two sequences were recorded in the NCBI database with the following gene bank accession numbers: PV470200 (D. haptospora) and PV470668 (D. phymatopaga). The resulting tree is displayed in Figure 6. The tree was created using the SYM + G4 substitution model and employed the maximum likelihood method, with statistical support estimated through 10,000 bootstrap replicates (indicated at the nodes). The species highlighted in red are the isolates from the present study, namely Dactylellina phymatopaga and D. haptospora. For comparison, the species Vermispora fusarina was included as an external group.

Figure 6.
Phylogenetic tree constructed using DNA sequence alignments from the ITS regions of nematophagous fungi isolates in the genus Dactylellina.
3.3. Predatory Activity of Isolated Fungi
The results about the predatory activity of the fungi against H. contortus infective larvae expressed as the means of recovered larvae in both groups of fungi, the mean of recovered larvae in the control group, and the reduction percentage attributed to the predatory effect of the fungi are shown in Table 4.

Table 4.
Results about the mean numbers of Haemonchus contortus (L3) recovered after the interaction with two nematophagous fungi, Dactylellina haptospora and D. phymatopaga, and reduction percentage attributed to the predatory activity of these fungi.
3.4. Nematocidal Effect of Liquid Culture Filtrates of Fungal Isolates
The mean of dead and total larvae and the larval mortality percentage occasioned by the effect of the fungal liquid culture filtrate at the different concentrations assessed is shown in Table 5.

Table 5.
Means of Haemonchus contortus dead and total larvae recovered after 72 h of being exposed to different concentrations of liquid culture filtrates from two nematophagous fungi, Dactylellina haptospora and D. phymatopaga, cultured in sweet potato–dextrose broth medium.
It is important to remark that after the exposure of H. contortus larvae to the liquid filtrates of both fungi, important changes in the integrity of some larvae were observed at the highest concentration of the liquid culture filtrates. For example, there was a narrowing in some parts of the larva’s body, giving the appearance of flattened areas and a loss of turgor in their external coat (Figure 7B) (Figure 7).

Figure 7.
(A) Microphotograph showing the appearance of a normal Haemonchus contortus infective larva in the control group; (B) larvae exposed to the liquid culture filtrates of Dactyllelina haptospora, which exhibit narrowing at both ends of the larval body; and (C) a, b, c flattened areas observed in a larva exposed to the liquid culture filtrates of D. phymatopaga.
4. Discussion
4.1. Morphological and Molecular Identification
The morphological analysis of key taxonomic structures indicates that our D1 isolate corresponds to the species D. haptospora. This conclusion is based on the presence of elongated, thin, cylindrical, and multi-septated conidia, which feature a small, globose distal cell resembling drumsticks. Additionally, the presence of adhesive knobs, along with the measurements of conidia and conidiophores, aligns with the description provided by Drechsler in 1940 [25]. Although Drechsler did not mention the presence of chlamydospores in his isolates, subsequent research by Yu et al. (2014) [26] has identified catenulated chlamydospores in D. haptospora. This finding further supports our taxonomical identification process.
We observed specific characteristics in our D2 isolate, including branched hyphae, erect and septate conidiophores, elongated and septate conidia with a central widening, and the presence of adhesive knobs. These features, along with the recorded measurements, align with the description of D. phymatopaga as outlined by Yu et al. (2014) [26]. Therefore, we have decided to classify our isolate within this genus and species.
Traditional morphological methods for taxonomic identification can effectively distinguish between genera and species of nematode-trapping fungi. However, the high phenotypic similarity among these species requires the use of molecular techniques, such as PCR, to achieve the most accurate taxonomic diagnosis.
In our findings, the D1 isolate exhibited over 92% similarity and 100% coverage, which confirmed our morphological taxonomic identification as Dactylellina haptospora (DQ999820.1). This was further supported by constructing a phylogenetic tree that included 20 additional sequences related to this genus. Conversely, our D2 isolate showed 99% coverage and over 97% similarity to D. phymatopaga (KT215203.1), and these results were corroborated by both morphological and phylogenetic analyses.
4.2. Assessment of Predatory Activity of Fungal Isolates Against Haemonchus contortus Infective Larvae
Both isolates, D. haptospora and D. phymatopaga, showed important predatory activities (95 and 69%) against H. contortus infective larvae, respectively. These results can be compared with the results obtained with other genera/species of nematophagous fungi against the same assessed nematode. In this regard, studies on the predatory activity of various nematophagous fungi show in vitro predatory efficacy ranging between 70% and 90% [10,31,32]. The present study pretends to search for potential nematode-trapping fungi for controlling H. contortus larvae. Although there is a large number of studies with other genera/species of nematode-trapping fungi focused on the control of nematodes of importance in the livestock industry, there is so far no study about the predatory activity of species of the genus Dactylellina against nematodes affecting small ruminants, so this could be the very first report about this potential biotechnological application.
In a comprehensive review of the existing literature, the authors of this study identified a limited number of records concerning the predatory behavior of nematophagous fungi species from the genus Dactylellina against nematodes that significantly impact agriculture and the livestock industry. Table 6 illustrates the effectiveness of the predatory activity of certain Dactylellina species against nematodes from various taxonomical groups.

Table 6.
Comparison of target nematodes, predatory efficacy percentages, and assessment conditions for different species of the genus Dactylellina spp. in various countries.
4.3. Assessment of In Vitro Nematocidal Activity of Fungal Isolates Against Haemonchus contortus Infective Larvae
The liquid culture filtrates of both assessed species of Dactylellina showed a low nematocidal activity at the highest concentration (100 mg/mL) after 72 h of interaction. Several factors can enhance or inhibit the production of nematocidal compounds released by nematophagous fungi in the liquid culture medium, i.e., the nutritional regime and the culture medium conditions [35], the concentration of compounds in the liquid culture filtrates [22], and the presence or absence of nematodes [36,37,38]. In this study, we only assessed the nematocidal activity of fungi cultured on sweet potato broth, and no highly lethal effect was found even at the highest concentration. Perhaps these two species could exhibit higher nematocidal activity if they are cultured in culture media supplying a different regime of nutrients; however, this hypothesis should be demonstrated in future studies.
If an important lethal effect of liquid culture filtrates is discovered, pure metabolites could be isolated using chromatographic techniques, followed by nuclear magnetic resonance [39,40,41].
Recent studies have demonstrated that the species D. haptotyla exhibits nematocidal activity. One of the active compounds identified is 2-furoic acid, which has been shown to kill phytonematodes, including the root knot nematode Meloidogyne incognita. The concentration required to achieve a 50% lethal effect (LE50) was determined to be 55.05 μg/mL [42]. It would be important to assess the potential activity of this and other compounds produced by D. haptotyla against H. contortus, searching for natural nematocidal compounds. The results of the predatory activity demonstrated by the assessed fungal species make these isolates potential candidates to be assessed in future in vivo studies. One strategy of using nematode-trapping fungi in the control of gastrointestinal parasitic nematodes of ruminants has been well established. This method consists of orally administering a suspension of spores/chlamydospores or mycelia of selected fungal strains to monitor their passage through the gastrointestinal tract of the animals. The goal of this step in the process is to determine whether fungi can survive after passing through the digestive tract of small ruminants and reaching the feces [43]. Once the spores or chlamydospores are in the fecal material, they germinate, colonize the feces, and produce their trapping devices. These fungi then engage in predatory activity within the feces, killing larvae, reducing forage contamination, and helping to prevent the spread of infection within the flock [44].
The importance of this study lies in the fact that both fungi demonstrated an important in vitro predatory activity of infective larvae of H. contortus, one of the most pathogenic parasitic nematodes affecting small ruminants globally.
In this context, the use of nematicides could be replaced by the use of nematophagous fungi, with all the benefits that this implies.
The nematophagous fungi can serve as a complementary strategy to both anthelmintic drugs and vaccines and even to other control strategies, as there is no reason to oppose the combined use of these alternative methods.
Exploring the use of nematophagous fungi as potential agents of biocontrol could offer several advantages over other methods of control. For example, it provides a natural control method, is environmentally safe since it does not contaminate the soil [45], is harmless, and avoids leaving chemical residues in milk, meat, or by-products intended for human consumption [46,47]. Additionally, it does not contribute to the development of anthelmintic resistance.
Nevertheless, because haemonchosis is a complex disease, using only one method of control is not enough to obtain the best results. So, the combination of alternative methods of control should be explored, searching for the most effective control strategy. In this context, the use of nematophagous fungi should be accompanied by other measurements of control, i.e., the use of plants/plant metabolites with anthelmintic activity [48], vaccines [49], and grazing management practices, among others [50]. The use of these strategies into an integrated control program should be evaluated, looking for the best control strategy [51]. The selection of “elite” strains as potential candidates for controlling parasitic nematodes involves a rigorous process. This process includes in vitro trials, in vivo assays, and assessments to ensure the absence of toxicity to flora and fauna, as well as no public health risks for the applicant or as residues in products for human consumption. Although this investigation is still in its early stages, it has the potential to yield promising results in the future.
5. Conclusions
The results of the present study lead to the conclusion that based on morphological and molecular analyses, the two isolates obtained from soil samples from the “El Texcal” Ecological Reserve in Morelos, Mexico, corresponded to the species D. haptospora and D. phymatopaga. These species are excellent predatory agents of H. contortus infective larvae under in vitro conditions, and although their liquid culture filtrates obtained in sweet potato–dextrose broth exerted very little nematocidal activity, these species could be considered in future in vivo assays as potential tools of control of sheep haemonchosis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/parasitologia5020026/s1, Video S1: A supplementary video (S1) showing the capture of H. contortus infective larvae by the fungus Dactylellina haptospora.
Author Contributions
Conceptualization and writing—original draft preparation: P.M.-d.G. and M.S.B.-A.; laboratory work: E.G.-M. and E.J.D.-N.; data curation: G.P.-A.; writing—review and editing, P.M.-d.G.; supervision and funding acquisition, M.E.L.-A. and A.Y.O.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received partial economic support by Consejo Nacional de Humanidades, Ciencia y Tecnología (CONAHCYT) Proyecto Ciencias de Frontera-2023 (grant number CF-2023-I-2309).
Institutional Review Board Statement
The Norma Oficial Mexicana (Official Mexican Standards) with official rule number NOM-052-ZOO-1995 (http://www.senasica.gob.mx accessed on 23 May 2022) and the Ley Federal de Sanidad Animal (Federal Law for Animal Health) DOF 07-06-2012 were strictly adhered to (https://www.gob.mx/cms/uploads/attachment/file/118761/LFSA.pdf accessed on 23 May 2022), following the ethical standards outlined by INIFAP. The animal management procedure was revised and eventually approved by the Bioethics Committee of the National Center for Disciplinary Research in Animal Health and Innocuity of INIFAP.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article and supplementary materials.
Acknowledgments
The authors would like to express their gratitude to biotechnologist Génesis Bautista García, David Emanuel Reyes Guerrero, biotechnologist María José Hernández Vega, and biotechnologist Ana Jatziri Torres Armendáriz for their assistance with various laboratory tasks.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van der Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.; De Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Eldridge, D.J.; Liu, Y.R.; Liu, Z.W.; Coleine, C.; Trivedi, P. Soil biodiversity and function under global change. PLoS Biol. 2025, 23, e3003093. [Google Scholar] [CrossRef]
- Lawal, I.; Fardami, A.Y.; Ahmad, F.I.I.; Yahaya, S.; Abubakar, A.S.; Sa’id, M.A.; Maiyadi, K.A. A review on nematophagus fungi: A potential nematicide for the biocontrol of nematodes. J. Environ. Bioremediat. Toxicol. 2022, 5, 26–31. [Google Scholar] [CrossRef]
- Nordbring-Hertz, B. Morphogenesis in the nematode-trapping fungus Arthrobotrys oligospora: An extensive plasticity of infection structures. Mycologist 2004, 18, 125–133. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, W.; Fan, Y.; Deng, W.; Zhang, L.; Wang, S.; Xiang, M. Septin AoCDC11 is involved in trap morphogenesis, conidiation, and vegetative growth in carnivorous Arthrobotrys oligospora. Fungal Genet. Biol. 2025, 177, 103971. [Google Scholar] [CrossRef]
- Hsueh, Y.P.; Gronquist, M.R.; Schwarz, E.M.; Nath, R.D.; Lee, C.H.; Gharib, S.; Sternberg, P.W. Nematophagous fungus Arthrobotrys oligospora mimics olfactory cues of sex and food to lure its nematode prey. Elife 2017, 6, e20023. [Google Scholar] [CrossRef]
- Rahman, M.U.; Chen, P.; Zhang, X.; Fan, B. Predacious strategies of nematophagous fungi as bio-control agents. Agronomy 2023, 13, 2685. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, L.; Zhang, H.; Ma, X.; Sun, Y.; Li, R.; Meng, Q. Proteomic insights into nematode-trapping fungi Arthrobotrys oligospora after their response to chitin. J. Vet. Res. 2025, 69, 71. [Google Scholar] [CrossRef]
- De Hoog, G.S.; van Oorschot, C.A.N. Taxonomy of the Dactylaria complex VI. Key to the genera and check-list of epithets. Stud. Mycol. 1985, 26, 97–122. [Google Scholar]
- Kumar, D. Evaluation of nematophagous and biocontrol efficacy of Dactylellina phymatopaga against Meloidogyne graminicola on rice (Oryza sativa L.). Biol. Control 2024, 188, 105425. [Google Scholar] [CrossRef]
- Xue, Y.J.; Li, E.L.; Wang, A.H.; Cai, K.Z. Predatory activity and passage of six nematophagous fungi species in gastrointestinal tract of trichostrongylide-infected sheep. Biocontrol Sci. Technol. 2018, 28, 654–662. [Google Scholar] [CrossRef]
- Abosse, J.S.; Terefe, G.; Teshale, B.M. Comparative study on pathological changes in sheep and goats experimentally infected with Haemonchus contortus. Surg. Exp. Pathol. 2022, 5, 14. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Shikoray, L.; Mohamed, M.Y.I.; Habib, I.; Matsumoto, T. Veterinary drug residues in the food chain as an emerging public health threat: Sources, analytical methods, health impacts, and preventive measures. Foods 2024, 13, 1629. [Google Scholar] [CrossRef]
- Vokřál, I.; Podlipná, R.; Matoušková, P.; Skálová, L. Anthelmintics in the environment: Their occurrence, fate, and toxicity to non-target organisms. Chemosphere 2023, 345, 140446. [Google Scholar] [CrossRef]
- De Lara, R.; Castro, T.; Castro, J.; Castro, G. Cultivo del nematodo Panagrellus redivivus (Goodey, 1945) en un medio de avena enriquecida con Spirulina sp. Rev. Biol. Mar. Oceanogr. 2007, 42, 29–36. [Google Scholar] [CrossRef]
- Olmedo-Juárez, A.; Delgado-Núñez, E.J.; Bahena-Vicencio, A.; Villa-Mancera, A.; Zamilpa, A.; González-Cortázar, M.; Mendoza de Gives, P. In vitro nematocidal properties from two extracts: Lippia graveolens leaves and Delonix regia flowers against eggs and infective larvae of Haemonchus contortus. J. Med. Food 2022, 25, 329–337. [Google Scholar] [CrossRef]
- Valcarcel-Sancho, F.V.; Vázquez, F.A.R.; García, Á.S.O.; Novillo, M.B.A.; Sopeña, L.M. Atlas de Parasitología Ovina; Servet: Fethiye/Muğla, Türkiye, 2009; 137p, ISBN 978-84-92569-05-2. [Google Scholar]
- Liébano-Hernández, E. Ecología de larvas de nematodos gastrointestinales de bovinos, ovinos y caprinos. Epidemiol. Enferm. Paras. Anim. Domést. 2011, 1, 267–268. [Google Scholar]
- Olmedo-Juárez, A.; Rojo-Rubio, R.; Zamilpa, A.; Mendoza de Gives, P.; Arece-García, J.; López-Arellano, M.E.; von Son-de Fernex, E. In vitro larvicidal effect of a hydroalcoholic extract from Acacia cochliacantha leaf against ruminant parasitic nematodes. Vet. Res. Commun. 2017, 41, 227–232. [Google Scholar] [CrossRef]
- ANP Texcal. Secretaría de Desarrollo Sustentable. Available online: https://sustentable.morelos.gob.mx/anp/el-texcal (accessed on 26 January 2017).
- Berhanu, M.; Gebeyaw, D.T.; Kefale, D.; Kang, Y. Overview of nematophagous fungi, isolation techniques, and their role in biological control of helminthic parasites: A literature review. Acta Entomol. Zool. 2024, 5, 133–143. [Google Scholar] [CrossRef]
- Bahena-Nuñez, D.S.; Ocampo-Gutiérrez, A.Y.; Mendoza-de Gives, P.; González-Cortázar, M.; Zamilpa, A.; Higuera-Piedrahita, R.I.; Hernández-Romano, J. Arthrobotrys oligospora (Fungi: Orbiliales) and its liquid culture filtrate myco-constituents kill Haemonchus contortus infective larvae (Nematoda: Trichostrongylidae). Biocontrol Sci. Technol. 2024, 34, 754–775. [Google Scholar] [CrossRef]
- Noman, E.; Al-Gheethi, A.A.; Rahman, N.K.; Talip, B.; Mohamed, R.; Kadir, O.A. Single spore isolation as a simple and efficient technique to obtain fungal pure culture. In IOP Conference Series: Earth and Environmental Science; IOP: Bristol, UK, 2018; Volume 140, p. 12055. [Google Scholar]
- Campbell, C.K.; Johnson, E.M. Identification of Pathogenic Fungi: Campbell/Identification of Pathogenic Fungi; Warnock, D.W., Ed.; Wiley-Blackwell: Chichester, UK, 2013; ISBN 9781444330700. [Google Scholar]
- Drechsler, C. Three fungi destructive to free-living terricolous nematodes. J. Wash. Acad. Sci. 1940, 30, 240–254. [Google Scholar]
- Yu, Z.; Mo, M.; Zhang, Y.; Zhang, K.Q. Taxonomy of nematode-trapping fungi from Orbiliaceae, Ascomycota. In Nematode-Trapping Fungi; Zhang, K.-Q., Hyde, K.D., Eds.; Springer Science & Business: Berlin/Heidelberg, Germany, 2014; Volume 23, pp. 41–210. [Google Scholar]
- White, T.J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Stielow, J.; Lévesque, C.; Seifert, K.; Meyer, W.; Irinyi, L.; Smits, D.; Renfurm, R.; Verkley, G.; Groenewald, M.; Chaduli, D.; et al. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia Mol. Phylogeny Evol. Fungi 2015, 35, 242–263. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Anzúrez, G.; Olmedo-Juárez, A.; Von-Son de Fernex, E.; Alonso-Díaz, M.Á.; Delgado-Núñez, E.J.; López-Arellano, M.E.; Mendoza-de Gives, P. Arthrobotrys musiformis (Orbiliales) kills Haemonchus contortus infective larvae (Trichostronylidae) through its predatory activity and its fungal culture filtrates. Pathogens 2022, 11, 1068. [Google Scholar] [CrossRef]
- Ocampo-Gutiérrez, A.Y.; Hernández-Velázquez, V.M.; Aguilar-Marcelino, L.; Cardoso-Taketa, A.; Zamilpa, A.; López-Arellano, M.E.; Mendoza-de Gives, P. Morphological and molecular characterization, predatory behavior and effect of organic extracts of four nematophagous fungi from Mexico. Fungal Ecol. 2021, 49, 101004. [Google Scholar] [CrossRef]
- Jaramillo-Tlalapango, J.; Gives, P.M.-D.; Isabel-Higuera-Piedrahita, R.; Ocampo-Gutiérrez, A.Y.; Eugenia-López-Arellano, M.; Pérez-Anzúrez, G.; Olmedo-Juárez, A.; Hernández-Romano, J.; Maza-Lopez, J.; Delgado-Núñez, E.J.; et al. Study of a Mexican isolate of Arthrobotrys musiformis (Orbiliales): Predatory behavior and nematocidal activity of liquid culture filtrates against Haemonchus contortus (Trichostrongylidae), protein profile and myco-constituent groups. Fungal Biol. 2023, 127, 1345–1361. [Google Scholar] [CrossRef]
- Campos, A.K.; Valadão, M.C.; Carvalho, L.M.; Araújo, J.V.d.; Guimarães, M.P. In vitro nematophagous activity of predatory fungi on infective larvae of Strongyloides papillosus. Acta Vet. Bras. 2017, 11, 213–218. [Google Scholar] [CrossRef]
- Wairimu, W.J.; Kimenjul, J.W.; Muiru, W.M.; Wachira, P.M. Interactions between soil additives and a variety of naturally occurring nematode-demolishing fungi in banana fields of Meru and Embu Counties, Kenya. Cell Biol. Dev. 2022, 6. [Google Scholar] [CrossRef]
- Wen, X.F.; Shi, T.T.; Zhang, Y.Q.; Wang, S.H.; Xiang, C.M.; Zhao, P.J. DHXT1, a Virulence Factor of Dactylellina haptotyla, Regulates Pathogenicity by Participating in Trap Formation and Metabolite Synthesis. Int. J. Mol. Sci. 2024, 25, 7384. [Google Scholar] [CrossRef]
- Pérez-Anzúrez, G.; Mendoza-de Gives, P.; Alonso-Díaz, M.Á.; von Son-de Fernex, E.; Paz-Silva, A.; López-Arellano, M.E.; Olmedo-Juárez, A. Lecanicillium psalliotae (Hypocreales: Cordycipitaceae) Exerts Ovicidal and Larvicidal Effects against the Sheep Blood-Feeding Nematode Haemonchus contortus through Its Liquid Culture Filtrates. Pathogens 2024, 13, 588. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.; Mittal, A.; Naik, S.N. Evidence for the involvement of nematocidal toxins of Purpureocillium lilacinum 6029 cultured on Karanja deoiled cake liquid medium. World J. Microbiol. Biotechnol. 2016, 32, 82. [Google Scholar] [CrossRef]
- Kuo, T.H.; Yang, C.T.; Chang, H.Y.; Hsueh, Y.P.; Hsu, C.C. Nematode-trapping fungi produce diverse metabolites during predator–prey interaction. Metabolites 2020, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-de Gives, P.; Braga, F.R.; Araújo, J.V.d. Nematophagous fungi, an extraordinary tool for controlling ruminant parasitic nematodes and other biotechnological applications. Biocontrol Sci. Technol. 2022, 32, 777–793. [Google Scholar] [CrossRef]
- Degenkolb, T.; Vilcinskas, A. Metabolites from nematophagous fungi and nematicidal natural products from fungi as an alternative for biological control. Part I: Metabolites from nematophagous ascomycetes. Appl. Microbiol. Biotechnol. 2016, 100, 3799–3812. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Dai, Z.; Zhang, K.; Li, G.; Zhao, P. Pathogenicity and metabolites of endoparasitic nematophagous fungus Drechmeria coniospora YMF1.01759 against nematodes. Microorganisms 2021, 9, 1735. [Google Scholar] [CrossRef]
- Dai, Z.; Gan, Y.; Zhao, P.; Li, G. Secondary metabolites from the endoparasitic nematophagous fungus Harposporium anguillulae YMF 1.01751. Microorganisms 2022, 10, 1553. [Google Scholar] [CrossRef]
- Lei, H.-M.; Wang, J.-T.; Hu, Q.-Y.; Li, C.-Q.; Mo, M.-H.; Zhang, K.-Q.; Li, G.-H.; Zhao, P.-J. 2-Furoic acid associated with the infection of nematodes by Dactylellina haptotyla and its biocontrol potential on plant root-knot nematodes. Microbiol. Spectr. 2023, 11, e01896-23. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Alvares, F.B.V.; Silva, J.T.; Ferreira, L.C.; da Costa, P.W.L.; Sarmento, W.F.; Feitosa, T.F.; de Araújo, J.V.; Braga, F.R.; Vilela, V.L.R. Predatory effects of the fungus Arthrobotrys cladodes on sheep gastrointestinal nematodes. Biocontrol Sci. Technol. 2020, 30, 830–839. [Google Scholar] [CrossRef]
- Mendoza-de Gives, P. Soil-Borne nematodes: Impact in agriculture and livestock and sustainable strategies of prevention and control with special reference to the use of nematode natural enemies. Pathogens 2022, 11, 640. [Google Scholar] [CrossRef]
- Nolinda, N.; Ikusika, O.O.; Akinmoladun, O.F.; Mpendulo, C.T. Impact of nematode infestation in livestock production and the role of natural feed additives–A review. Open Agric. 2024, 9, 20220234. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Durjava, M.; Dusemund, B.; Kouba, M.; López-Alonso, M.; Puente, S.L.; et al. Safety of a feed additive consisting of Duddingtonia flagrans NCIMB 30336 (BioWorma®) for all grazing animals (International Animal Health Products Pty Ltd.). EFSA J. 2023, 21, e8465. [Google Scholar]
- Ma, Y.; Jiang, L.; Fan, Z.; Hao, L.; Li, Z.; Zhang, Y.; Li, Q.; Wang, R.; Luo, H. Nematode controlling effects and safety tests of Duddingtonia flagrans biological preparation in sheep. Sci. Rep. 2025, 15, 1843. [Google Scholar] [CrossRef] [PubMed]
- Chavarría-Joya, L.; Alonso-Díaz, M.Á.; Olmedo-Juárez, A.; von Son-de Fernex, E.; Mendoza-de-Gives, P. Assessing the individual and combined use of Caesalpinia coriaria (Plantae: Fabaceae) and Duddingtonia flagrans (Fungi: Orbiliaceae) as sustainable alternatives of control of sheep parasitic nematodes. Biocontrol Sci. Technol. 2022, 32, 1260–1274. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Li, J.; Liu, F.; Ye, L.; Liu, X.; Hu, M. Identification and validation of protective glycoproteins in Haemonchus contortus H11. Front. Immunol. 2025, 16, 1521022. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.; Ellis, K. Parasite control in regenerative livestock farming. Livestock 2023, 28, 112–120. [Google Scholar] [CrossRef]
- Siddique, A.; Whitley, N.; Samples, O.M.; Burke, J.M.; Terrill, T.H.; Miller, J.E.; Connell, T. Exploring the Role of Duddingtonia flagrans in Integrated Parasite Management: A Systematic Review of Current Research and Applications. preprints 2025, 2025021078. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).