Abstract
A new species of giant petrel, Macronectes tinae sp. nov., is described from the Pliocene deposits of South Taranaki, New Zealand. The holotype is a near complete skull and the paratype a fragmentary left humerus; both come from the Tangahoe Formation, dating from the late Pliocene (Piacenzian or “Waipipian”; age estimated as ca. 3.36–3.06 Ma). The new species of giant petrel is the first fossil Macronectes ever reported. It is morphologically similar to the two present-day Macronectes spp., but it was a smaller bird. The skull is diagnosed by its overall smaller size, a proportionately longer apertura nasi ossea, and potentially by a shorter os supraocciptale. The humerus is diagnosed from both species by a proportionately less deep shaft, a more prominent medial portion of the epicondylus ventralis, and a larger and fusiform fossa medialis brachialis. The Tangahoe Formation is proving to be a remarkable source of marine vertebrate fossils and an important piece of the puzzle in understanding the evolution and biogeography of seabirds.
1. Introduction
Giant petrels (Macronectes Richmond, 1905) are the largest birds in the family Procellariidae, identifiable by their heavyset body and beak. They are represented by two living species, Macronectes giganteus (Gmelin, 1789) and M. halli (Mathews, 1912) [1,2]. Macronectes halli was originally described as a subspecies of M. giganteus; its status as a separate species was only attained in the second half of the 20th century [3,4,5] (see also [6]).
Both species of giant petrels are distributed around the Southern Hemisphere, ranging from Antarctica to the subtropics [1,7]. They are fulmarine petrels, phylogenetically close to the fulmar species (Fulmarus Stephens, 1826), their sister taxa, and to the Cape petrel Daption capense (Linnaeus, 1758), Antarctic petrel Thalassoica antarctica (J.F. Gmelin 1789), and snow petrel Pagodroma nivea (G. Forster, 1777) [6].
Macronectes has no fossil representatives known so far, aside from the bones of undetermined taxa in Pleistocene and Holocene deposits (e.g., [8,9]). A skull and a humerus belonging to Macronectes were recently found in the Pliocene deposits of the Tangahoe Formation in New Zealand. The Tangahoe Formation is a sequence of alternating marine sandstones, siltstones, and shell beds, located in the sedimentary Whanganui Basin in the western portion of New Zealand’s North Island (see [10] for lithologic and stratigraphic descriptions of its fossiliferous sites in the western coastal section in South Taranaki). These deposits have been dated through biostratigraphic correlation and magnetostratigraphy at 3.36–3.06 Ma, late Pliocene [10,11,12].
In the present paper, we analyse those new Macronectes fossils, compare them to the skeletons of living giant petrels, and assign them to a new species based on their morphological characters: Macronectes tinae sp. nov.
2. Materials and Methods
The only two fossils known of the herein-described new species of giant petrel were recovered in the coastal deposits of South Taranaki. They consist of a fragmentary left humerus (proximal end not preserved) and a nearly complete skull, which in all likelihood belonged to distinct individuals because the humerus was found about 2 km south of the skull. The fossils are housed in the fossil vertebrate collection of the Museum of New Zealand Te Papa Tongarewa (NMNZ, Wellington, New Zealand) under the registration numbers NMNZ S.048502 (skull) and NMNZ S.048870 (humerus).
The fossils were compared to recent specimens of Procellariiformes in the NMNZ to determine their affinities. The osteological nomenclature used here follows [13,14,15]. After its identity as a giant petrel (Macronectes Richmond, 1905) was established (see Systematics section), a more detailed comparison with congeners was conducted. The common names of the bird species used herein follow [2].
Measurements of the specimens (left humeri and skulls) were taken with a digital calliper (0.01 mm precision, rounded to the nearest 0.1 mm), except for the total length of the skull, which was taken with a metallic ruler, as it exceeded the length of the calliper. The following abbreviations are used for the measurements. Humerus: DW, distal width; SbW, shaft base width; and SbD, shaft base depth (measured from the ventral side). Furthermore, simple proportions were calculated to aid in the comparison, namely, [DW/SBW] and [SbW/SbD]. Skull: FTD, distance between fossae temporalium; MFF, minimum interorbital width (taken between the fossae glandulae nasalis); MIF, minimum interorbital width (including the fossae glandulae nasalis); MUW, maxillary unguis width; NL, nasal aperture (apertura nasi ossea) length; NW, nasal aperture (apertura nasi ossea) width; PFNW, nasal process width (taken at the level of the processus frontalis nasalis); PoW, postorbital width (taken at the level of the processus postorbitalis); PrW, preorbital width (taken at the level of the processus supraorbitalis of the os lacrimale); and TL, total length (from the prominentia cerebellaris to the tip of the beak. Skull measurements follow [16], with the addition of MIF and MUW. The measurements can be found in the Supplementary Material (Table S1).
Principal component analyses (PCAs) were conducted in PAST (v.4.03; [17] to aid in the visualisation of morphometric differences. Two analyses were conducted, one for measurements taken from the humerus and one for cranial measurements. The total length (TL) of the skull was excluded from the PCA, given the disproportionate effect it has on the results due to its higher order of magnitude. The results of the PCA analysis can be found in the Supplementary Material (skull: Table S2; humerus: Table S3).
List of comparative Macronectes spp. material measured: Macronectes giganteus (Gmelin, 1789): OR.028535, OR.030370, and OR.30745; Macronectes halli Mathews, 1912: OR.012449, OR.013563, OR.015278, OR.015606, OR.021100, and OR.026443; and Macronectes sp.: OR.014613, OR.015877, OR.015878, OR.017608, OR.025650, OR.028597, OR.029141, OR.029173, OR.030014, OR.030324, OR.030385, OR.030687, OR.031078, S.000704, S.000742, S.000744, S.000746, S.000748, S.000923, and S.000949.
3. Systematics
The fossil skull (Figure 1) is a clear indication of generic affinity, given the characteristic large bulbous bill shape, resulting from a wider and enlarged corpus ossis premaxillaris and a deeper proximal premaxilla (Figure 2; [16]). Furthermore, compared to other Procellariidae (including fulmars), the apertura nasi ossea (nasal aperture) is proportionately shorter (in relation to bill length) in Macronectes spp., and the region between the orbits and nasal aperture is more elongated [18].
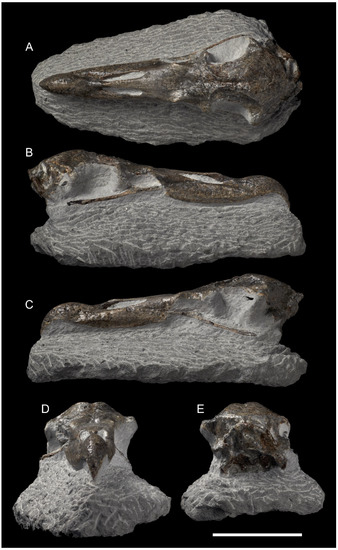
Figure 1.
Skull (holotype, NMNZ S.048502) of Macronectes tinae sp. nov., partially embedded in matrix, in different views; scale bar = 5 cm. (A) Dorsal view. (B) Lateral view (right). (C) Lateral view (left). (D) Anterior view. (E) Caudal view.
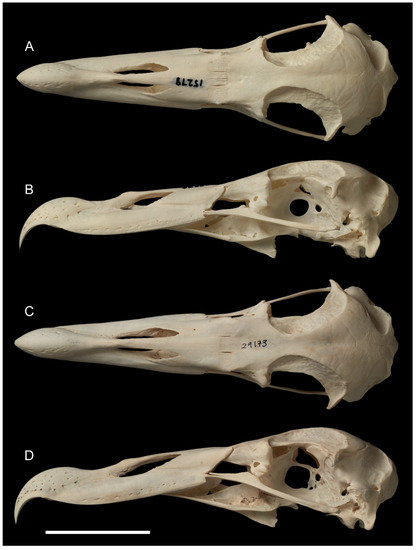
Figure 2.
Skulls (except mandible) of Macronectes spp.; scale bar = 5 cm. (A,B) M. giganteus, NMNZ OR.015278. (C,D) M. halli, NMNZ OR.029173.
The classification of the fossil humerus (Figure 3) in Procellariidae can be inferred based on: (1) the spur-like, extended processus supracondylaris dorsalis ([14]; the “ectepycondilar prominence” sensu [13]); and (2) the epicondylus ventralis (“ectepicondyle” sensu [13]; “convex bulge” sensu [19]) that craniocaudally slopes, forming a protrusion on the ventral margin [19]. It differs from larger Procellariiformes, such as Diomedea spp. and Thalasarche spp., by a greater expansion of the distal end of the humerus, a more proximally positioned processus supracondylaris dorsalis (ectepicondylar process), and a deeper fossa medialis brachialis (Figure 4).

Figure 3.
Left humeri of Macronectes spp. in cranial view; scale bar = 5 cm. (A) Macronectes tinae sp. nov., paratype NMNZ S.048870 (proximal end not preserved). (B) M. giganteus, NMNZ OR.029141. (C) M. halli, NMNZ OR.029173.
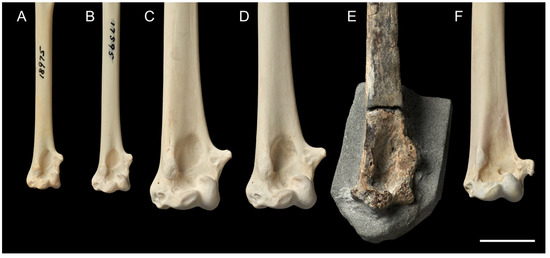
Figure 4.
Detail of proximal end of the left humeri of selected Procellariiformes in cranial view; scale bar = 2 cm. (A) Antarctic petrel Thalassoica antarctica (Gmelin, 1789), NMNZ OR.018975. (B) Antarctic fulmar Fulmarus glacialoides (Smith, 1840), NMNZ OR.017595. (C) Macronectes giganteus, NMNZ OR.029141. (D) Macronectes halli, NMNZ OR.029173. (E) Macronectes tinae sp. nov., paratype NMNZ S.048870. (F) Indian yellow-nosed albatross Thalasarche carteri (Rothschild, 1903), NMNZ OR.028477.
The defining feature of the humerus in the genus Macronectes is, unfortunately, at the proximal end: a weakly developed second (dorsal) fossa pneumotricipitalis [19]. Nevertheless, the dorsal end of the fossil humerus (Figure 3) bears a closer resemblance to Macronectes spp. than to other Procellariidae, including fulmars (the sister taxa; [6]) and other fulmarine petrels, which are much smaller birds overall. However, it is notable that, like the fossil, smaller fulmarine petrels have a slightly more oval fossa medialis brachialis and a more bulbous epicondylus ventralis (Figure 4). The position and general shape of the condyles are the same in the fossil and the two modern species of Macronectes (Figure 4). It is worth noting, however, that the humeri of modern Macronectes spp. have a proportionately shorter and wider distal end compared with the fossil and other fulmarine petrels (Figure 4).
The fusion of cranial and humeral elements (lacking visible sutures) indicates that these bones belonged to adult individuals. The differences in the size, proportions, and morphological structures of the fossils in comparison to living congeners allow the description of a new species (see below for a detailed comparison).
- Order Procellariiformes Fürbringer, 1888
- Family Procellariidae Leach, 1820
- Genus Macronectes Richmond, 1905
- Macronectes tinae sp. nov.
- ZooBank reg. nr.: urn:lsid:zoobank.org:act:EB59C374-6AE6-4FB8-8D22-0F2747CD6F3A
- Holotype: NMNZ S.048502 (col. Alastair Johnson, 2017): largely complete skull (Figure 1).
- Paratype: NMNZ S.048870 (col. Alastair Johnson, 2019): left humerus, fragmentary, only the shaft and distal end remaining (Figure 3A). The shaft is to be broken near where the crista deltopectoralis would terminate.
- Type locality and stratum: New Zealand, North Island, southern Taranaki, Hāwera. Tangahoe Formation. The holotype and paratype were surface collected as beach boulders and do not have an exact Fossil Record Electronic Database number, but see Q21/f0002 for nearby location.
- Age: Late Pliocene, Piacenzian (“Waipipian Stage” in the New Zealand scale): constrained to 3.36–3.06 Ma, based on the oxygen isotope stage and magnetic polarity data [10].
- Etymology: The specific epithet honours Tina King, the late partner of fossil collector Alastair Johnson. This giant petrel skull was her favourite fossil, hence the homage.
- Measurements: Skull: FTD = 7.2 mm; MFF = 6.9 mm; MIF = 20.5 mm; MUW = 12.6 mm; NL = 23.1 mm; NW = 4.5 mm; PFNW = 22.2 mm; PoW = 48.8 mm (estimate); PrW = 34.9 mm (estimate); TL = 148 mm. Humerus: total (preserved) length = 166 mm; DW = 26.8 mm; SbW = 14.7 mm; SbD = 6.8 mm.
- Diagnosis: Skull: overall smaller size; proportionately longer apertura nasi ossea; apparently shallower os supraocciptale. Humerus: shaft proportionately craniocaudally less deep, with more delicate appearance than congeners; medial portion of epicondylus ventralis more prominent; fossa medialis brachialis proportionately larger, elongated, and nearly fusiform.
- Differential diagnosis: There is little intraspecific and interspecific variation in the skull and humerus morphology between M. giganteus and M. halli ([16,18]; this study: Table S1), barring sexual dimorphism (males are larger; [16,20,21]) and the slightly smaller average size of M. halli (the size range of the two species completely overlap; [1,7]). That is to be expected from taxa with little genetic distinction [6]. As such, morphological comparisons can be made between M. tinae sp. nov. and both living Macronectes spp. simultaneously.
- Skull: The skull of M. tinae sp. nov. (Figure 1) is smaller than all Macronectes spp. in the NMNZ collection (Table S1) and can be instantly diagnosed by its size. Barring the size difference, almost all other structures are the same as in living Macronectes spp. (Figure 2), with two exceptions: the fossa temporalium and the os supraocciptale.
According to the PCA, PC1 explains circa 55% of variance, PC2 26%, and PC3 8% (Table S2). PC1 values are strongly related to almost all measurements, except FTD (distance between fossae temporalium); larger values of PC1 mean larger sizes. PC2 is mostly related to FTD, with larger PC2 values indicating larger FTD. PC3 is related to MFF (minimum interorbital width) and NL (length of the nasal aperture); larger PC3 values indicate larger MFF, but smaller NL. In a PC1 × PC2 plot (Figure 5B), there is not much difference between M. tinae sp. nov. and living Macronectes spp. (which greatly overlap). However, a PC1 × PC3 plot (Figure 5C) shows M. tinae sp. nov. is separated from the two recent species due to its low PC3 value (potentially due to NL).
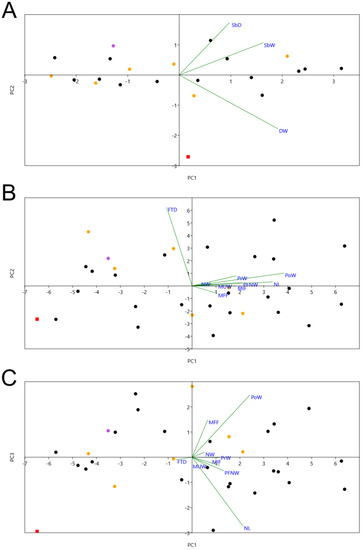
Figure 5.
Plots of PC1 × PC2 from the PCA of (A) humerus and (B) skull, and (C) plot of PC1 × PC3 (skull). The red square represents M. tinae sp. nov.; purple circle is M. giganteus; orange circles are M. halli; and black circles are Macronectes sp. See Materials and Methods for abbreviations.
The length of the nasal aperture (Table S1) of M. tinae sp. nov. is roughly the same as in living giant petrels, making it proportionately larger in the fossil (in relation to the rest of the skull).
Furthermore, the os supraocciptale (supraocciptal bone) of M. tinae sp. nov. (Figure 1) is apparently shallower than in living Macronectes spp., even considering the smallest specimens of the latter. The depth of this bone in M. tinae sp. nov. is likely somewhere between 1/2 and 2/3 of the depth observed in recent Macronectes spp. (Figure 2) and could be an important diagnostic feature. However, due to the light deformation of the fossil, this cannot be stated with precision, and no reliable measurements could be taken from this bone to include in the PCA. As such, this must remain as a qualitative comparison for the moment.
Finally, the crista nuchalis transversa is apparently more prominent in M. tinae sp. nov. than in its living congeners, although this feature might have been exacerbated in the present fossil due to preservation (caudal end of skull lightly crushed; Figure 1).
- Humerus: The humerus of M. tinae sp. nov. is about as big as the smallest Macronectes spp. in the NMNZ collection (e.g., NMNZ OR.015606; Table S1). The distal end of the fossil is more delicate than that of living Macronectes spp., with its shaft being proportionately less deep (Figure 3; Table S1: larger [SbW/SbD] measure in M. tinae sp. nov.). According to the PCA, PC1 explains 81% of variance and PC2 17% (Table S3). Larger PC1 values mean greater W, SbW, and SbD, while larger PC2 values mean greater SbW and SbD (thicker shaft), but lower W (smaller distal end). By plotting PC1 × PC2 (Figure 5A), it is clear that M. tinae sp. nov. is separated from the two recent species (which largely overlap) due to its different proportions, as explained above.
The base of the processus supracondylaris dorsalis of the fossil has the same shape as found in living Macronectes spp., but its spur-like extension is broken off (Figure 3A). Likewise, the condylus dorsalis (dorsal/external condyle), condylus ventralis (ventral condyle), and epicondylus ventralis (ventral epicondyle) are all worn, but what remains of them has similar shapes to the equivalent condyles found in the living species (Figure 3). The epicondylus ventralis appears to extend further distally than the condylus dorsalis in the fossil (Figure 3A). This may be partly due to damage; nevertheless, it appears that the epicondylus ventralis was more developed in M. tinae sp. nov. than in its congeners (Table S1: larger [DW/SBW] measure in M. tinae sp. nov.). Other fulmarine petrel genera tend to have a slightly more prominent epicondylus ventralis than Macronectes spp. (Figure 4). The fossa medialis brachialis (brachial fossa) of M. tinae sp. nov. is proportionately larger than in its congeners and has an elongated, nearly fusiform shape (Figure 3A); it is more circular in living Macronectes spp. (Figure 3B,C). The fossa medialis brachialis in other fulmarine petrel genera is usually similar in shape to that found in modern Macronectes spp.; however, a few Thalassoica spp. show a more elongate shape, approaching that seen in M. tinae sp. nov. (Figure 4). The caudal view of the distal humerus remains obstructed by sediment (Figure 3A).
4. Discussion
The skeletal differences between M. tinae sp. nov. and its living congeners are easily observable and sufficient to establish a new extinct species. The functional significance (if any) of these differences, however, remains unknown. Nevertheless, given the morphological similarities and the young age of the fossil (late Pliocene, ca. 3.36–3.06 Ma), it can be expected that M. tinae sp. nov. had a generally similar anatomy and habits to its congeners, the present-day giant petrels (Figure 6).

Figure 6.
Artistic reconstruction of Macronectes tinae sp. nov. in its palaeoenvironment. Illustration by Simone Giovanardi, © Te Papa (CC-BY 4.0). A darker plumage was chosen for the reconstruction because a darker colouration in giant petrels seems to be related to warmer regions [7], as Taranaki had warmer temperatures during the Pliocene [22].
All evidence from the size of the fossils (Table S1) suggests that individuals of M. tinae sp. nov. had smaller bodies than both M. halli and M. giganteus (including the smaller-bodied potential subspecies M. g. solanderi (Mathews, 1912) from the southern Atlantic [23], which is currently not accepted as a separate taxon [2]). The slenderer and more delicate humerus is also an indication that members of Macronectes in the Pliocene had not yet achieved the bulk of recent species (the largest birds among the Procellariidae) and that the ancestors of Macronectes were smaller, as might be predicted because their closest relatives are much smaller. One possible reason for the smaller size of the fossil species is that the species occupied relatively warm waters, in a similar way to the Diomedea exulans group sensu lato, where the smaller taxa occupy more northern breeding sites [24]. The average temperatures of the Tangahoe Formation palaeoenvironment were higher than in the present [22]. However, while living Macronectes spp. generally nest in the subantarctic, both species range into tropical waters, particularly as juveniles [7].
Giant petrels are more littoral birds than other Procellariidae and, notably, are the only species in the family that can effectively stand and walk on land [1,25]. On land, they tend to be gregarious opportunistic scavengers and predators, depending largely on seal and penguin colonies on the shore for both carcasses and chicks [7,20,26,27]. Giant petrels also prey upon other seabirds, and hunt cephalopods and fish near the water surface, also taking krill to feed their chicks [1,25,28,29]. The diet of females has a larger proportion of fish, cephalopods, and crustaceans [27].
The Tangahoe Formation in Taranaki represents a coastal palaeoenvironment [10] suitable for giant petrels. It contains fossils of colonial mammals and seabirds that would have provided a ready food source for them (Figure 6), such as the monk seal Eomonachus belegaerensis (Rule et al., 2020) and the dawn crested penguin Eudyptes atatu (Thomas, Tennyson, Scofield & Ksepka, 2020) [30,31].
The seabird fauna of the Tangahoe Formation is starting to be studied in more depth and is proving to be quite diverse. Besides the penguin, there are fossils of three other Procellariiformes: Pom’s shearwater Ardenna davealleni (Tennyson & Mannering, 2018), the deep-billed petrel Procellaria altirostris (Tennyson & Tomotani, 2021), and Alastair’s albatross Aldiomedes angustirostris (Mayr & Tennyson, 2020) [32,33,34]. The fossils of all three species were found in the same outcrops as the penguins, seals, and the presently described giant petrel. There are also undescribed Pelagornithidae fossils [2,35], an extinct family of seabirds.
Macronectes tinae sp. nov. is the first pre-Quaternary fossil giant petrel ever reported. The location where the fossils were found in New Zealand is within the species’ present at-sea distribution [7]; therefore, Macronectes tinae sp. does not have clear biogeographical implications.
It has been speculated [36] that Macronectes originated in the late Oligocene. Fulmar species (Fulmarus spp.), the sister taxa of Macronectes, have a fossil record spanning back to the middle Miocene of California, USA (ca. 16.0–15.2 Ma; [37,38,39]), Fulmarus hammeri (Howard, 1968) and F. miocaenus (Howard, 1984); this would imply that Fulmarus and Macronectes had already split before that date. One molecular estimate put the divergence of these two genera at around 7.8 Ma, while the divergence between the clades Fulmarus+Macronetes and Thalassoica+Pagodroma was estimated at around 15.9 Ma [6]. Another molecular study put the divergence date between Macronectes and Fulmarus at 1.8 Ma [40]. The presence of Macronectes tinae sp. nov. in the Pliocene and the Fulmarus species in the Miocene indicate that both molecular studies have underestimated the divergence dates.
No fossils of Pagodroma, Thalassoica, or Daption are known, but a Fulmarinae gen et sp. indet. from the Pliocene of South Africa is considered to be closely related to Daption [41] (see also the fossil Procellariidae gen. et sp. Indet. Mentioned by [42]). Daption is the sister clade to all other fulmarine petrels, with an estimated divergence from them at circa 26.2 Ma [6], so that fossil does not add much information.
5. Conclusions
A new species of extinct giant petrel, Macronectes tinae sp. nov., from the late Pliocene (Piacenzian) of Taranaki, New Zealand, is described herein. The Tangahoe Formation continues to provide outstanding seabird fossils and is becoming an important piece of the puzzle to understand the evolution and biogeography of seabirds in New Zealand and beyond. New Zealand, in particular, is considered a global centre of procellariiform diversity [43], a status that was probably already in place in the late Pliocene.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/taxonomy3010006/s1, Table S1: Specimen measurements; Table S2: PCA results (skull); Table S3: PCA results (humerus).
Author Contributions
Conceptualization, A.J.D.T.; methodology, investigation, data curation, writing (original draft preparation), writing (review and editing), R.B.S. and A.J.D.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Te Papa Collection Development Fund (New Zealand).
Data Availability Statement
All data can be found within the article and its Supplementary Materials.
Acknowledgments
We thank Jean-Claude Stahl (NMNZ) for the photographs used herein; Alastair Johnson and John Buchanan-Brown for their expert preparation work on the fossil Macronectes specimens; Simone Giovanardi for the beautiful illustration; and the three anonymous reviewers for their helpful comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carboneras, C. Family Procellariidae (petrels and shearwaters). In Handbook of the Birds of the World; del Hoyo, J., Elliot, A., Sargatal, J., Eds.; Lynx: Barcelona, Spain, 1992; pp. 216–239. [Google Scholar]
- Checklist Committee OSNZ. Checklist of the Birds of New Zealand, 5th ed.; Ornithological Society of New Zealand Occasional Publication No. 1; Ornithological Society of New Zealand: Wellington, New Zealand, 2022; 335p. [Google Scholar]
- Bourne, W.P.; Warham, J. Geographical variation in the giant petrels of the genus Macronectes. Ardea 1966, 54, 45–67. [Google Scholar]
- Voisin, J.-F. Les pétrels géants Macronectes giganteus et M. halli de l’île de la Possession (archipel Crozet). Oiseau 1968, 38, 95–122. [Google Scholar]
- Rheindt, F.E.; Austin, J. Major analytical and conceptual shortcomings in a recent taxonomic revision of the Procellariiformes—A reply to Penhallurick and Wink (2004). Emu-Austral Ornithol. 2005, 105, 181–186. [Google Scholar] [CrossRef]
- Penhallurick, J.; Wink, M. Analysis of the taxonomy and nomenclature of the Procellariiformes based on complete nucleotide sequences of the mitochondrial cytochrome b gene. Emu 2004, 104, 125–147. [Google Scholar] [CrossRef]
- Marchant, S.; Higgins, P.J. Handbook of Australian, New Zealand and Antarctic Birds. Vol. 1: Ratites to Ducks, Part A—Ratites to Petrels. Oxford University Press: Melbourne, Australia, 1990; 736p. [Google Scholar]
- Avery, G.A.; Klein, R.G. Review of fossil phocid and otariid seals from the southern and western coasts of South Africa. Trans. R. Soc. S. Afr. 2011, 66, 14–24. [Google Scholar] [CrossRef]
- Tennyson, A.J.D. Holocene bird bones found at the subantarctic Auckland Islands. Notornis 2020, 67, 269–294. [Google Scholar]
- Naish, T.R.; Wehland, F.; Wilson, G.S.; Browne, G.H.; Cook, R.A.; Morgans, H.E.G.; Rosenberg, M.; King, P.R.; Smale, D.; Nelson, C.S.; et al. An integrated sequence stratigraphic, palaeoenvironmental, and chronostratigraphic analysis of the Tangahoe Formation, southern Taranaki coast, with implications for mid-Pliocene (c. 3.4–3.0 Ma) glacio-eustatic sea-level changes. J. R. Soc. N. Z. 2005, 35, 151–196. [Google Scholar] [CrossRef]
- McKee, J. Geology and vertebrate palaentology of the Tangahoe Formation, South Taranaki Coast, New Zealand. Geol. Soc. N. Z. Misc. Publ. B 1994, 80, 63–91. [Google Scholar]
- Thomas, D.B.; Tennyson, A.J.D.; Scofield, R.P.; Heath, T.A.; Pett, W.; Ksepka, D.T. Ancient crested penguin constrains timing of recruitment into seabird hotspot. Proc. R. Soc. B Boil. Sci. 2020, 287, 20201497. [Google Scholar] [CrossRef]
- Gilbert, B.M.; Martin, L.D.; Savage, H.G. Avian Osteology; Modern Printing: Laramie, WY, USA, 1981; 252p. [Google Scholar]
- Baumel, J.J.; Witmer, L.M. Osteologia. In Handbook of Avian Anatomy: Nomina Anatomica Avium; Baumel, J.J., King, A.S., Breazile, J.E., Evans, H.E., Vanden Berge, J.C., Eds.; Nuttall Ornithological Club: Cambridge, UK, 1993; pp. 45–132. [Google Scholar]
- Livezey, B.C.; Zusi, R.L. Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy. II. Analysis and discussion. Zool. J. Linn. Soc. 2007, 149, 1–95. [Google Scholar] [CrossRef]
- Piro, A.; Hospitaleche, C.A. Skull morphology and ontogenetic variation of the Southern Giant Petrel Macronectes giganteus (Aves: Procellariiformes). Polar Biol. 2019, 42, 27–45. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]
- Mazzochi, M.S.; Carlos, C.J. Skull morphology of four Antarctic fulmarine petrels (Aves: Procellariiformes): Insights into their feeding biology. Polar Biol. 2022, 45, 191–201. [Google Scholar] [CrossRef]
- Mayr, G.; Smith, T. Phylogenetic affinities and taxonomy of the Oligocene Diomedeoididae, and the basal divergences amongst extant procellariiform bird. Zool. J. Linn. Soc. 2012, 166, 854–875. [Google Scholar] [CrossRef]
- Conroy, J.W.H. Ecological aspects of the biology of the Giant Petrel, Macronectes giganteus (Gmelin), in the maritime Antarctic. Br. Antarct. Surv. Sci. Rep. 1972, 75, 1–74. [Google Scholar]
- Carlos, C.J.; Voisin, J.F. Identifying giant petrels, Macronectes giganteus and M. halli, in the field and in the hand. Seabird 2008, 21, 1–15. [Google Scholar]
- Hendy, A.J.; Kamp, P.J.; Vonk, A.J. Late Miocene turnover of molluscan faunas, New Zealand: Taxonomic and ecological reassessment of diversity changes at multiple spatial and temporal scales. Palaeogeogr. Palaeoclim. Palaeoecol. 2009, 280, 275–290. [Google Scholar] [CrossRef]
- Salomon, M.; Voisin, J.-F. Ecogeographical variation in the Southern Giant Petrel (Macronectes giganteus). Can. J. Zool. 2010, 88, 195–203. [Google Scholar] [CrossRef]
- Schodde, R.; Tennyson, A.J.; Groth, J.G.; Lai, J.; Scofield, R.; Steinheimer, F.D. Settling the name Diomedea exulans Linnaeus, 1758 for the Wandering Albatross by neotypification. Zootaxa 2017, 4236, 135–148. [Google Scholar] [CrossRef]
- Prince, P.A.; Morgan, R.A. Diet and feeding ecology of Procellariiformes. In Seabird: Feeding Ecology and Role in Marine Ecosystems; Croxall, J.P., Ed.; Cambridge University Press: Cambridge, UK, 1987; pp. 135–171. [Google Scholar]
- Hunter, S. The role of giant petrels in the Southern Ocean ecosystem. In Antarctic Nutrient Cycles and Food Webs; Siegfried, W.R., Condy, P.R., Laws, R.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 534–542. [Google Scholar]
- Reisinger, R.R.; Carpenter-Kling, T.; Connan, M.; Cherel, Y.; Pistorius, P.A. Foraging behaviour and habitat-use drives niche segregation in sibling seabird species. R. Soc. Open Sci. 2020, 7, 200649. [Google Scholar] [CrossRef]
- Obst, B.S. Densities of Antarctic Seabirds at Sea and the Presence of the Krill Euphausia superba. Ornithology 1985, 102, 540–549. [Google Scholar] [CrossRef]
- Harper, P.C. Feeding behaviour and other notes on 20 species of Procellariiformes at sea. Notornis 1987, 34, 169–192. [Google Scholar]
- Rule, J.P.; Adams, J.W.; Marx, F.G.; Evans, A.R.; Tennyson, A.J.D.; Scofield, R.P.; Fitzgerald, E.M.G. First monk seal from the Southern Hemisphere rewrites the evolutionary history of true seals. Proc. R. Soc. B Boil. Sci. 2020, 287, 20202318. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.B.; Ksepka, D.T.; Holvast, E.J.; Tennyson, A.J.D.; Scofield, P. Re-evaluating New Zealand’s endemic Pliocene penguin genus. N. Z. J. Geol. Geophys. 2020, 63, 324–330. [Google Scholar] [CrossRef]
- Tennyson, A.J.D.; Mannering, A.A. A new species of Pliocene shearwater (Aves: Procellariidae) from New Zealand. Tuhinga 2018, 29, 1–19. [Google Scholar]
- Mayr, G.; Tennyson, A.J.D. A small, narrow-beaked albatross from the Pliocene of New Zealand demonstrates a higher past diversity in the feeding ecology of the Diomedeidae. Ibis 2020, 162, 723–734. [Google Scholar] [CrossRef]
- Tennyson, A.J.D.; Tomotani, B.M. A new fossil species of Procellaria (Aves: Procellariiformes) from the Pliocene of New Zealand. Papéis Avulsos De Zool. 2021, 61, e20216116. [Google Scholar] [CrossRef]
- McKee, J.W.A. A pseudodontorn (Pelecaniformes: Pelagornithidae) from the middle Pliocene of Hawera, Taranaki, New Zealand. N. Z. J. Zool. 1985, 12, 181–184. [Google Scholar] [CrossRef]
- Imber, M.J. Origins, phylogeny and taxonomy of the gadfly petrels Pterodroma spp. Ibis 1985, 127, 197–229. [Google Scholar] [CrossRef]
- Howard, H. Tertiary birds from Laguna Hill, Orange County, California. Los Angeles Cty. Mus. Contrib. Sci. 1968, 142, 1–21. [Google Scholar] [CrossRef]
- Howard, H. Additional avian records from the Miocene of Kern County, California with the description of a new species of fulmar. Bull. South. Calif. Acad. Sci. 1984, 83, 84–89. [Google Scholar]
- Prothero, D.R.; Sanchez, F.; Denke, L.I. Magnetic stratigraphy of the early to middle Miocene Olcese Sand and Round Mountain Silt, Kern County, California. New Mex. Mus. Nat. Hist. Sci. Bull. 2008, 44, 357–363. [Google Scholar]
- Techow, N.; O’Ryan, C.; Phillips, R.; Gales, R.; Marin, M.; Patterson-Fraser, D.; Quintana, F.; Ritz, M.; Thompson, D.; Wanless, R.; et al. Speciation and phylogeography of giant petrels Macronectes. Mol. Phylogenetics Evol. 2010, 54, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.L. An early Pliocene marine avifauna from Duinefontein, Cape Province, South Africa. Ann. South Afr. Mus. 1985, 95, 147–164. [Google Scholar]
- Olson, S.L. Early Pliocene Procellariiformes (Aves) from Langebaanweg, south-western Cape Province, South Africa. Ann. South Afr. Mus. 1985, 95, 123–145. [Google Scholar]
- Dickinson, E.C.; Remsen, J.V. The Howard & Moore Complete Checklist of the Birds of the World, 4th ed.; A & C Black: London, UK, 2013; 752p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).