The Origin of the Mangrove and Saltmarsh Snail Ellobium (Eupulmonata, Ellobiidae) †
Abstract
1. Introduction
1.1. The Habitat of Ellobium
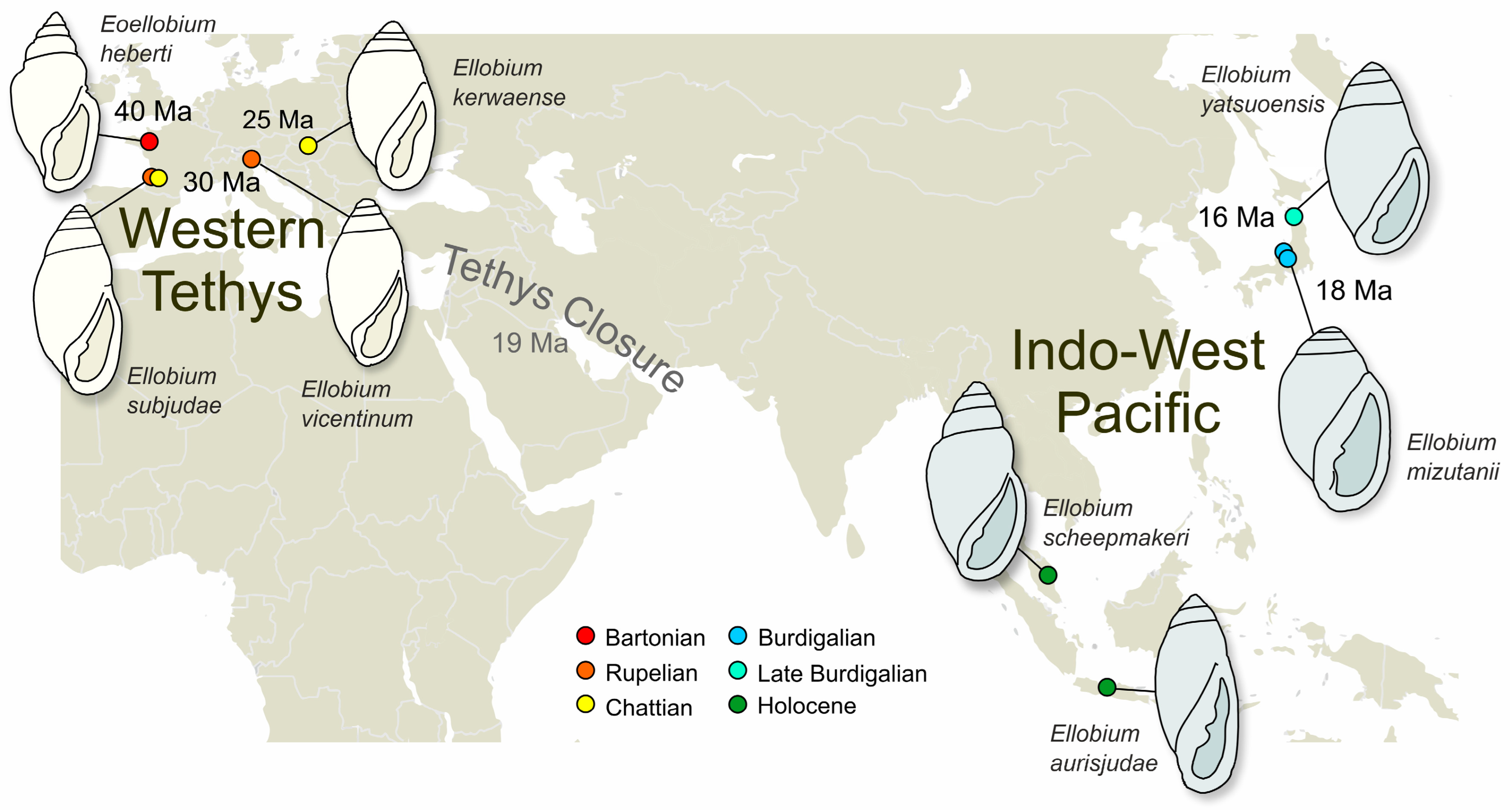
1.2. The Fossil Record of Ellobium
2. Material and Methods
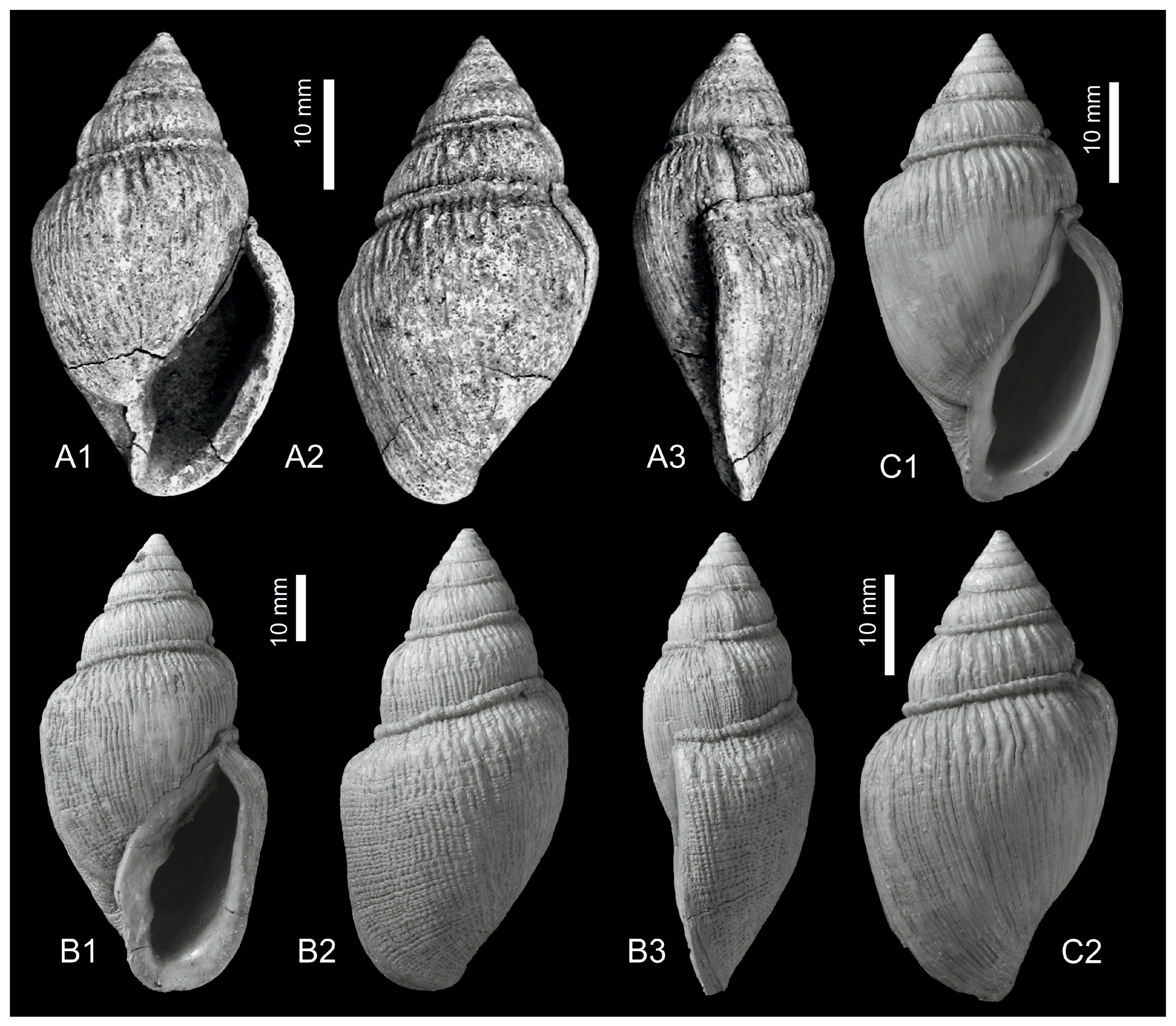
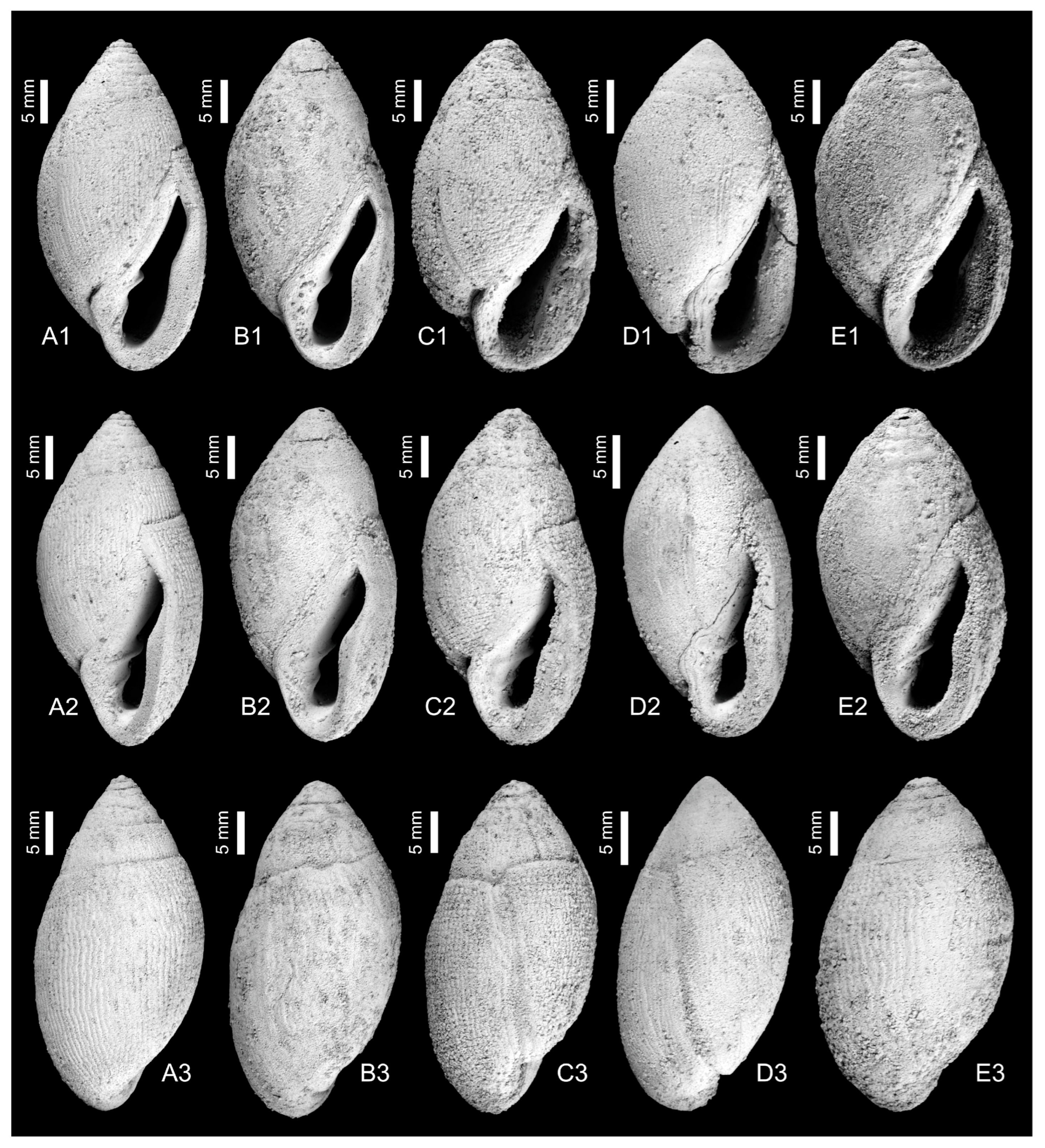
3. Systematics
4. Discussion
The Extinction of Ellobium in European Seas
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NHMW | Natural History Museum Vienna (Austria) |
| MNHN.F. | Muséum national d’Histoire naturelle, Paris (France) |
| RGM | Naturalis Biodiversity Center, Leiden (Netherlands) |
References
- Morton, J.E. The evolution of the Ellobiidae with a discussion on the origin of the Pulmonata. Proc. Zool. Soc. Lond. 1955, 125, 127–168. [Google Scholar] [CrossRef]
- Ellison, A.M.; Farnsworth, E.J.; Merkt, R.E. Origins of mangrove ecosystems and the mangrove biodiversity anomaly. Glob. Ecol. Biogeogr. 1999, 8, 95–115. [Google Scholar] [CrossRef]
- Teoh, H.W.; Sasekumar, A.; Ismael, M.H.; Chong, V.C. Trophic discrimination factor and the significance of mangrove litter to benthic detritivorous gastropod, Ellobium aurisjudae (Linnaeus). J. Sea Res. 2018, 131, 79–84. [Google Scholar] [CrossRef]
- Tan, S.K.; Tan, S.H.; Low, M.E.Y. On Ellobium aurismalchi (Müller, 1774) (Mollusca: Ellobiidae). Nat. Singap. 2009, 2, 357–359. Available online: https://lkcnhm.nus.edu.sg/wp-content/uploads/sites/10/app/uploads/2017/04/2009nis357-359.pdf (accessed on 27 January 2023).
- Lim, S.-Y.; Lee, C.-S.; Kim, M.-S.; Yoo, S.-H. The conservation value of endangered marine species: The case of the Ellobium chinense. J. Korean Soc. Mar. Environ. Saf. 2015, 21, 645–654. [Google Scholar] [CrossRef]
- MolluscaBase (Ed.) MolluscaBase. Ellobium Röding, 1798. 2022. Available online: http://molluscabase.org/aphia.php?p=taxdetails&id=206412 (accessed on 5 November 2022).
- Linnaeus, C. Systema Naturae per Regna Tria Naturae… Editio Decima, Reformata; Laurentii Salvii: Holmiae, Sweden, 1758; Volume 1, 823p, Available online: https://www.biodiversitylibrary.org/item/10277#page/3/mode/1up (accessed on 27 January 2023).
- Pfeiffer, L. Drei neue Auriculaceen. Malakozool. Blätter 1855, 2, 7–8. Available online: https://www.biodiversitylibrary.org/page/15864748 (accessed on 27 January 2023).
- Petit de la Saussaye, M. Description de Coquilles Nouvelles. J. De Conchyliol. 1850, 1, 402–406. Available online: https://www.biodiversitylibrary.org/page/25136220 (accessed on 27 January 2023).
- Preston, H.B. New species and varieties of terrestrial and fluviatile shells from Equatorial Africa. Rev. De Zool. Afr. 1913, 3, 47–62, 212. Available online: https://www.biodiversitylibrary.org/item/103048#page/63/mode/1up (accessed on 27 January 2023).
- Orbigny, A. D’ Synopsis terrestrium et fluviatilium molluscorum, in suo per Americam meridionalem itinere collectorum. Mag. De Zool. 1835, 5, 1–44. Available online: https://www.biodiversitylibrary.org/page/2633132 (accessed on 27 January 2023).
- Keen, A.M. Sea Shells of Tropical West America. Marine Mollusks from Baja California to Peru, 2nd ed.; Stanford University Press: Stanford, CA, USA, 1971; 1064p. [Google Scholar]
- Férussac, A.E.J.P.F. d’Audebard de. Tableau systématique des Pulmonés Géhydrophiles, tableau systématique de la famille des Auricules. In Deuxième Partie. Tableaux Particuliers des Mollusques Terrestres et Fluviatiles, Présentant Pour Chaque famille les Genres et Espèces Qui la Composent. Classe des Gastéropodes. Ordre des Pulmonés Sans Opercules; Bertrand: Paris, France, 1821; pp. 96–114. [Google Scholar]
- Martins, A.M.F. Anatomy and Systematics of the Western Atlantic Ellobiidae (Gastropoda: Pulmonata). Malacologia 1996, 37, 163–356. Available online: https://www.biodiversitylibrary.org/page/13113594#page/179/mode/1up (accessed on 27 January 2023).
- Rosenberg, G.; Moretzsohn, F.; García, E.F. Gastropoda (Mollusca) of the Gulf of Mexico. In Gulf of Mexico–Origins, Waters, and Biota; Felder, D.L., Camp, D.K., Eds.; Texas A&M Press: College Station, TX, USA, 2009; pp. 579–699. [Google Scholar]
- Groh, K. Ellobiidae. In Philippine Marine Mollusks; Poppe, G.T., Ed.; Coch Books: Hackenheim, Germany, 2010; Volume 3, pp. 446–457. [Google Scholar]
- Raven, H.; Vermeulen, J.J. Notes on molluscs from NW Borneo and Singapore. 2. A synopsis of the Ellobiidae (Gastropoda, Pulmonata). Vita Malacol. 2007, 4, 29–62. [Google Scholar]
- Piamklad, S.; Tuntiwaranurak, C.; Dumrongrojwattana, P. Malacofauna diversity and distribution (Mollusca: Gastropoda, Bivalvia) at Pak Nam Pran Mangrove Forest in Pran Buri District, Prachuap Khiri Khan Province, Thailand. In Proceedings of the Burapha University International Conference, Pattaya, Thailand, 3–4 July 2014; Burapha University: Chon Buri, Thailand, 2014. STP839-36. [Google Scholar]
- Faezah, P.; Farah, H.S. Composition of gastropods in mangroves of Tanjung Dawai and Pulau Sayak, Kedah. Malays. Appl. Biol. 2011, 40, 13–17. [Google Scholar]
- Gmelin, J.F. Vermes. Caroli a Linnaei Systema Naturae per Regna Tria Naturae, 13th ed.; Beer, G.E.: Lipsiae, Germany, 1971; pp. 3021–3910. Available online: http://www.biodiversitylibrary.org/item/83098#5 (accessed on 27 January 2023).
- Reeve, L.A. Monograph of the genus Nerita. In Conchologia Iconica, or, Illustrations of the Shells of Molluscous Animals; L. Reeve & Co., Ltd.: London, UK, 1855; Volume 9, Available online: https://www.biodiversitylibrary.org/page/9141840 (accessed on 27 January 2023).
- Manullang, T.; Bakti, D.; Leidonald, R. Structure of gastropod communities at mangrove ecosystem in Lubuk Kertang village, West Berandan District, Langkat Regency, North Sumatera Province. IOP Conf. Ser. Earth Environ. Sci. 2018, 122, 012103. [Google Scholar] [CrossRef]
- Setyadi, G.; Rahayu, D.L.; Pribadi, R.; Hartati, R.; Wijayanti, D.; Sugianto, D.; Darmawan, A. Crustacean and mollusk species diversity and abundance in the mangrove communities of Mimika District, Papua, Indonesia. Biodiversitas J. Biol. Divers. 2021, 22, 4146–4157. [Google Scholar] [CrossRef]
- Ismail, M.H.; Chong, V.C.; Ramli, R.; Sasekumar, A. Rediscovery of Ellobium scheepmakeri (Petit De La Saussaye, 1850) (Mollusca: Gastropoda: Ellobiidae), a rare ellobiid in Bukit Belimbing mangrove forest, Peninsular Malaysia. Molluscan Res. 2017, 37, 222–226. [Google Scholar] [CrossRef]
- Petit de la Saussaye, M. Descriptions of a new species of shells belonging to the genus Auricula, collected by H. Cuming, Esq. Proc. Zool. Soc. Lond. 1843, 1842, 201–202. Available online: https://www.biodiversitylibrary.org/page/30679963 (accessed on 27 January 2023).
- Mohapatra, A. Mollusca. In Fauna of Godavari Estuary Andhra Pradesh; Estuarine Ecosystem Series; The Zoological Survey of India: Kolkota, India, 2001; Volume 4, pp. 55–82. ISBN 978-8185874531. [Google Scholar]
- Kimura, T. Why an endangered snail Ellobium chinense aggregates on the uppermost tidalflat? Nippon. Suisan Gakkaishi 2011, 77, 119. [Google Scholar] [CrossRef]
- Shin, C.R.; Choi, E.H.; Kim, G.; Baek, S.Y.; Park, B.; Hwang, J.; Jun, J.; Kil, H.J.; Oh, H.; Lee, K.; et al. Characterization of metapopulation of Ellobium chinense through Pleistocene expansions and four covariate COI guanine-hotspots linked to G-quadruplex conformation. Sci. Rep. 2021, 11, 12239. [Google Scholar] [CrossRef]
- Ngoc, H. Gastropod (Mollusca: Gastropoda) in mangrove forest ecosystem on Northern coastal area of Vietnam. J. Sci. Hnue Nat. Sci. 2008, 53, 151–158. [Google Scholar]
- Rodrigues, C.A.L.; Ribeiro, R.P.; Santos, N.B.; Almeida, Z.S. Patterns of mollusc distribution in mangroves from the São Marcos Bay, coast of Maranhão State, Brazil. Acta Amaz. 2016, 46, 391–400. [Google Scholar] [CrossRef]
- Wingard, G.L.; Stackhouse, B.L.; Daniels, A.M. Using mollusks as indicators of restoration in nearshore zones of south Florida’s estuaries. Bull. Mar. Sci. 2022, 98, 351–380. [Google Scholar] [CrossRef]
- Wenz, W. Fossilium Catalogus I: Animalia. Gastropoda Extramarina Tertiaria; Junk, W.: Berlin, Germany, 1923; Pars 21; pp. 1069–1420. Available online: https://www.biodiversitylibrary.org/item/125704#page/49/mode/1up (accessed on 27 January 2023).
- Glibert, M. Euthyneura et Pulmonata fossiles du Cénozoïque étranger des collections de l’Institut Royal des sciences naturelles de Belgique. Mémoires De L’institut R. Des Sci. Nat. De Belg. 1962, 70, 1–140. Available online: http://www.vliz.be/imisdocs/publications/252233.pdf (accessed on 27 January 2023).
- Glibert, M. Révision des Gastropoda du Danien et du Montien de la Belgique, tome 1: Les Gastropoda du calcaire de Mons. Mémoires De L’institut R. Des Sci. Nat. De Belg. 1973, 173, 7–116. Available online: https://biblio.naturalsciences.be/rbins-publications/memoirs-of-the-royal-belgian-institute-of-natural-sciences-first-series/173-1973/vol-173-024e9bx-text.pdf (accessed on 27 January 2023).
- Plaziat, J.-C. Contribution à l’étude de la faune et de la flore du Sparnacien des Corbières septentrionales. Cah. De Paléontologie Du Cent. Natl. De La Rech. Sci. 1970, 9, 1–121. [Google Scholar]
- Huckriede, R. Molluskenfaunen mit limnischen und brackischen Elementen aus Jura, Serpulit und Wealden NW-Deutschlands und ihre paläogeographische Bedeutung. Geol. Jahrb. Beih. 1967, 67, 1–263. [Google Scholar]
- Briart, A.; Cornet, F.L. Description des fossiles du Calcaire grossier de Mons. Gastéropodes. 4e partie. Mémoires De L ‘Académie R. Des Sci. Des Lett. Et Des Beaux-Arts De Belg. 1887, 47, 1–128. Available online: http://www.vliz.be/imisdocs/publications/112983.pdf (accessed on 27 January 2023).
- Cossmann, M. Mollusques éocèniques de la Loire-Inférieure. Tome 2, Deuxième fascicule. Gastropodes (suite et fin). Bull. De La Société Des Sci. Nat. De L’ouest De La Fr. 1902, 2, 5–159. Available online: https://www.biodiversitylibrary.org/page/5853400 (accessed on 27 January 2023).
- Vasseur, G. Recherches Géologiques sur les Terrains Tertiaires de la France Occidentale; Paléontologie, Atlas: Quinsac, Toulouse, France, 1881; pl. 4 and 19. [Google Scholar]
- de Morgan, J. Observations sur les Auriculidés du Falunien de la Touraine. Bull. De La Société Géologique De Fr. 1917, 16, 21–49. Available online: https://biodiversitylibrary.org/page/31119282 (accessed on 27 January 2023).
- Tournouër, R. Diagnoses de deux Auriculidae fossiles des faluns du S. O. de la France. J. De Conchyliol. 1871, 19, 360–361. Available online: https://www.biodiversitylibrary.org/page/15136381 (accessed on 27 January 2023).
- Degrange-Touzin, A. Étude sur la faune terrestre, lacustre et fluviatile de l’Oligocène supérieur et du Miocène dans le Sud-Ouest de la France et principalement dans la Gironde. Actes De La Société Linnéenne De Bordx. 1892, 45, 125–230. Available online: https://www.biodiversitylibrary.org/page/33424078 (accessed on 27 January 2023).
- de Carle Sowerby, J. The Mineral Conchology of Great Britain; or, Coloured Figures and Descriptions of Those Remains of Testaceous Animals or Shells, which Have Been Preserved at Various Times and Depths in the Earth; Sowerby: London, UK, 1821–1823; Volume 4, pp. 1–160. Available online: https://www.biodiversitylibrary.org/page/14392771 (accessed on 27 January 2023).
- Ceulemans, L.; Van Dingenen, F.; Landau, B.M. The lower Pliocene gastropods of Le Pigeon Blanc (Loire-Atlantique, northwest France). Part 5—Neogastropoda (Conoidea) and Heterobranchia (fine). Cainozoic Res. 2018, 18, 89–176. [Google Scholar]
- Deshayes, G.-P. Encyclopédie Méthodique ou par Ordre de Matières. Histoire Naturelle des Vers et Mollusques. 1830. Volume 2. Available online: https://www.biodiversitylibrary.org/bibliography/8638#/summary (accessed on 27 January 2023).
- Sandberger, C.L.F. Die Land- und Süßwasser-Conchylien der Vorwelt; Kreidel, C.W.: Wiesbaden, Germany, 1870; pp. 1–96, 1871, pp. 97–160; 1873, pp. 257–352; Available online: https://www.biodiversitylibrary.org/item/112229#page/7/mode/1up (accessed on 27 January 2023).
- Wenz, W. Zur Nomenklatur tertiärer Land- und Süßwassergastropoden. IV. Senckenbergiana 1922, 4, 5–7. Available online: https://www.biodiversitylibrary.org/page/30068315 (accessed on 27 January 2023).
- d’Orbigny, A. Prodrome de Paléontologie. Stratigraphique Universelle des Animaux Mollusques et Rayonnés Faisant Suite au Cours Élémentaire de Paléontologie et de Géologie Stratigraphique; Victor Masson: Paris, France, 1852; Volume 3, pp. 191, 196. Available online: https://www.biodiversitylibrary.org/page/31730040 (accessed on 27 January 2023).
- Fuchs, T. Beiträge zur Kenntnis der Conchylienfauna des Vicentinischen Tertiärgebirges. Denkschr. Der Math.-Nat. Cl. Der Kais. Akad. Der Wiss. 1870, 30, 137–216. Available online: https://www.zobodat.at/pdf/DAKW_30_2_0137-0216.pdf (accessed on 27 January 2023).
- Kawase, M.; Ichihara, T. A new species of Ellobium (Gastropoda: Panpulmonata: Ellobiidae) from the Miocene Mizunami Group in Gifu Prefecture, central Japan. Bull. Mizunami Foss. Musum 2021, 48, 1–7. [Google Scholar] [CrossRef]
- Tsuda, K. New Miocene molluscs from the Kurosedani Formation, in Toyama prefecture, Japan. J. Fac. Sci. Niigata Univ. 1959, 3, 67–110. [Google Scholar]
- Vasseur, G. Recherches Géologiques sur les Terrains Tertiaires de la France Occidentale; Paléontologie, Atlas: Quinsac, Toulouse, France, 1882; pls 1–3 and 5–11 [plate 4 and 19 see Vasseur, 1881a]. [Google Scholar]
- Cossmann, M. Mollusques éocèniques de la Loire-Inférieure. (Tome 1, fascicule 1). Bull. De La Société Des Sci. Nat. De L’ouest De La Fr. 1895, 5, 159–197. Available online: https://www.biodiversitylibrary.org/item/28858#page/245/mode/1up (accessed on 27 January 2023).
- Cox, L.R. General characteristics of Gastropoda. In Treatise on Invertebrate Paleontology, Part I, Mollusca 1, Mollusca—General Features, Scaphopoda, Amphineura, Monoplacophora, Gastropoda—General Features, Archaeogastropoda and some (mainly Paleozoic) Caenogastropoda and Opisthobranchia; Knight, J.B., Cox, L.R., Keen, A.M., Smith, A.G., Batten, R.L., Yochelson, E.L., Ludbrook, N.H., Robertson, R., Yonge, C.M., Moore, R.C., Eds.; The Geological Society of America and The University of Kansas Press: Lawrence, KS, USA, 1960; pp. 169–185. [Google Scholar]
- Haszprunar, G.; Huber, G. On the central nervous system of Smeagolidae and Rhodopidae, two families questionably allied with the Gymnomorpha (Gastropoda, Euthyneura). J. Zool. Lond. 1990, 220, 185–199. Available online: http://dx.doi.org/10.1111/j.1469-7998.1990.tb04302.x (accessed on 27 January 2023).
- Pfeiffer, L. Synopsis Auriculaceorum. Malakozool. Blätter 1854, 1, 145–156. Available online: https://www.biodiversitylibrary.org/page/15864608 (accessed on 27 January 2023).
- Crosse, H. Mollusques Éocèniques de la Loire-Inférieure, par M. Cossmann [Article review]. J. De Conchyliol. 1896, 44, 106–108. Available online: https://www.biodiversitylibrary.org/item/81049#page/146/mode/1up (accessed on 27 January 2023).
- Lebrun, P.; Courville, P.; Pacaud, J.-M. Les mollusques des sables éocènes de Bois-Gouët (Loire-Atlantique). In Les coquillages de l’Éocène du Bassin Parisien, un Trésor Inestimable Vieux de Plusieurs Dizaines de Millions d’années. Fossiles, Revue française de Paléontologie; Lebrun, P.: Paris, France, 2012; Volume 3, pp. 92–97. [Google Scholar]
- Vasseur, G. Recherches géologiques sur les terrains tertiaires de la France occidentale. Ann. Des Sci. Géologiques 1881, 13, 1–432. [Google Scholar]
- Doncieux, L. Catalogue descriptive des fossiles nummulitiques de l’Aude et de l’Hérault. Deuxième Partie (Fascicule 1). Corbières septentrionales. Ann. De L’université De Lyon N.S. 1908, 22, 1–250. Available online: https://www.biodiversitylibrary.org/item/186558#page/11/mode/1up (accessed on 30 January 2023).
- Calzada, S.; Urquiola, M.M. Algunos gasterópodos de El Bruc. Batalleria 1995, 5, 29–36. [Google Scholar]
- Röding, P.F. Museum Boltenianum Sive Catalogus Cimeliorum e Tribus Regnis Naturæ Quæ Olim Collegerat Joa. Fried Bolten, M.D. p. d. per XL. Annos Proto Physicus Hamburgensis. Pars Secunda Continens Conchylia Sive Testacea Univalvia, Bivalvia & Multivalvia; Trapp: Hamburg, Germany, 1798; Volume viii, 199p, Available online: https://www.biodiversitylibrary.org/page/16230659 (accessed on 27 January 2023).
- Gray, J.E. A list of the Recent Mollusca, their synonyms and types. Proc. Zool. Soc. Lond. 1847, 15, 129–219. Available online: https://www.biodiversitylibrary.org/item/46217#page/631/mode/1upa (accessed on 27 January 2023).
- Sasaki, T.; Maekawa, Y.; Takeda, Y.; Atsushiba, M.; Chen, C.; Noshita, K.; Uesugi, K.; Hoshino, M. 3D Visualization of Calcified and Non-calcified Molluscan Tissues Using Computed Tomography. In Biomineralization; Endo, K., Kogure, T., Nagasawa, H., Eds.; Springer: Singapore, 2018; pp. 83–93. [Google Scholar] [CrossRef]
- Báldi, T.; Cságoly, É. Faziostratotypus: Máriahalom. Sand pit. In OM Egerien. Chronostratigraphie und Neostratotypen 5; Báldi, T., Seneš, J., Eds.; Veda: Bratislava, Slovakia, 1975; pp. 134–137. [Google Scholar]
- Báldi, T. A Dunántúli Középhegység és Észak-Magyarország oligocénjének korrelációja. Födtani Közlöny 1976, 106, 407–424. Available online: http://epa.oszk.hu/01600/01635/00217/pdf/EPA01635_foldtani_kozlony_1976_106_4_407-424.pdf (accessed on 27 January 2023).
- Janssen, A.W. Late Oligocene molluscs from a sandpit near Máriahalom (Hungary): A preliminary study. Ann. Univ. Sci. Bp. De Rolando Eötvös Nomin. Sect. Geol. 1984, 24, 109–150. [Google Scholar]
- Réka, O. Életnyomok és patológiás elváltozások az unyi homokbányában gyűjtött puhatestűek mészvázain. Malakológiai Tájékoztató (Malacol. Newsl.) 1996, 15, 11–27. Available online: https://library.hungaricana.hu/en/view/MEGY_HEVE_Malakot_15_1996/?query=piliscsaba&pg=12&layout=s (accessed on 27 January 2023).
- Harbeck, T. Die Evolution der Archaeopulmonata. Zool. Verh. Leiden 1996, 305, 1–133. Available online: https://repository.naturalis.nl/pub/317550 (accessed on 27 January 2023).
- Vicián, Z.; Kovács, Z. A new Egerian (Upper Oligocene—Lower Miocene) gastropod fauna from the Esztergom Basin (NE Transdanubia, Hungary). Földtani Közlöny 2016, 146, 233–256. Available online: https://foldtan.hu/sites/default/files/04_KovacsZ.pdf (accessed on 27 January 2023).
- Harzhauser, M.; Piller, W.E. Benchmark data of a changing sea—Palaeogeography, Palaeobiogeography and events in the Central Paratethys during the Miocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 253, 8–31. [Google Scholar] [CrossRef]
- Brocchi, G. Conchiologia Fossile Subapennina, con Osservazioni Geologiche Sugli Apennini e sul Suolo Adiacente; Stamperia Reale: Milano, Italy, 1814; 712p, Available online: https://www.biodiversitylibrary.org/item/43873#page/9/mode/1up (accessed on 27 January 2023).
- Bruguière, J.G. Encyclopédie Méthodique ou par Ordre de Matières. Histoire Naturelle des vers, Volume 1; Pancoucke: Paris, France, 1792; pp. 345–757. Available online: https://biodiversitylibrary.org/page/8892006 (accessed on 27 January 2023).
- Harzhauser, M.; Mandic, O.; Büyükmeriç, Y.; Neubauer, T.A.; Kadolsky, D.; Landau, B.M. A Rupelian mangrove swamp mollusc fauna from the Thrace Basin in Turkey. Arch. Für Molluskenkd. 2016, 145, 23–58. [Google Scholar] [CrossRef]
- de Grateloup, J.P.S. Description de plusieurs espèces de coquilles fossiles des environs de Dax (Landes). Bull. D’histoire Nat. De La Société Linnéenne De Bordx. 1827, 2, 3–26. Available online: https://www.biodiversitylibrary.org/item/110491#page/7/mode/1up (accessed on 27 January 2023).
- de Grateloup, J.P.S. Tableau des coquilles fossiles qu’on rencontre dans les terrains calcaires tertiaires (Faluns) des environs de Dax, dans le département des Landes. 1er article. Bull. D’histoire Nat. De La Société Linnéenne De Bordx. 1828, 2, 72–109. Available online: https://www.biodiversitylibrary.org/item/110491#page/82/mode/1up (accessed on 27 January 2023).
- de Grateloup, J.P.S. Conchyliologie fossile du Bassin de l’Adour. 5ème mémoire. Famille des Plicacés (Trachélipodes). Description des genres et des espèces de coquilles fossiles, appartenant à cette famille de Trachélipodes, qu’on observe dans les couches des terrains marins supérieurs du bassin de l’Adour, aux environs de Dax (Landes). Actes De La Société Linnéenne De Bordx. 1838, 10, 251–290. Available online: https://www.biodiversitylibrary.org/item/101658#page/269/mode/1up (accessed on 27 January 2023).
- de Grateloup, J.P.S. Conchyliologie Fossile des Terrains Tertiaires du Bassin de l’Adour, (Environs de Dax). Tome Ier. Univalves. Atlas; Th. Lafargue: Bordeaux, France, 1847; Available online: https://archive.org/details/conchyliologief00gratgoog (accessed on 27 January 2023).
- Pictet, F.J. Traité de Paléontologie ou Histoire Naturelle des Animaux Fossiles Considérés Dans Leurs Rapports Zoologiques et Géologiques; Baillières: Paris, France, 1855; Volume 3, 727p, Available online: https://www.biodiversitylibrary.org/item/48930#page/9/mode/1up (accessed on 27 January 2023).
- Raulin, V. Distribution géologique des animaux vertébrés et des mollusqucs terrestres et fluviatiles fossiles de 1′Aquitane, précédée d’une note sur les divers faluns de la Gironde. Recl. Des Actes De L‘Académie Impériale Des Sci. Belles-Lett. Et Arts De Bordx. 1856, 18, 363–408. [Google Scholar]
- Tournouër, R. Auriculidées fossiles des faluns. J. De Conchyliol. 1872, 20, 77–116. Available online: https://www.biodiversitylibrary.org/item/53466#page/81/mode/1up (accessed on 27 January 2023).
- Benoist, E.A. Description de coquilles fossiles des terrains tertiaires moyens. Actes De La Société Linnéenne De Bordx. 1875, 30, 47–73. Available online: https://www.biodiversitylibrary.org/item/106216#page/709/mode/1up (accessed on 27 January 2023).
- Hoernes, R. Elemente der Palaeontologie (Palaeozoologie), 8th ed.; Veit & Co., Ltd.: Leipzig, Germany, 1884; Volume 16, 594p, Available online: https://www.zobodat.at/pdf/MON-GEO_0049_0001-0594.pdf (accessed on 27 January 2023).
- Zittel, K.A. Handbuch der Palaeontologie. I. Abtheilung, Palaeozoologie; Oldenbourg: München, Germany; Leipzig, Germany, 1885; Volume 2, 893p, Available online: https://www.biodiversitylibrary.org/item/79170#page/6/mode/1up (accessed on 27 January 2023).
- Raulin, V. Sur la faune Oligocène de Gaas (Landes). Bull. De La Société Géologique De Fr. 1896, 23, 546–555. Available online: https://www.biodiversitylibrary.org/item/97296#page/555/mode/1up (accessed on 27 January 2023).
- Raulin, V. Succession des Mollusques terrestres et d’eau douce dans le basin tertiaire de l’Aquitaine. Bull. De La Société Géologique De Fr. Ser. 3 1900, 28, 45–54. Available online: https://www.biodiversitylibrary.org/item/96117#page/51/mode/1up (accessed on 27 January 2023).
- Magne, A. Note sur la faune terrestre et fluviatile de la marnière de Bis, à Gaas. Procès-Verbaux Des Séances De La Société Linnéenne De Bordx. 1936, 88, 36–38. Available online: https://www.biodiversitylibrary.org/page/54716234 (accessed on 27 January 2023).
- Oppenheim, P. Beiträge zur Kenntniss des Oligozäns und seiner Fauna in den venetischen Voralpen. Z. Der Dtsch. Geol. Ges. 1900, 52, 243–326. Available online: https://www.biodiversitylibrary.org/item/148377#page/287/mode/1up (accessed on 27 January 2023).
- Romero, P.E.; Pfenninger, M.; Kano, Y.; Klussmann-Kolb, A. Molecular phylogeny of the Ellobiidae (Gastropoda: Panpulmonata) supports independent terrestrial invasions. Mol. Phylogenetics Evol. 2016, 97, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Itoigawa, J.; Shibata, H.; Nishimoto, H. Molluscan fossils of the Mizunami Group. Bull. Mizunami Foss. Mus. 1974, 1, 43–203. Available online: http://www2.city.mizunami.gifu.jp/bulletin/list/15/pdf/2/1/BMFM01-002Itoigawaetal.%201974.pdf (accessed on 27 January 2023).
- Itoigawa, J.; Shibata, H.; Nishimoto, H.; Okumura, K. Miocene fossils of the Mizunami Group, central Japan. 2. Molluscs. Monogr. Mizunami Foss. Mus. 1981, 3A, 1–53. Available online: http://www2.city.mizunami.gifu.jp/bulletin/list/8/pdf/1/1/MMFM03A-Itoigawaetal.,1981.pdf (accessed on 27 January 2023).
- Itoigawa, J.; Shibata, H.; Nishimoto, H.; Okumura, K. Miocene fossils of the Mizunami Group, central Japan. 2. Molluscs (continued). Monogr. Mizunami Foss. Mus. 1982, 3B, 1–330. Available online: http://www2.city.mizunami.gifu.jp/bulletin/list/8/pdf/2/1/MMFM03B-Itoigawaetal.,1982.pdf (accessed on 27 January 2023).
- Matsubara, T.; Komori, K. The first record of fossil Ellobium (Gastropoda: Ellobiidae) from northeastern Japan. Venus 2007, 65, 325–331. [Google Scholar] [CrossRef]
- Dharma, B. Recent and Fossil Indonesian Shells; Conchbooks: Hackenheim, Germany, 2005; 424p. [Google Scholar]
- Kase, T.; Yolanda, M.A.; Koji, K.; Ryoji, W. A mangrove-forest dwelling gastropod Ellobium aurismidae Linnaeus Ellobiidae, Pulmonata from the Mapulo Formation, Batangas Province, southern Luzon, Philippines. Natl. Sci. Mus. Monogr. 2004, 24, 187–195. [Google Scholar]
- Oyama, K. Studies of fossil molluscan biocoenosis, No. 1. Biocoenological studies on the mangrove swamps, with descriptions of new species from Yatuo Group. Rep. Geol. Surv. Jpn. 1950, 132, 1–16. [Google Scholar]
- Woodring, W.P. Geology and Paleontology of Canal Zone and adjoining parts of Panama description of Tertiary Mollusks (Gastropods: Eulimidae, Marginellidae to Helminthoglyptidae). Geol. Surv. Prof. Pap. 1970, 306-D, 299–452. Available online: https://pubs.usgs.gov/pp/0306d/report.pdf (accessed on 27 January 2023).
- Harzhauser, M.; Mandic, O.; Piller, W.E.; Reuter, M.; Kroh, A. Tracing back the origin of the Indo-Pacific mollusc fauna—Basal Tridacninae from the Oligocene and Miocene of the Sultanate of Oman. Palaeontology 2008, 51, 199–213. [Google Scholar] [CrossRef]
- Harzhauser, M.; Kroh, A.; Mandic, O.; Piller, W.E.; Göhlich, U.; Reuter, M.; Berning, B. Biogeographic responses to geodynamics: A key study all around the Oligo-Miocene Tethyan Seaway. J. Comp. Zool. 2007, 246, 241–256. [Google Scholar] [CrossRef]
- Yasuhara, M.; Huang, H.H.; Reuter, M.; Tian, S.Y.; Cybulski, J.D.; O’Dea, A.; Mamo, B.L.; Cotton, L.J.; Di Martino, E.; Feng, R.; et al. Hotspots of Cenozoic tropical marine biodiversity. Oceanogr. Mar. Biol. Annu. Rev. 2022, 60, 243–300. [Google Scholar] [CrossRef]
- Kronenberg, G.C.; Lee, H.G. Genera of American strombid gastropods (Gastropoda: Strombidae) and remarks on their phylogeny. Veliger 2007, 49, 256–264. Available online: https://www.biodiversitylibrary.org/page/42498213 (accessed on 27 January 2023).
- Harzhauser, M. Marine und brachyhaline Gastropoden aus dem Karpatium des Korneuburger Beckens und der Kreuzstettener Bucht (Österreich, Untermiozän). Beiträge Zur Paläontologie 2002, 27, 61–159. Available online: https://www.zobodat.at/pdf/Beitr-Palaeontologie_27_0061-0159.pdf (accessed on 27 January 2023).
- Zuschin, M.; Harzhauser, M.; Hengst, B.; Mandic, O.; Roetzel, R. Long-term ecosystem stability in a lower Miocene estuarine succession. Geology 2014, 42, 7–10. [Google Scholar] [CrossRef]
- Roberts, A.P.; Wilson, G.S.; Harwood, D.M.; Verosub, K.L. Glaciation across the Oligocene–Miocene boundary in southern McMurdo Sound, Antarctica: New chronology from the CIROS-1 drill hole. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 198, 113–130. [Google Scholar] [CrossRef]
- Greenop, R.; Sosdian, S.; Henehan, M.J.; Wilson, P.A.; Lear, C.; Foster, G.L. Orbital forcing, ice volume, and CO2 across the Oligocene-Miocene Transition. Paleoceanogr. Paleoclimatology 2019, 34, 316–328. [Google Scholar] [CrossRef]
- von Schlotheim, E.F. Die Petrefactenkunde Auf Ihrem Jetztigen Standpunkte Durch die Beschreibung Seiner Sammlung Versteinerter und Fossiler Überreste des Thier- und Pflanzenreichs der Vorwelt; Becker: Gotha, Germany, 1820; pp. lxii + 437; Available online: http://books.google.at/books/about/Die_Petrefactenkunde_auf_ihrem_jetzigen.html?id=4SQ-AAAAcAAJ&redir_esc=y (accessed on 27 January 2023).
- Esu, D.; Girotti, O. The Late Oligocene Molluscan Fauna from Otranto (Apulia, Southern Italy): An example of alternating freshwater, lagoonal and emerged environments. Palaeontology 2010, 53, 137–174. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harzhauser, M.; Pacaud, J.-M.; Landau, B.M. The Origin of the Mangrove and Saltmarsh Snail Ellobium (Eupulmonata, Ellobiidae). Taxonomy 2023, 3, 68-84. https://doi.org/10.3390/taxonomy3010007
Harzhauser M, Pacaud J-M, Landau BM. The Origin of the Mangrove and Saltmarsh Snail Ellobium (Eupulmonata, Ellobiidae). Taxonomy. 2023; 3(1):68-84. https://doi.org/10.3390/taxonomy3010007
Chicago/Turabian StyleHarzhauser, Mathias, Jean-Michel Pacaud, and Bernard M. Landau. 2023. "The Origin of the Mangrove and Saltmarsh Snail Ellobium (Eupulmonata, Ellobiidae)" Taxonomy 3, no. 1: 68-84. https://doi.org/10.3390/taxonomy3010007
APA StyleHarzhauser, M., Pacaud, J.-M., & Landau, B. M. (2023). The Origin of the Mangrove and Saltmarsh Snail Ellobium (Eupulmonata, Ellobiidae). Taxonomy, 3(1), 68-84. https://doi.org/10.3390/taxonomy3010007






