Extracting Lithium from Brazilian α-Spodumene via Chlorination Roasting
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedures and Methods
2.2. Experimental Conditions of Chlorination
2.3. Experimental Conditions for Extraction of Lithium
2.4. Chemical and Physical Analyses
3. Results and Discussion
3.1. Characterization of Sample
3.2. Tests of MgCl2/CaCl2 Molar Ratio
3.3. Studies on Spodumene:Chloride Mass Ratio
3.4. Studies on Roasting Chlorination Time
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BRAZIL Resolution N. 2 of June 18, 2021. Defines the List of Strategic Minerals for the Country, in Accordance with the Criteria Set Out in Art. 2 of Decree No. 10,657, of March 24, 2021. Available online: https://www.gov.br/mme/pt-br/assuntos/noticias/mme-lanca-relatorio-anual-do-comite-interministerial-de-analise-de-projetos-de-minerais-estrategicos/resolucao2CTAPME.pdf (accessed on 2 February 2025).
- Asif, A.H.; Li, C.; Lim, H.; Sun, H. Australia’s Spodumene: Advances in Lithium Extraction Technologies, Decarbonization, and Circular Economy. Ind. Eng. Chem. Res. 2024, 63, 2073–2086. [Google Scholar] [CrossRef]
- Lee, D.; Joo, Y.Y.; Shin, D.J.; Shin, S.M. Recovery of Lithium from Beta-Spodumene Through Serial Calcination and Water Leaching with CaO. JOM 2024, 76, 1477–1484. [Google Scholar] [CrossRef]
- BRAZIL BRAZIL, Decree No. 11,120, of July 5, 2022. Allows Foreign Trade Operations of Lithium Minerals and Ores and Their Derivatives. Available online: https://www.planalto.gov.br/ccivil_03/_ato2019-2022/2022/decreto/d11120.htm (accessed on 19 February 2025).
- United States Geological Survey (USGS). Mineral Commodity Summaries 2023; USGS: Reston, VA, USA, 2023; 200p. Available online: https://pubs.usgs.gov/periodicals/mcs2023/mcs2023-lithium.pdf (accessed on 19 February 2025).
- Sun, Z.; Cao, H.; Xiao, Y.; Sietsma, J.; Jin, W.; Agterhuis, H.; Yang, Y. Toward sustainability for recovery of critical metals from electronica waste: The hydrochemistry processes. ACS Sustain. Chem. Eng. 2016, 5, 21–40. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, J.; Li, H.; Chen, Y.; Wang, C. A promising approach for the recovery of high value-added metals from spent lithium-ion batteries. J. Power Sources 2017, 351, 192–199. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, X.; Cao, H.; Zhao, C.; Lin, X.; Ning, P.; Zhang, Y.; Jin, W.; Sun, Z. A closed-loop process for selective metal recovery from spent lithium iron phosphate batteries through mechanochemical activation. ACS Sustain. Chem. Eng. 2017, 5, 9972–9980. [Google Scholar] [CrossRef]
- Yu, J.; He, Y.; Ge, Z.; Li, H.; Xie, W.; Wang, S. A promising physical method for recovery of LiCoO2 and graphite from spent lithium-ion batteries: Grinding flotation. Sep. Purif. Technol. 2018, 190, 45–52. [Google Scholar] [CrossRef]
- Meng, F.; McNeice, J.; Zadeh, S.; Ghahreman, A. Review of Lithium Production and Recovery from Minerals, Brines, and Lithium-Ion Batteries. Miner. Process. Extr. Metall. Rev. 2019, 42, 123–141. [Google Scholar] [CrossRef]
- Tran, T.; Luong, V.T. Chapter 3—Lithium Production Processes. In Lithium Process Chemistry; Chagnes, A., Światowska, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 81–124. [Google Scholar] [CrossRef]
- Rögener, F.; Tetampel, L. Electrodialysis for the Concentration of Lithium-Containing Brines—An Investigation on the Applicability. Membranes 2022, 12, 1142. [Google Scholar] [CrossRef]
- Kuang, G.; Li, H.; Hu, S.; Jin, R.; Liu, S.; Guo, H. Recovery of aluminium and lithium from gypsum residue obtained in the process of lithium extraction from lepidolite. Hydrometallurgy 2015, 157, 214–218. [Google Scholar] [CrossRef]
- Dessemond, C.; Lajoie-Leroux, F.; Soucy, G.; Laroreche, N.; Magnan, J.F. Spodumene: The Lithium Market, Resources and Processes. Minerals 2019, 9, 334. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, T.; He, L.; Zhao, Z.; Liu, X. A promising approach for directly extracting lithium from α-spodumene by alkaline digestion and precipitation as phosphate. Hydrometallurgy 2019, 189, 105141. [Google Scholar] [CrossRef]
- Barbosa, L.I.; Valente, G.; Orosco, R.P.; González, J.A. Lithium extraction from β-spodumene through chlorination with chlorine gas. Miner. Eng. 2014, 56, 29–34. [Google Scholar] [CrossRef]
- Choubey, P.K.; Kim, M.S.; Srivastava, R.R.; Lee, J.C.; Lee, J.Y. Advance review on the exploitation of the prominent energy-storage element: Lithium. Part I: From mineral and brine resources. Miner. Eng. 2016, 89, 119–137. [Google Scholar] [CrossRef]
- Qiu, Y.; Wu, D.; Yan, L.; Zhou, Y. Recyclin spodumene slag: Preparation of green polymer composites. RSC Adv. 2016, 43, 36942–36953. [Google Scholar] [CrossRef]
- Kuang, G.; Liu, Y.; Li, H.; Xing, S.; Li, F.; Guo, H. Extraction of lithium from β-spodumene using sodium sulfate solution. Hydrometallurgy 2018, 177, 49–56. [Google Scholar] [CrossRef]
- Rosales, G.D.; Ruiz, M.D.C.; Rodriguez, M.H. Novel process for the extraction of lithium from -spodumene by leaching with HF. Hydrometallurgy 2014, 147–148, 1–6. [Google Scholar] [CrossRef]
- Rezaee, M.; Shihua, H.; Sagzhanov, D.; Hassas, B.V.; Slawecki, T.M.; Agrawal, D.; Akbari, H.; Mensah-Biney, R. Microwave-assisted calcination of spodumene for efficient, low-cost and environmentally friendly extraction of lithium. Powder Technol. 2022, 397, 116992. [Google Scholar] [CrossRef]
- Li, H.; Eksteen, J.; Kuang, G. Recovery of lithium from mineral resources: State-of-the-art and perspectives—A review. Hydrometallurgy 2019, 189, 105129. [Google Scholar] [CrossRef]
- Braga, P.F.A.; França, S.C.A.; Gonçalves, C.C.A.; Ferraz, P.F.V.; Neumann, R. Extraction of lithium from a montebrasite concentrate: Applied mineralogy, pyro- and hydrometallurgy. Hydrometallurgy 2020, 191, 105249. [Google Scholar] [CrossRef]
- Lajoie-Leroux, F.; Dessemond, C.; Soucy, G.; Laroche, N.; Magnan, J. Impact of the impurities on lithium extraction from β-spodumene in the sulfuric acid process. Miner. Eng. 2018, 129, 1–8. [Google Scholar] [CrossRef]
- Han, Z.; Wu, Y.; Hao, P.; Tan, X.; Wei, M.; Lv, X. Efficient lithium recovery from lithium-containing spent aluminium electrolyte via NaF fluorination roasting and Al2(SO4)3 leaching. J. Environ. Chem. Eng. 2023, 11, 110948. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Q.; Chen, B.; Shi, X.; Liao, T. Precipitation of lithium carbonate from spodumene by a sodium carbonete autoclave process. Hydrometallurgy 2011, 109, 43–46. [Google Scholar] [CrossRef]
- Napier, A.; Griffith, C. Process for Recovering Lithium Phosphate and Lithium Sulfate from Lithium-Bearing Silicates. U.S. Patent Application WO2019227158, 12 May 2019. [Google Scholar]
- Barbosa, L.I.; González, J.A.; Ruiz, M.d.C. Extraction of lithium from β-spodumene using chlorination roasting with calcium chloride. Thermochim. Acta 2015, 605, 63–67. [Google Scholar] [CrossRef]
- Fosu, A.; Kanari, N.; Vaugham, J.; Chagnes, A. Literature review and thermodynamic modelling of roasting processes for lithium extraction from spodumene. Metals 2020, 10, 1312. [Google Scholar] [CrossRef]
- Xing, C.M.; Wang, C.Y.; Wang, H. Magmatic-hydrothermal processes recorded by muscovite andcolumbite-group minerals from the Bailongshan rare-element pegmatites in the West Kunlun-Karakorum orogenic belt, NW China. Lithos 2020, 364–365, 105507. [Google Scholar] [CrossRef]
- Barrios, O.C.; González, Y.C.; Barbosa, L.I.; Orosco, P. Chlorination roasting of the cathode material contained in spent lithium-ion batteries to recover lithium, manganese, nickel and cobalt. Miner. Eng. 2022, 176, 107321. [Google Scholar] [CrossRef]
- Dunn, W.E.; Van Jahnke, J. Cyclical Vacuum Chlorination Processes, Including Lithium Extraction. U.S. Patent US7588741B2, 15 September 2009. Available online: https://patents.google.com/patent/US7588741B2/en (accessed on 19 February 2025).
- Jena, P.K.; Brocchi, E.A. Metal extraction through chlorine metallurgy. Miner. Process. Extr. Metall. Rev. 1996, 16, 211–237. [Google Scholar] [CrossRef]
- Ramos, I.D.E.; Orosco, P.R.; Barrios, O.C.; Jerez, A.L.P.; Sham, E.L.; Tesio, A.Y. Forsterite and magnesium aluminate spinel synthesis and silver extraction by calcination of silicon solar cell from discarded solar Pv panels with bischofite. Silicon 2023, 15, 2581–2595. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Wang, Z.; Wang, J.; Guo, H.; Hu, Q.; Peng, W.; Wu, X. Extraction of lithium from lepidolite using chlorination roastingíwater leaching process. Trans. Nonferrous Met. Soc. China 2012, 22, 1753–1759. [Google Scholar] [CrossRef]
- El-Naggar, M.M.A.A.; Medina, L.F. An alternative method for the recovery of lithium from spodumene. Metall. Trans. B 1984, 15, 725–726. [Google Scholar] [CrossRef]
- El-Naggar, M.M.A.A.; Medina, L.F.; Espídola, A. The reaction between spodumene and tachyhydrite. Metall. Trans. B 1988, 19, 663–668. [Google Scholar] [CrossRef]
- Krishnan, R.; Gopan, G. A comprehensive review of lithium extraction: From historical perspectives to emerging technologies, storage, and environmental considerations. Clean. Eng. Technol. 2024, 20, 100749. [Google Scholar] [CrossRef]
- Meshrama, P.; Pandey, B.D.; Mankhandb, T.R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Timich, M. Process Mineralogy of a Lithium Enriched Pegmatite Combining Mineral Separation and SEM Based Automated Mineralogy. Master’s Thesis, Departamento de Engenharia de Minas e Petróleo, Escola Politécnica da Universidade de São Paulo, São Paulo, Brasil, 2021. [Google Scholar]
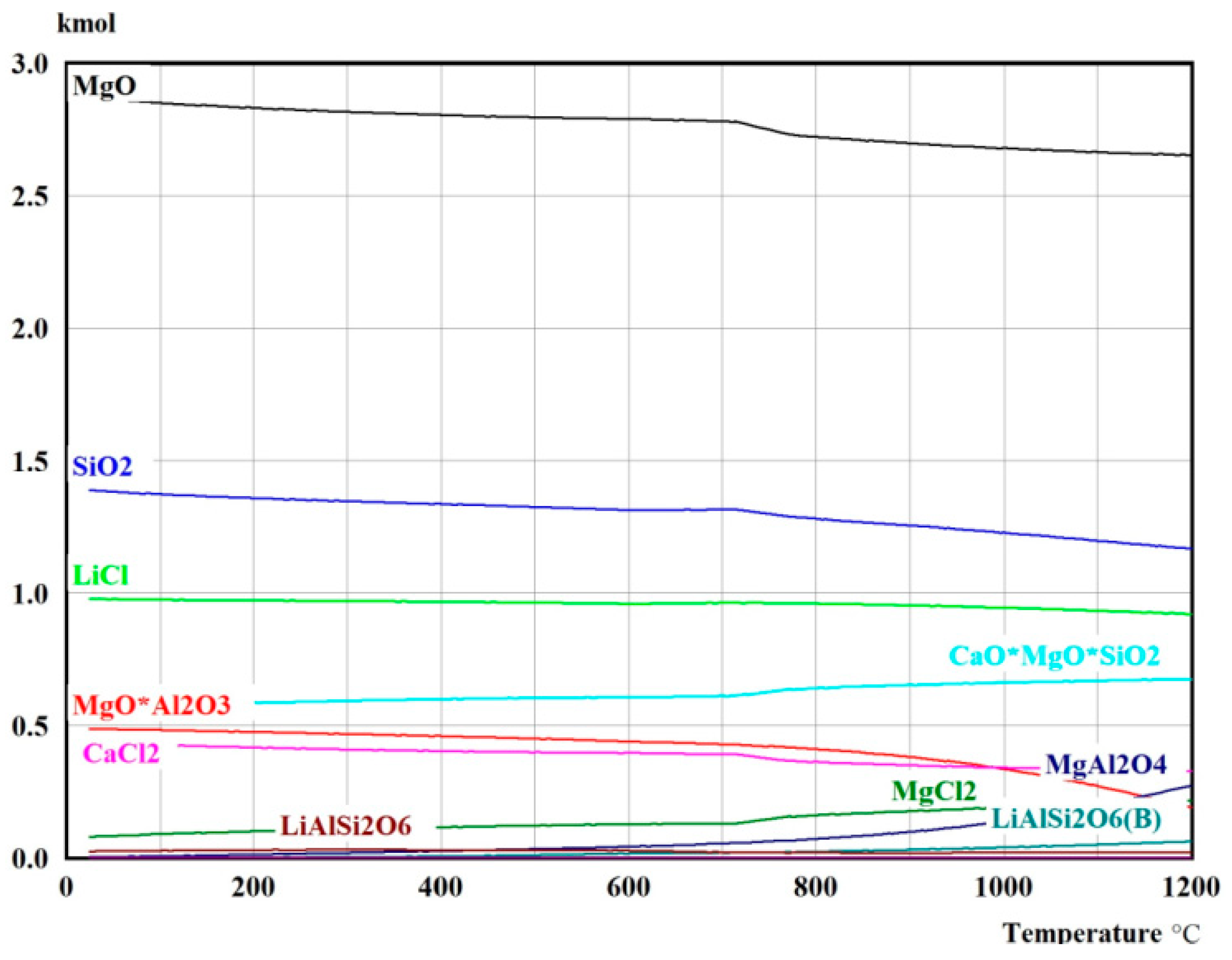


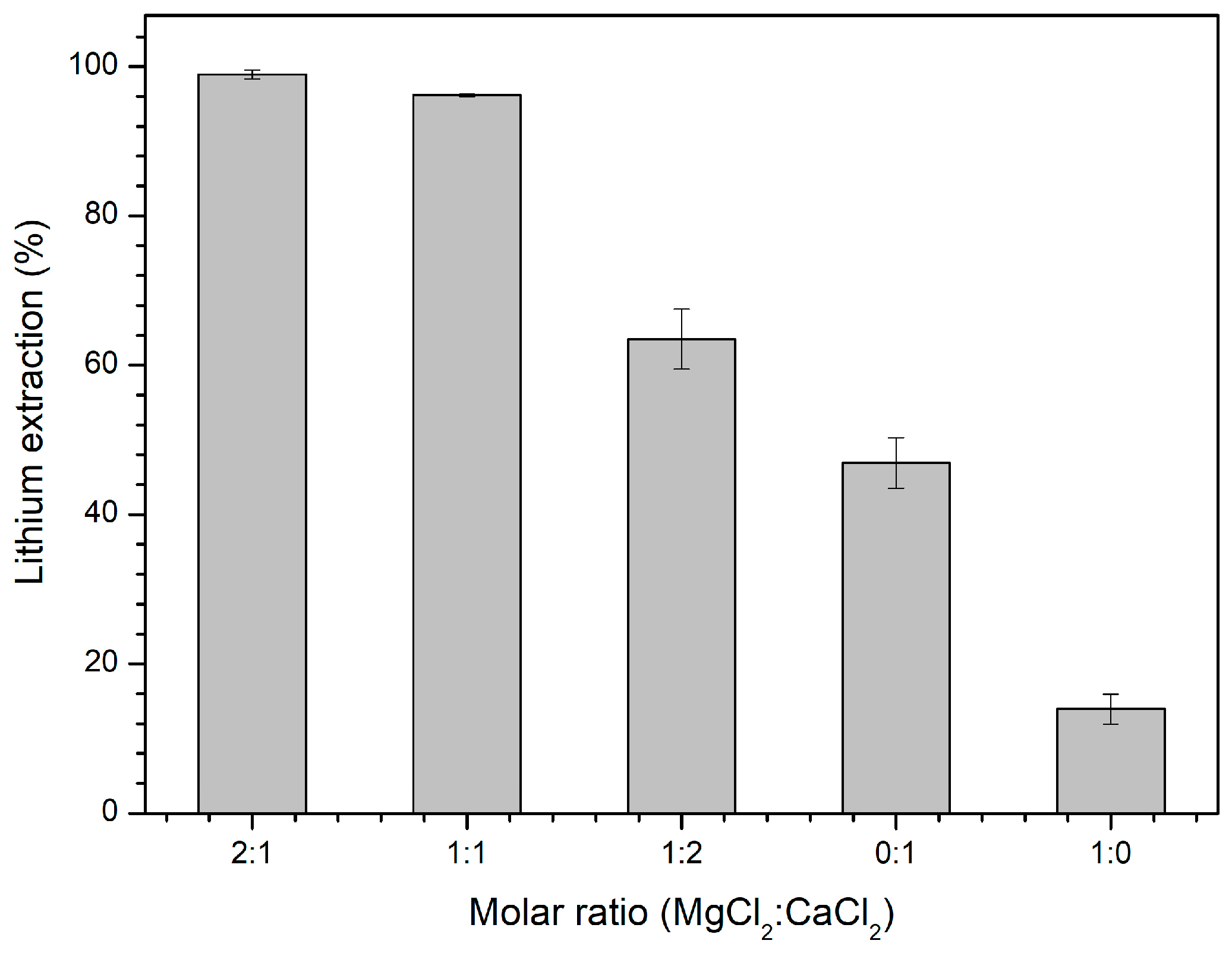


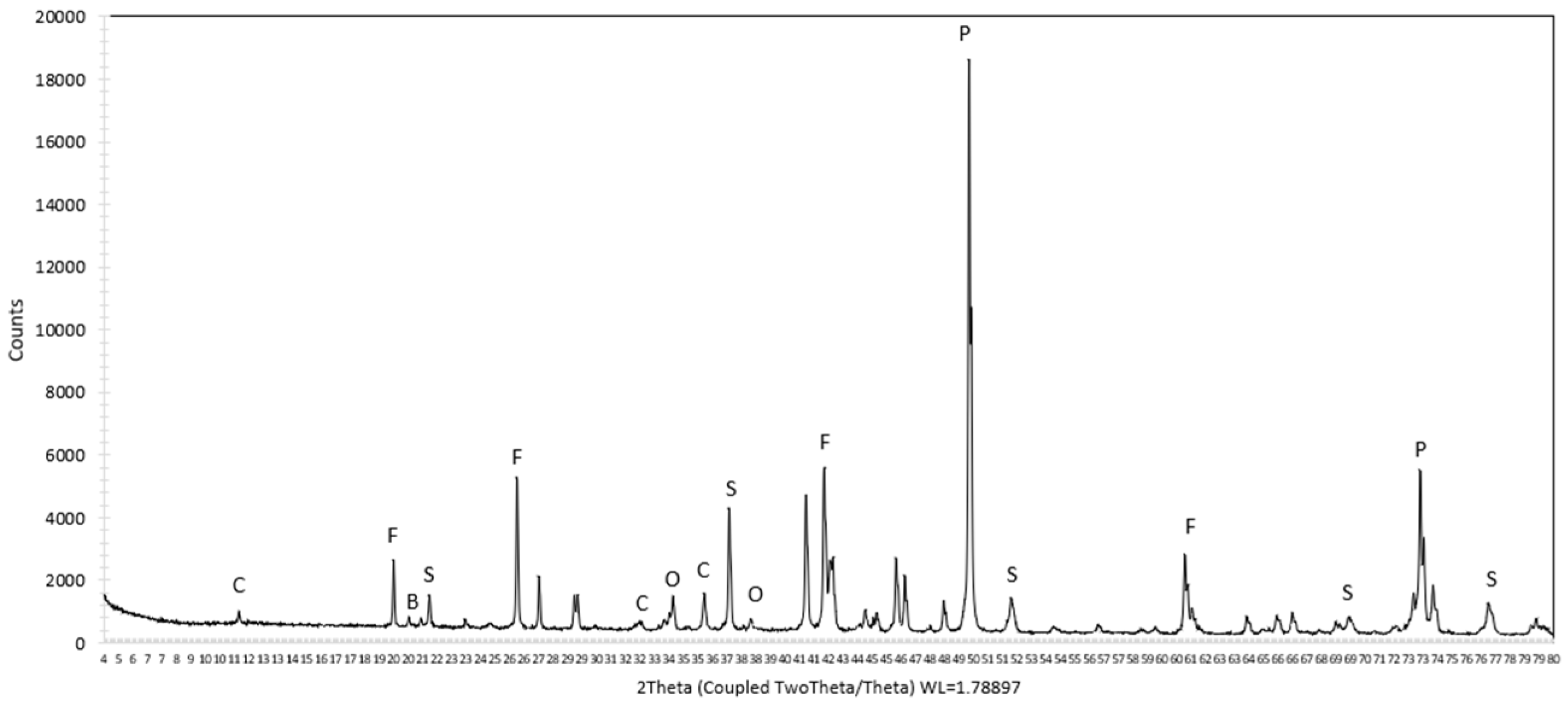

| Method | Mine Li | Additive | Reaction Time | Temperature | Lithium Extraction (%) |
|---|---|---|---|---|---|
| Acid roasting [20,21,22,23,24,25] | β-spodumene (>1050 °C) | H2SO4, HF, HCl | 20–30 min | 75–225 °C | 90–97 |
| Alkaline process [3,15,19,26,27] | β-spodumene (>1050 °C) | Na2CO3 CaO, NaOH | 60–360 min | 225–250 °C | 90.7–96 |
| Chlorination roasting [16,28] | α-spodumene (>25 °C) | CaCl2, Cl2 | 120 min | 900 °C | 90.2 |
| Test | Spodumene: Chlorides | Molar Ratio | Chlorination Roasting |
|---|---|---|---|
| MgCl2:CaCl2 | t (min) | ||
| 1 | 1:8 | 0:1 | 120 |
| 2 | 1:8 | 1:0 | 120 |
| 3 | 1:8 | 1:2 | 120 |
| 4 | 1:8 | 1:1 | 120 |
| 5 | 1:8 | 2:1 | 120 |
| 6 | 1:4 | 1:1 | 120 |
| 7 | 1:4 | 2:1 | 120 |
| 8 | 1:6 | 1:1 | 120 |
| 9 | 1:6 | 2:1 | 120 |
| 10 | 1:6 | 2:1 | 90 |
| 11 | 1:6 | 2:1 | 60 |
| 12 | 1:6 | 2:1 | 30 |
| Component | Al2O3 | Li2O | K2O | MgO | Mn2O3 | Fe2O3 | Na2O | P2O5 | CaO |
| wt/wt% | 23.26 | 5.67 | 1.40 | 0.17 | 0.14 | 1.07 | 0.79 | 0.65 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga, P.F.A.; Brigido, C.R.d.S.; Pinto, C.P.; França, S.C.A.; Rosales, G.D. Extracting Lithium from Brazilian α-Spodumene via Chlorination Roasting. Mining 2025, 5, 19. https://doi.org/10.3390/mining5010019
Braga PFA, Brigido CRdS, Pinto CP, França SCA, Rosales GD. Extracting Lithium from Brazilian α-Spodumene via Chlorination Roasting. Mining. 2025; 5(1):19. https://doi.org/10.3390/mining5010019
Chicago/Turabian StyleBraga, Paulo F. A., Caroline R. dos S. Brigido, Camila P. Pinto, Silvia C. A. França, and Gustavo D. Rosales. 2025. "Extracting Lithium from Brazilian α-Spodumene via Chlorination Roasting" Mining 5, no. 1: 19. https://doi.org/10.3390/mining5010019
APA StyleBraga, P. F. A., Brigido, C. R. d. S., Pinto, C. P., França, S. C. A., & Rosales, G. D. (2025). Extracting Lithium from Brazilian α-Spodumene via Chlorination Roasting. Mining, 5(1), 19. https://doi.org/10.3390/mining5010019






