Abstract
Background: Carcinoma cuniculatum (CC) is a rare subtype of squamous cell carcinoma that is difficult to diagnose owing to the lack of cellar atypia and/or associated oral epithelial dysplasia. The prognosis is good given proper resection, but it often has a poor prognosis with recurrence. We present the case of a 78-year-old man who visited our department with an ulcer around the implant in tooth 35. With Nikolsky’s phenomenon in the gingiva, a detailed examination revealed pemphigus vulgaris. Steroid administration remarkably improved the oral symptoms but caused osteomyelitis and rapid bone destruction, leading to pathological fracture. After multiple biopsies, mandibular segment resection was performed with a diagnosis of mandibular osteomyelitis, and no malignant findings were found. Four months later, the wound reopened, a white keratinized lesion appeared, and a biopsy revealed CC. Unresectable tumor infiltration was already observed, but the patient died of aspiration pneumonia 15 months after diagnosis. Conclusions: It took 20 months to make a definitive diagnosis of CC in this case. Pemphigus vulgaris may have made the diagnosis particularly difficult. Since other CCs are often diagnosed with osteomyelitis or odontogenic keratocyst preoperatively, we recommend keeping in mind the possibility of CC in refractory cases.
1. Introduction
Oral carcinoma cuniculatum (CC) is one of the very rare variants of oral squamous cell carcinoma (SCC) that was added to the WHO classification in 2005. Unlike normal SCC, it has little cytological morphology and contains well-differentiated epithelial cells, making a definitive diagnosis difficult. Oral CC is rarely metastasized but exhibits a unique locally aggressive tissue structure such as cuniculatum [1,2]. Cuniculatum is derived from the Latin “cun- iculus,” which means “rabbit warren” because of the multiple sinuses and crypts found in this tumor. The tumor was first described by Gottron in 1932 as “papillomatosis cutis” in the European literature [3].
Many cases of CC were reported on the soles of feet [1]. Oral CC was first reported in 1977 [4], with more than half of the cases affecting the mandibular gingiva [2]. Although the etiology specific to CC has not yet been elucidated and may be similar to other oral cancers, the recurrence rate of CC is relatively low and metastases are rare; thus, the prognosis after appropriate resection is often good. However, with recurrence, it is resistant to treatment and often follows a turning point with a poor prognosis [5]. There is a strong tendency for aggressive local infiltration into the bone with multiple microabscesses, which is often difficult to diagnose and surgically resect [6].
We report a case of oral CC initially diagnosed as refractory mandibular osteomyelitis derived from peri-implantitis with pemphigus vulgaris (PV) and requiring nine histopathological examinations before a definitive diagnosis was made.
2. Case Presentation
A 78-year-old man visited our department for a detailed examination of a left mandibular gingival ulcer around an implant in tooth 35. About 8 months prior, he noticed irritation and pain in his left mandibular gingiva and a gingival ulcer was pointed out by his referring dentist. Although antibacterial drugs were administered, the ulcer did not improve, and he was referred to our department. He had a history of pancytopenia of unknown cause, smoked for 10 pack-years in his 20 s, and still drinks heavily.
On the first visit, we found ulcerative lesions around the dental implant in tooth 35, oral lichenoid lesions of the bilateral buccal mucosa, and Nikolsky’s phenomenon in the gingiva (Figure 1). A blood test showed an anti-Dsg1 antibody > 3.0 U/mL and an anti-Dsg3 antibody of 44.2 U/mL, and tissue biopsy showed IgG deposition in the epithelial intercellular space via direct immunofluorescence, resulting in a diagnosis of PV (Figure 2). A systemic search was performed on suspicion of possible paraneoplastic pemphigus (PNP), and 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) showed the accumulation of SUVmax = 6.646 in the left mandibular molar and SUVmax = 6.078 in the posterior right mandible. In the right mandible, 47 severe periodontal lesions and Nikolsky’s phenomenon of the gingiva but no ulcerative lesion were observed; thus, it was considered an inflammatory lesion. No other findings suggestive of malignant disease were found outside the oral cavity. When the dermatologist started steroid treatment (1 mg/kg/day, 60 mg/day), all oral lesions improved rapidly and steroid use was gradually reduced.
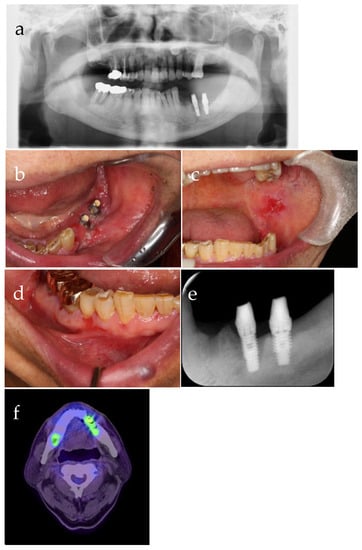
Figure 1.
Intraoral photograph from the first visit: (a) Peri-implant ulcer, (b) oral lichenoid lesions of the bilateral buccal mucosa, (c) Nikolsky’s phenomenon of the gingiva, (d) panorama X-ray photograph (X-P), (e) dental X-P, and (f) 18F-fluorodeoxyglucose positron emission tomography.
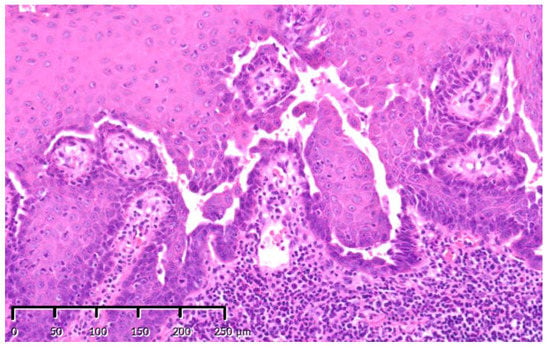
Figure 2.
Initial biopsy specimens: representative images of initial gingival biopsy specimens that were interpreted as pemphigus vulgaris. The features presented include gaps in the epithelial basal cells and intracavitary Tzanck cells with no epithelial cell dysplasia. Stain, hematoxylin and eosin (H&E); magnification, original ×200.
One month after the start of steroid treatment, the implant in tooth 35 showed marked instability and was removed (at that time, prednisolone was administered at 40 mg/day). The implant was easily removed without osteotomy, and histopathological examination of the erosive lesions remaining around the implant revealed PV without malignant findings. Healing was delayed, and bone exposure continued, so inflammation was considered to have been prolonged due to peri-implantitis. Antibiotics were administered orally, and re-curettage was performed. No malignant findings were found during the third histopathological examination. Since there was little evidence to consider switching to steroid-saving immunosuppressive agents for PSL, PSL administration was continued, albeit gradually decreasing the dose. Three months later, he noticed left chin hypoesthesia and severe pain in his left mandibular premolar. While scrutinizing a malignant lesion, such as primary intraosseous carcinoma (PIOC), drainage of the 35-part appeared and the osteolysis image centered on the 35-part were markedly enlarged (Figure 3). A biopsy showed no malignant findings (Figure 4), and we diagnosed refractory purulent mandibular osteomyelitis. Left mandibular anti-inflammatory surgery and removal of the implant in tooth 36 were performed when the administration of prednisolone was at 9 mg/day, but no malignant findings were found. Since prednisolone administration continued and the diagnosis was considered refractory osteomyelitis, hyperbaric oxygen therapy (HBO) was used in combination before and after surgery. One month later, diffuse swelling of the lower left jaw and drainage of the oral cavity occurred, and 3 weeks later, a pathological fracture of the left mandible occurred (Figure 5). HBO has performed again with intermaxillary fixation, which provided the local area with some rest, but his condition did not improve. Thus, mandibular segment resection, split-thickness skin grafting, and tracheostomy without reconstruction were performed. No malignant findings were found in the histopathological examination (Figure 6). Four months after the segmental resection, a fistula was formed in the lower part of the chin; occasional tingling pain recurred in the left mandible; and the wound on the floor of the oral cavity re-opened of its own accord, with a fine granular white lesion being exposed (Figure 7a). A biopsy revealed CC (Figure 7b,c) on the ninth histopathological examination. Computed tomography (CT) and additional biopsy revealed that CC had already infiltrated the surrounding tissue, and we diagnosed it as unresectable (Figure 7d,e). After that, he received the best supportive care without further cancer treatment, survived for 15 months after initial diagnosis, and died of aspiration pneumonia. No findings suggestive of cervical lymph nodes or distant metastases were found after the diagnosis of CC. (Timeline: Table 1).
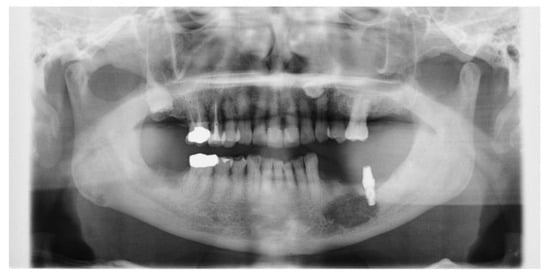
Figure 3.
Panorama X-P. The osteolytic lesion of the left mandible was significantly enlarged.
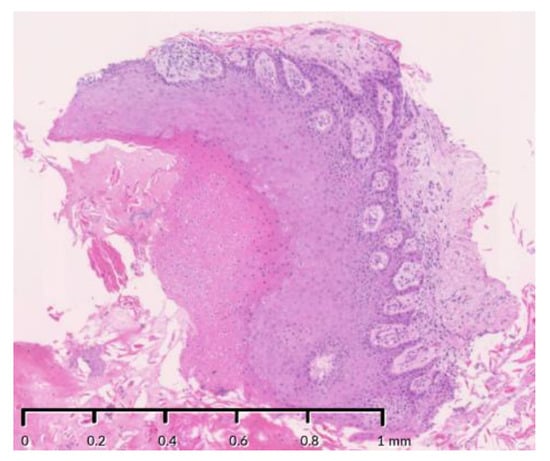
Figure 4.
Photomicrograph of the biopsy. Epithelial components were found in the lesions in the mandible, but there were few findings to diagnose it as neoplastic, and pemphigus vulgaris and osteomyelitis was considered to have spread from peri-implantitis. Stain, hematoxylin and eosin (H&E); magnification, original ×125.
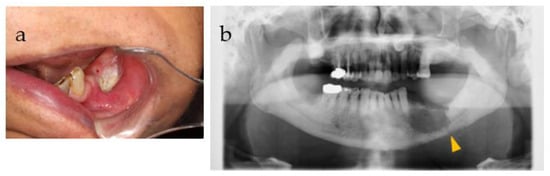
Figure 5.
(a) Intraoral photograph and (b) panorama X-P before and after a pathological fracture. A keratinized substance was found in the oral cavity, and the sixth histopathological examination confirmed that it was a keratinized substance. Furthermore, a pathological fracture was observed (yellow triangle).
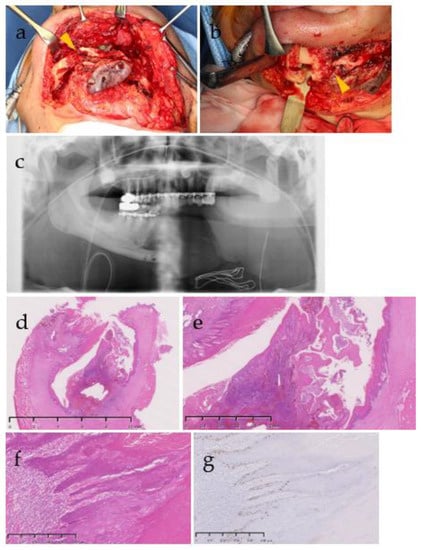
Figure 6.
(a,b) Intra-operative images of mandibular resection, (c) panorama X-P after the mandibular resection, and (d–g) photomicrograph of mandibular segment resection. Yellow arrowheads indicate pathological fractures (a,b). Invagination of the stratified squamous epithelium was observed in the mandible (d) and the epithelium was observed along the rotting bone (e), but epithelial atypia was scarce (f) and about 14% of Ki-67-positive cells were relatively localized in the basal layer and parabasal layer (g). There were a few findings to diagnose a neoplastic lesion, and osteomyelitis was considered to have spread from peri-implantitis. Stain, hematoxylin and eosin (H&E) (a–c), ki-67 immunohistochemistry staining (e); magnification, original ×5 (b), 20 (c), 100 (d,e).
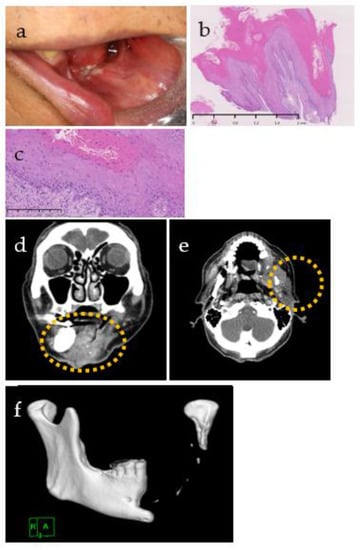
Figure 7.
(a) Intraoral photograph, (b,c) photomicrograph and computed tomography, (d–f) before and after the diagnosis of CC. (a) The wound re-opened, and white keratin appeared. Papillary hyperplasia of baking soda (b) and mild atypia in the epithelial cells (c) were observed, leading to a diagnosis of carcinoma cuniculatum. Stain, hematoxylin and eosin (H&E); magnification, original ×20 (b) and ×200 (c). (d–f) Wide extension beyond the mandible of an irregular soft tissue mass (yellow dotted circle) with a contrasting effect was observed, and obvious osteolytic changes in the stump of the remaining left mandibular condyle were also observed.

Table 1.
Summary of the clinical course until the diagnosis of carcinoma cuniculatum.
3. Discussion
In this case, it took 20 months and 9 biopsies to make a definitive diagnosis of CC. Due to the significant differentiation, dysplasia could not be identified on histopathological examination and PV was complicated, which is a difficult factor for diagnosis. In general, CC, which is a subtype of well-differentiated SCC, is often difficult to diagnose. Of the 65 cases of oral CC reported in English by January 2021, 29 patients were diagnosed with malignant lesions before surgery and 19 of them were diagnosed with CC (Table 2) [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. The average time to diagnosis was 15.9 months (3 weeks to 9 years). The presence or absence of pain and growth patterns varied and were inconsistent. Many of them showed cyst-like radiological findings, and most of the 17 cases diagnosed as non-malignant lesions were diagnosed as osteomyelitis of the jaw or odontogenic keratocyst (OKC). OKCs are prone to recurrence and are reported to recur in 25%, as they may show small satellite cysts or solid islands in the wall or may have budding of the basal layer ([32], pp. 234–242). Among the 65 patients who underwent surgery, 6 had a description of postoperative local recurrence. Although there was no mention of cytopathology in previous reports, in this case, cytopathology was performed multiple times in addition to nine histopathological examinations. For example, in the sixth histopathological examination, the possibility of a tumor was diagnosed as a keratinizing substance, and dysplasia was pointed out by the cytopathology, leading to the seventh histopathological examination. At the time of the biopsy, tissues were excised from the surface layer and from deeper parts on the skin side and on the oral side, but dysplasia was not detected. If diagnosis and treatment are difficult, it is recommended to consider biopsy from deeper tissues more frequently.

Table 2.
List of oral carcinoma cuniculatum.
The pathogenesis of CC includes trauma, chronic inflammation, irradiation [8], and p53 mutation [13] in addition to a history of drinking and/or smoking, which is a similar pathogenesis to that of cancer in general. However, these etiologies are not always recognized. The patient was a heavy drinker and had a history of smoking and peri-implantitis (chronic inflammation). A p53 mutation was found during the ninth biopsy, which provided the diagnosis of CC with atypia. In addition, there is a histopathological diagnosis that shows an increase in Ki-67, but it is difficult to distinguish it from an increase in reactivity due to inflammation, so it is necessary to always consider it together with clinical findings.
Pemphigus is clinically classified as PV (forms blisters on mucosal skin), pemphigus foliaceus (forms blisters only on the skin), and PNP. PNP is frequently associated with hematological malignancies, thymoma, and Castleman’s disease, among others, often having a poor prognosis. It presents with severe and diverse oral mucosal and cutaneous symptoms, often first in the oral cavity, especially on the lips [33]. In this case as well, PNP was suspected, but no lesions suggestive of a tumor were found other than the left mandibular lesion, and there were no such symptoms such as lip erosion or ulcer formation. Therefore, we considered that this case was not PNP but simultaneous PV and CC.
This is the first detailed report on a case of CC with PV. In the only case reported by Barrett et al., PV was mentioned as a differential diagnosis upon first histopathological examination, but CC was diagnosed during the second histopathological examination [26]. In this case, the diagnostic criteria for PV were met, steroid treatment improved the symptoms, and the symptoms except for the gingival ulcer were completely cured. Therefore, it is probable that the symptoms for CC were obscured by those of PV. It is unclear which one developed first, but CC also developed and likely coexisted from an early stage, and it is presumed that CC became apparent as PV improved with steroid treatment.
In this case, steroids were administered for the treatment of PV. Ricciuti et al. pointed out that high doses of steroids used to alleviate cancer-related symptoms might be associated with a poor prognosis [34]. However, it was also reported that, in the case of non-small cell lung cancer, it was not associated with poor prognosis when administered for the treatment of exacerbations of autoimmune diseases and chronic obstructive pulmonary disease unrelated to cancer or for the prevention of hypersensitivity reactions [34]. However, the possible effects of steroids are controversial because CC is characterized by multiple microabscesses. We also request dermatologists to reduce the dose of steroids as early as possible because of concerns about the effects of steroids during treatment for refractory osteomyelitis.
PIOC was also suspected because the bone permeation was markedly enlarged in this case, as viewed in the images, but PIOC is a type of cancer derived from epithelial remnants in the mandible ([32], pp. 206–212). In this case, vertical bone resorption of the implant in tooth 35 was observed during the first visit, the bone resorption progressed, the mobility of the implant increased, and the bone permeation expanded after implant removal. Therefore, we considered that CC, not PIOC, developed around the implant and infiltrated into the mandible from around the implant.
Surgical resection is recommended as a treatment for CC, with a relatively low recurrence rate and rare metastases. However, with recurrence, it is resistant to treatment and often has a poor prognosis [4]. There have been no reports of successful cases of chemotherapy and only one report of radiation therapy that eliminated the accumulation of 18F-FDG PET [24]. Radiation therapy for CC is not recommended, with the previous report not mentioning the post-irradiation course but rather pointing to the possibility of transformation into undifferentiated cancer by irradiation. There are some reports on chemotherapy but no reports on its success [5]. The patient in this case survived with cancer for 15 months after the diagnosis of CC. Although there is no detailed report on the long-term prognosis of untreated CC cases, it is basically indolent and may have a relatively long prognosis. The first recommendation for CC treatment is complete resection, and there is no basis for recommending chemotherapy or radiation therapy at this time.
4. Conclusions
In refractory osteomyelitis or OKC cases, the possibility of CC should also be kept in mind.
Author Contributions
C.M., K.-i.S., C.O., M.S., K.Y., J.S., A.S. and Y.K. participated directly in the care and diagnosis of the patient; K.Y., J.S. and A.S. reviewed and edited the manuscript; A.M. performed the pathology studies; C.M. and A.M. visualized the study; and C.M. and K.-i.S. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Consent for publication was obtained from the patient.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge Ken Natsuga and Hideyuki Ujiie of the Department of Dermatology, Hokkaido University, and their colleagues for their contributions to the diagnosis and treatment of PV.
Conflicts of Interest
The authors declare no conflict of interest.
References
- El-Naggar, A.K.; Takata, T.; Slootweg, P.J.; Chan, J.K.C.; Grandis, J. Tumours of the Oral Cavity and Mobile Tongue. In WHO Classification of Head and Neck Tumours; International Agency for Research on Cancer, IARC Press: Lyon, France, 2017; pp. 109–111. [Google Scholar]
- Farag, A.F.; Abou-Alnour, D.A.; Abu-Taleb, N.S. Oral carcinoma cuniculatum, an unacquainted variant of oral squamous cell carcinoma: A systematic review. Imaging Sci. Dent. 2018, 48, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Coldiron, B.M.; Brown, F.C.; Freeman, R.G. Epithelioma cuniculatum (carcinoma cuniculatum) of the thumb: A case report and literature review. J. Dermatol. Surg. Oncol. 1986, 12, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Flieger, S.; Owiński, T. Epithelioma cuniculatum an unusual form of mouth and jaw neoplasm. Czas. Stomatol. 1977, 30, 395–401. [Google Scholar]
- Sun, Y.; Kuyama, K.; Burkhardt, A.; Yamamoto, H. Clinicopathological evaluation of carcinoma cuniculatum: A variant of oral squamous cell carcinoma. J. Oral Pathol. Med. 2012, 41, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Hashimoto, S.; Watanabe, K.; Takahashi, K.; Usubuchi, H.; Suzuki, H. Carcinoma cuniculatum mimicking leukoplakia of the mandibular gingiva. Auris Nasus Larynx. 2012, 39, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Allon, D.; Kaplan, I.; Manor, R.; Calderon, S. Carcinoma cuniculatum of the jaw: A rare variant of oral carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 94, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.L.D.; Graetz, K.W. Carcinoma Cuniculatum: A Rare Entity in the Oral Cavity. J. Craniofac. Surg. 2009, 20, 1270–1272. [Google Scholar] [CrossRef]
- Hutton, A.; McKaig, S.; Bardsley, P.; Monaghan, A.; Parmar, S. Oral Carcinoma Cuniculatum in a Young Child. J. Clin. Pediatr. Dent. 2010, 35, 89–94. [Google Scholar] [CrossRef]
- Pons, Y.; Kerrary, S.; Cox, A.; Guerre, A.; Bertolus, C.; Gruffaz, F.; Capron, F.; Goudot, P.; Ruhin-Poncet, B. Mandibular cuniculatum carcinoma: Apropos of 3 cases and literature review. Head Neck-J. Sci. Spec. Head Neck. 2012, 34, 291–295. [Google Scholar] [CrossRef]
- Thavaraj, S.; Cobb, A.; Kalavrezos, N.; Beale, T.; Walker, D.M.; Jay, A. Carcinoma Cuniculatum Arising in the Tongue. Head Neck Pathol. 2012, 6, 130–134. [Google Scholar] [CrossRef]
- Fonseca, F.P.; Pontes, H.A.R.; Pontes, F.S.C.; de Carvalho, P.L.; Sena, M.; Jorge, J.; Santos-Silva, A.R.; de Almeida, O.P. Oral carcinoma cuniculatum: Two cases illustrative of a diagnostic challenge. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.H.; Venkateswaran, K.; Leow, P.C.; Loh, K.S.; Thamboo, T.P.; Petersson, F. Carcinoma Cuniculatum of the Esophagus and Tongue: Report of Two Cases, Including TP53 Mutational Analysis. Head Neck Pathol. 2014, 8, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Padilla, R.J.; Murrah, V.A. Carcinoma cuniculatum of the oral mucosa: A potentially underdiagnosed entity in the absence of clinical correlation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.C.; Wong, B.; O’Brien, M.J.; Salama, A. Mandibular Destruction Secondary to Invasion by Carcinoma Cuniculatum. J. Oral Maxillofac. Surg. 2015, 73, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Shay, S.; Choy, W.; Christensen, R.E.; St. John, M.A. Extensive carcinoma cuniculatum of the mandible. Am. J. Otolaryngol. 2015, 36, 446–450. [Google Scholar] [CrossRef]
- Datar, U.V.; Kale, A.; Mane, D. Oral Carcinoma Cuniculatum: A New Entity in the Clinicopathological Spectrum of Oral Squamous Cell Carcinoma. J. Clin. Diagn. Res. 2017, 11, ZD37–ZD39. [Google Scholar] [CrossRef]
- Prasad, R.S.; Moorthy, A.; Bhadranna, A.; Pai, A. Proliferative endophytic lesion of the maxilla: A diagnostic challenge. J. Oral Maxillofac. Pathol. 2018, 22 (Suppl. 1), S82–S86. [Google Scholar] [CrossRef]
- Thibouw, F.; Anna, H.; Levasseur, J.; Mondoloni, C.; Aubriot-Lorton, M.H.; Zwetyenga, N. Carcinoma cuniculatum of the lip: A case report. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 224–228. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Hu, Y.H.; Tian, Z.; Zhu, L.; Zhang, C.P.; Li, J. Oral carcinoma cuniculatum presenting with moth-eaten destruction of the mandible. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, E86–E93. [Google Scholar] [CrossRef]
- Sivapathasundharam, B.; Kavitha, B.; Padmapriya, V.M. Carcinoma Cuniculatum of the Alveolar Mucosa: A Rare Variant of Squamous Cell Carcinoma. Head Neck Pathol. 2019, 13, 652–655. [Google Scholar] [CrossRef]
- Ramos, G.O.; Meyer, G.L.; Visioli, F.; Manoela, M.D.; Oliveira, M.G. Carcinoma cuniculatum in the tongue of a patient with oral lichen planus: Unusual presentation. Indian J. Dent. Res. 2018, 29, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Broly, E.; Barthelemy, P.; Ciftci, S.; Borel, C.; Broly, M.; Gros, C.I.; Marcellin, L.; Bornert, F. Hidden intra-mandibular carcinoma cuniculatum appearing in a patient with metastatic prostate cancer: A case report. BMC Oral Health. 2019, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dejust, S.; El Farsaoui, K.; Bellefqih, S.; Lalire, P.; Morland, D. 18F-FDG PET/CT in Oral Cuniculatum Carcinoma. Clin. Nucl. Med. 2019, 44, 741–742. [Google Scholar] [CrossRef]
- Allon, I.; Vered, M.; Kaplan, I.; Nahlieli, O.; Yahalom, R.; Shalmon, B.; Srouji, S.; Livoff, A. Rare variants of head and neck squamous cell carcinoma-differential immunohistochemical profiles. Acta Histochem. 2019, 121, 151444. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.W.; Garg, M.; Armstrong, D.; Bisase, B.S.; Newman, L.; Norris, P.M.; Shelley, M.; Tighe, J.V.; Hyde, N.H.C.; Chaston, N.J.; et al. Cystic Squamous Cell Carcinomas of the Jaws: Twelve Cases Highlighting Histopathological Pitfalls. Int. J. Surg. Pathol. 2020, 28, 624–630. [Google Scholar] [CrossRef]
- Lee, N.V.; Ben Kang, E.T.; Senger, C.; Poh, C.F. Oral cancer in a 5-year-old boy: A rare case report and review of literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, E10–E19. [Google Scholar] [CrossRef]
- Niklander, S.; Sernuda, L.M.; Martinez, R. Unusual case of carcinoma cuniculatum affecting the oral cavity of an 11-year-old boy. An. Bras. Dermatol. 2021, 96, 799–801. [Google Scholar] [CrossRef]
- Yadav, S.; Bal, M.; Rane, S.; Mittal, N.; Janu, A.; Patil, A. Carcinoma Cuniculatum of the Oral Cavity: A Series of 6 Cases and Review of Literature. Head Neck Pathol. 2021. [CrossRef]
- Janardhanan, M.; Rakesh, S.; Savithri, V.; Aravind, T.; Mohan, M. Carcinoma Cuniculatum of Mandible Masquerading as Odontogenic Keratocyst: Challenges in the Histopathological Diagnosis. Head Neck Pathol. 2021, 15, 1313–1321. [Google Scholar] [CrossRef]
- Elangovan, E.; Banerjee, A.; Abhinandan; Roy, B. Oral carcinoma cuniculatum. J. Oral Maxillofac. Pathol. 2021, 25, 163–166. [Google Scholar] [CrossRef]
- El-Naggar, A.K.; Takata, T.; Slootweg, P.J.; Chan, J.K.C.; Grandis, J. Odontogenic and maxillofacial bone tumours. In WHO Classification of Head and Neck Tumours; International Agency for Research on Cancer, IARC Press: Lyon, France, 2017; pp. 206–212, 234–242. [Google Scholar]
- Fujita, K.; Sato, H.; Kato, S.; Saisu, H.; Ikeura, K.; Kodaka, R.; Tsunoda, H.; Mori, T.; Yamagami, J.; Amagai, M.; et al. Clinical Characterization of Oral Symptoms in 6 Paraneoplastic Pemphigus Patients. J. Jpn. Oral Med. 2017, 23, 1–8. [Google Scholar] [CrossRef][Green Version]
- Ricciuti, B.; Dahlberg, S.E.; Adeni, A.; Sholl, L.M.; Nishino, M.; Awad, M.M. Immune Checkpoint Inhibitor Outcomes for Patients With Non-Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J. Clin. Oncol. 2019, 37, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).