Abstract
Aims. The purpose of this systematic review was to evaluate studies that assessed denture–mucosa pressure distribution and pressure–pain threshold and their methodology, used to measure such pressure distributions, mainly in complete and implant overdentures. Materials and methods. An electronic search of the relevant full-text peer-reviewed literature on denture–mucosa pressure distribution was done. Searches were performed independently by two researchers by using the OVID Medline, PubMed and Web of Science databases from 1 January 1946 to 31 December 2021 using the following MeSH terms; (denture OR complete dentures OR implant supported dentures) AND (mucosa OR mucous membrane) AND (pressure OR hydrostatic pressure). Only those publications in the English language were included. Furthermore, a manual search of the citations of the included studies was done to ensure a thorough search was conducted. Results. A full text review resulted in a total of eighteen studies. Of these, seven evaluated various intraoral pressures, two investigated the pressure–pain threshold in edentulous oral mucosa, five measured intraoral pressure through finite element analysis/FEA studies, two demonstrated pressure transducer and pressure measuring systems, and two investigated the comparison between implant-overdentures and complete dentures. Conclusions. To date, there is no study that assesses the pressure distribution on oral mucosa to provide a standardised and validated baseline pressure range which can be used to improve the designs and materials used for fabricating complete dentures. The relationship between pressure on the oral mucosa and the pain threshold of denture-wearing patients still remains poorly understood. There is yet no baseline data which can be universally applied for future studies; to correlate the oral mucosa pressure and pain threshold of edentulous patients encourages further research, especially comparing mucosa pressure under different denture designs for both complete and implant overdentures.
1. Introduction
It has been well established that some edentulous patients experience psychosocial and functional complications [,] which can adversely affect the patient’s quality of life. This is a significant global problem as the prevalence of edentulism remains as the worldwide demographic transitions into an ageing population, which is most commonly evident in industrialised countries. The World Health Organisation (WHO) reported that by 2050 [], approximately 22% of the population would be in the age group of 60 years and above. Consequently, an exponential demand for ongoing oral health care would be expected to improve, maintain and promote the oral health of this ageing population.
Complete dentures, implant overdentures and partial dentures are often fabricated to restore the aesthetic and functional demands of edentulous patients. As the various prostheses rely on the support from the oral mucosa and residual ridges, it is critical to minimise any excessive residual ridge resorption [] by evenly distributing occlusal loads []. As the oral mucosa of an edentulous arch is comprised of mainly epithelial and connective tissue [], the histological reaction to wearing dentures is notably significant in the mechanical and physiological capacity of the mucosa. This also relates to patients’ denture-wearing experience as any excessive localised pressure can lead to pain or discomfort [], leading to unnecessary soft and hard tissue damage affecting the masticatory function, nutritional intake and overall oral health-related quality of life.
In the absence of natural teeth, mechanoreceptive function [] and proprioception occur through continuous stimulation between the denture base and oral mucosa. Therefore, patterns of intraoral pressures within the oral cavity depend on whether it is acceptable functional behaviours, such as mastication, swallowing and speech, or parafunctional behaviours, such as bruxism []. Management of the amount and pattern of pressure exerted on oral mucosa is an important aspect for denture treatment, to minimise any excessive residual ridge resorption impacting on the stability and retention of removable prostheses. As the capacity of the oral mucosa under continuous loading is variable [] and dependent on the level and duration of the mechanical load during denture wearing, there is limited knowledge on the physiological parameters for the oral mucosa’s pain threshold. Notably, the pressure–pain threshold (PPT), is a major area of interest within denture treatment as it is the maximum pressure before pain is experienced by the patient [,].
Previous studies have reported on the maximum ability that the edentulous mucosa can bear when subjected to masticatory stress [,]. However, as the local variability of the relationship between pressure on the mucosa and the poor ability of methods to report this local variability, which depend also on mucosa properties at this specific site, the pain threshold of denture-wearing patients still remains poorly understood. Although various findings correlating hydrostatic pressure to soft-tissue induced bone resorption have been investigated [,], there are still some uncertainties about the mechanical and physiological capacity of the oral mucosa. Consequently, there is a need for such reviews in order to understand the correlation between the denture–mucosa pressure distribution, PPT and how this affects the patient’s oral health-related quality of life.
Therefore, the purpose of this systematic review was to gather the quantitative evidence that assessed the denture–mucosa pressure distribution using conventional complete dentures or implant overdentures and how this correlated to the pressure–pain threshold. Furthermore, the secondary aim was to evaluate the methodologies used to measure such pressure distributions.
2. Materials and Methods
An electronic search using Ovid MEDLINE, PubMed and Web of Science was performed with the following MESH (Medical Subject Headings) terms: (denture OR complete dentures OR implant supported dentures) AND (mucosa OR mucous membrane) AND (pressure OR hydrostatic pressure). The overall search strategy is presented in Table 1. The evaluation criteria were defined in accordance with the PICO(S) (Patient or Population, Intervention, Control or Comparison, Outcome and Study types) criteria. The review included all studies involving completely edentulous patients with conventional complete dentures, or implant overdentures. There were no restrictions regarding the sex or age of the participants. The selection inclusion criteria included publications in English language only. Studies using in vitro and in silico methods were also included. Studies with animal models, studies irrelevant to the focus question, those where only the abstracts were available, as well as reviews were excluded from the selection. Full-text articles were included to ensure thorough review of the respective studies. This was completed to identify any gaps within the current literature to supplement the development of our methodology to measure the denture–mucosa pressure distribution in a typical edentulous patient. Outcome parameters were defined with respect to existing reviews; the main outcome parameters of the included studies according to the denture–mucosa pressure distribution in terms of PPT, as well as an evaluation of the methodology used to measure pressure, including the standardisation and validity of the results. The lower limit on the publication date was 1 January 1946 and the search included up to 31 December 2021. Titles and abstracts of potential studies were assessed independently by two reviewers (AP, JJEC) according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Studies that met the inclusion and exclusion criteria were evaluated for its relevance for the purpose of the current systematic review. A further manual search was also conducted using the citations of the included studies. Furthermore, the two reviewers evaluated the full-text studies independently and cross-referenced the individually selected literature to reduce the risk of bias of each included study. Inter-rater reliability was evaluated using SPSS ver 26. (IBM) using Cohen’s Kappa Coefficient.

Table 1.
Systematic search strategy.
3. Results
The search strategy details are illustrated in a PRISMA flow chart in Figure 1. The systematic database searches led to a total of 76 abstracts initially. According to the exclusion criteria, 59 studies were excluded due to being review articles, conference proceeding abstracts and being not relevant to the topic. Thus a total of 17 selected articles were included in the final analysis. Six articles were further identified to be relevant via a manual search. During the full-text screening, five articles were excluded since they were out of the scope for this systematic review, being clinical studies conducted with dentate patients. Thus, a total of 18 studies [,,,,,,,,,,,,,,,,,] met the inclusion and exclusion criteria to be included in the final analysis (Table 2. A strong inter-examiner agreement was found during the full-text screening and article final selection (Cohen’s Kappa, 0.95). All 18 eligible studies were either in vivo studies (12 articles) and in vitro/in silico studies (8 articles) that were well-designed and found to have a low risk of bias for most criteria, except for the participant selection bias for mainly in vivo studies. For most studies, the risk of bias assessment (Table 2), especially for participant selection was difficult to identify due to the lack of information provided regarding the participant selection process (identified as “unclear”).
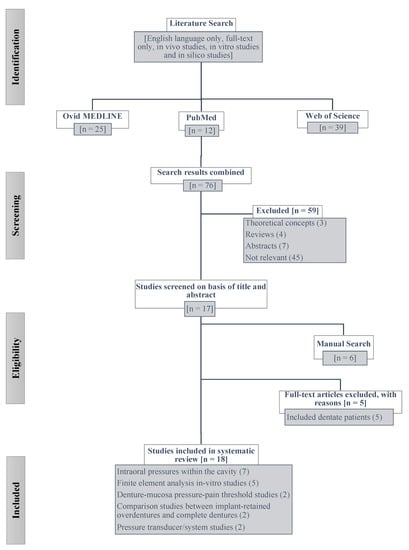
Figure 1.
Flowchart of the systematic literature search.

Table 2.
Risk of bias summary.
The purpose, methods (type of studies) and pressure range results of all 18 studies included in the final analysis are summarised in Table 3. Due to the different pressure units used in each study, the last column in Table 3 presents the data in a standardised unit, such as kPa, for an easier comparison.

Table 3.
Summary of full-text literature review.
The studies were characterised into five categories based on their objectives; firstly, 7 evaluated various intraoral pressures [,,,,,,]. A large range of intraoral pressure was observed between the studies after the results were converted into a standardised unit (kPa), ranging from 3.99 kPa [] to the highest of 461.6 kPa []. Out of these 7 studies, the mean intraoral pressure result of 4 studies [,,,] were not possible to be converted into kPa due to results presented in an image rather than numerical values.
Two studies investigated the pressure–pain threshold (PPT) in the edentulous oral mucosa [,]. Ogawa et al. (2004) [] evaluated the PPT in 15 patients and reported that different areas of the edentulous oral mucosa have a different PPT which varies depending on intraoral locations. Tanaka et al. (2004) [] also reported that PPT varies around the areas, with an increase in the pain threshold from the anterior to posterior alveolus in both the maxilla and mandible, but decreased from the anterior palate to the posterior palate. The mid palate showed 200–300% higher PPT than the buccal alveolar mucosa.
Five studies were found to measure intraoral pressure through finite element analysis (FEA) studies (Table 4) [,,,,]. Two studies used three-dimensional FEA methods [,], whereas the rest of the studies used either two-dimensional or non-linear FEA methods [,,] to analyse the pressure distribution in oral mucosa. Despite the simulation or modelling technique used, all studies reported a higher pressure distribution in implant overdenture groups than complete denture groups. Avci et al. (2009) [] and Watson et al. (1984) [] demonstrated the pressure distribution in the oral mucosa using a hydraulic pressure transducer and strain-gauge pressure measuring systems, respectively. They reported that the area of the lower denture was about half that of the maxillary denture and suggested that pressures could become exceedingly high on the lower denture-bearing mucosa during chewing. Two studies [,] specifically investigated the comparison between implant-overdentures and complete dentures’ pressure distribution. Ahmad et al. (2015) [] focused on comparing the implant overdenture and conventional overdentures and the biomechanics of each system. In their in vivo study, the complete denture group developed a more even pressure distribution at an average of 17.7 ± 4.81 kPa with lower ridge resorption. The implant overdenture group generated an uneven distribution of hydrostatic pressure and resulted in at least twice as much residual resorption than the complete denture groups. In their in vitro study, Sato et al. (2020) [] focused on determining the appropriate attachment number and design in a mandibular overdenture and reported that ball attachments exerted the greatest effects on support and bracing—suitable for reducing oral mucosa pressure during mastication. A significant decrease in the oral mucosa pressure value and an increase in support and bracing when 2-implant dentures were applied—compared with complete dentures.

Table 4.
In vitro and/or in silico studies investigating the pressures developed beneath dentures.
4. Discussion
Numerous in vivo, in vitro and in silico studies have attempted to evaluate the pressures under complete denture bases; however, because of the complexity and difficulty of replicating intraoral cavity conditions, results from this topic of interest can be variable, inaccurate and unreliable. Moreover, there is another layer of complexity as past studies investigate different designs and combinations of dentures, such as complete dentures, implant overdentures with a different number of implant supports.
An initial observation of the in vivo studies [,,,,,,,,,,,] demonstrates that there is no consistency in regards to the number of participants nor the types of participants involved, as differences in age and gender were also observed (Table 5). Several studies only looked at a single patient for their study [,,,], in which they evaluated pressure measuring systems and validated their study using data obtained from a singular individual. In contrast, Józefowicz [] observed 50 patients in the first part of the study focusing on numerous landmarks of the edentulous maxillae, and then 475 patients in the second part of the investigation, which only looked at one region. Józefowicz [] investigated the gum softness by measuring pressure yielding of the maxillary mucoperiosteum. Although he did not directly measure the pressure or pain threshold, the findings indicate the ability of oral mucosa to conform to change under pressure and demonstrates how this can ultimately affect the construction of the denture.

Table 5.
In vivo studies investigating the pressures developed beneath dentures.
The extent of edentulism differed across in vivo studies and this meant that there were some variations in the design of prostheses as some studies included conventional complete dentures or implant overdentures [,,,], whereas partially edentulous patients were rehabilitated with removable partial dentures [,,]. While it is acknowledged that the amount and pattern of pressure exerted by removable prostheses would differ between tooth-mucosa supported and mucosa-support only situations, there is yet to be any clinical report investigating the correlation between the extent of edentulism and pressure distribution on the edentulous ridges.
Various pressure sensor systems, such as pressure transducers [], tactile sensor sheets [,], pressure algometers including pressure sensitive strain gauges [], pressure gauges [] and hydraulic pressure transducers [], were used to measure pressure or the pressure–pain threshold. While it is useful to see the various results associated with different pressure sensor systems, these devices are designed to focus on a single intraoral position (i.e., the hard palate) and may not be able to provide continuous pressure readings during normal intraoral function. It would be more beneficial to use appropriate pressure measuring systems in various locations so that the amount of pressure and its distributions can be observed. This would then give further insights into the correlation between the pressure measurements and pain threshold.
There were various methods to induce intraoral pressure such as counting one to ten, chewing assorted textured foods or swallowing []. While these methods utilise everyday functional intraoral movement, they also have limitations in that the frequency, intensity and pattern of force produced by individuals vary. Therefore, the methodology should be standardised between participants. Another method using an algometer to apply pressure to the patient’s oral mucosa to measure the PPT has the advantage that the rate at which manual force is applied is consistent to provide reliability in the results [,].
A recent study by Sato et al. (2020), which measured the oral mucosa pressure caused by mandibular implant overdentures and various attachment systems [], provides adequate reference for future studies investigating oral mucosa pressure. However, there were some limitations within this study. A precision universal testing machine, Instron 8874, was used to measure the in vitro pressures yet there was no regard to specifying the size of the load cell used in conjunction with the configuration testing. Moreover, the study used only acrylic complete dentures as their prosthesis of choice and did not use a comparison group, such as using any mucosal tissue to simulate accurate clinical environment, which further resulted in a lack of validity in their findings.
In more recent years, FEA simulations have been used in in silico studies when investigating the biomechanical responses of edentulous mucosa [] by estimating the load of the masticatory force experienced beneath the prosthesis []. However, due to the nature of FEA studies and the finite element modelling (FEM) software, clinical data are required to mimic the function and anatomy of the edentulous jaws. For this reason, any assumptions made within the respective FEA studies are critical as they influence the accuracy and validity of the experiment [,]. Źmudzki et al. [] further highlighted the inconsistencies associated with FEA research and suggested errors when the pressures measured beneath dentures were lower than the average PPT. An alternative method in validating results from FEA investigations was demonstrated by Chen et al. [], where clinical data were used to characterise the mucosal tissue and the results from their simulated study were subsequently corroborated with clinical data. This assisted in determining the biomechanical parameters, Poisson’s ratio of oral mucosa and the friction coefficient between the denture base and mucosa, using an inverse method. The mechanical properties of other materials were based on data from previous FE studies. Although in vitro studies and FEA offer a feasible approach to simulating in vivo situations, there are certain limitations, such as applying assumptions on the responses of the biological system or the difficulty of setting up a clinically accurate environment. Therefore, any conclusions made based on these measurements cannot be extrapolated to the entirety of the edentulous population and, hence, it is essential that in vivo measurements are used as the baseline data for in vitro studies as part of the validation process.
Two studies reported that the PPT of an edentulous patient was approximately 630 kPa [,] and that there was a strong relationship between PPT and different areas of the edentulous oral mucosa [], such as the mandibular crest recording the greatest PPT measurement [] due to the location of the occlusal load during masticatory function. Nevertheless, the PPT variable between individuals [] and the changes in intraoral pressure were based on the different patient activities, such as mastication, speech and swallowing []. There also seemed to be a generalised pressure pattern occurring between the duration of denture-wearing and age, in which lower pressure ranges were observed for long-term denture wearers []. Older patients seemed to produce lower pressure ranges as they were more skillful in controlling their dentures during normal function. For this reason, the correlation between denture–mucosa pressure and PPT, and how these results affect the outcome of the patient’s quality of life, has yet to be fully investigated (although few studies have referred to it).
The pressure ranges measured were variable among included studies. Numerous studies presented their pressure range and distribution results using graphical and/or diagrammatical measurements [,,,,,,,,], which is difficult to interpret accurately as well as objectively. Additionally, across all studies, the pressure units were not standardised which led to the inability to compare the measurements recorded in units other than mPa or kPa [,].
There is still a lack of methodology where the ranges and distribution of pressure developed under the denture base and applied to the edentulous mucosa can be measured. A future study should therefore focus on developing a standardised way of measuring the mucosal pressure related to edentulous patients.
5. Conclusions
According to a systematic review conducted, past studies that attempted to measure the denture–mucosa pressure distribution varied greatly in their methodology which limited a valid comparison. Despite the clinical significance, there is yet to be any baseline value which can be universally applied for future studies to correlate the oral mucosa pressure PPT of edentulous patients. The authors encourage further clinical and in vitro/in silico research evaluating the pressure distribution in oral mucosa, especially comparing different designs of prosthesis under a controlled setting.
Author Contributions
Conceptualization, A.P. and J.J.E.C.; methodology, A.P., S.M., J.N.W., J.J.E.C.; formal analysis, A.P. and J.J.E.C.; investigation, A.P., S.M., J.N.W., J.J.E.C.; resources, J.J.E.C.; data curation, A.P. and J.J.E.C.; writing—original draft preparation, A.P., S.M., J.N.W., J.J.E.C.; writing—review and editing, A.P., S.M., J.N.W., J.J.E.C. All authors have read and agreed to the published version of the manuscript.
Funding
No financial support for this work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Conflicts of Interest
The author declares no conflict of interest.
References
- Emami, E.; De Souza, R.F.; Kabawat, M.; Feine, J.S. The Impact of Edentulism on Oral and General Health. Int. J. Dent. 2013, 2013, 498305. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E.; Kandelman, D.; Arpin, S.; Ogawa, H. Global oral health of older people—Call for public health action. Community Dent. Health 2010, 27 (Suppl. 2), 257–268. [Google Scholar] [PubMed]
- World Health Organisation (WHO). Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 25 March 2020).
- Źmudzki, J.; Chladek, G.; Kasperski, J. The influence of a complete lower denture destabilization on the pressure of the mucous membrane foundation. Acta. Bioeng. Biomech. 2012, 14, 67–73. [Google Scholar] [PubMed]
- Chen, J.; Ahmad, R.; Suenaga, H.; Li, W.; Swain, M.; Li, Q. A comparative study on complete and implant retained denture treatments: A biomechanics perspective. J. Biomech. 2015, 48, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Ogimoto, T.; Koyano, K.; Ogawa, T. Denture wearing and strong bite force reduce pressure pain threshold of edentulous oral mucosa. J. Oral Rehabil. 2004, 31, 873–878. [Google Scholar] [CrossRef]
- Ogawa, T.; Tanaka, M.; Ogimoto, T.; Okushi, N.; Koyano, K.; Takeuchi, K. Mapping, profiling and clustering of pressure pain threshold (PPT) in edentulous oral mucosa. J. Dent. 2004, 32, 219–228. [Google Scholar] [CrossRef]
- Cutright, D.E.; Brudvik, J.S.; Gay, W.D.; Selting, W.J. Tissue pressure under complete maxillary dentures. J. Prosthet. Dent. 1976, 35, 160–170. [Google Scholar] [CrossRef]
- Shi, S.G.; Wu, S.; Lu, L.; Imai, A.; Tanaka, M.; Kawazoe, T. The stress-bearing ability of mucosa in complete denture-wearers. Chin. J. Dent. Res. Off. J. Sci. Sect. Chin. Stomatol. Assoc. CSA 1998, 1, 41–45. [Google Scholar]
- Berg, T., Jr.; Chase, W.W.; Ray, K. Denture base pressure tests. J. Prosthet. Dent. 1967, 17, 540–548. [Google Scholar] [CrossRef]
- Józefowicz, W. Pressure yielding of the maxillary mucoperiosteum. J. Prosthet. Dent. 1972, 27, 600–606. [Google Scholar] [CrossRef]
- Kubo, K.; Kawata, T.; Suenaga, H.; Yoda, N.; Shigemitsu, R.; Ogawa, T.; Sasaki, K. Development of in vivo measuring system of the pressure distribution under the denture base of removable partial denture. J. Prosthodont. Res. 2009, 53, 15–21. [Google Scholar] [CrossRef]
- Roedema, W.H. Relationship between the width of the occlusal table and pressures under dentures during function. J. Prosthet. Dent. 1976, 36, 24–34. [Google Scholar] [CrossRef]
- Watson, C.J.; Huggett, R. Pressures recorded at the denture base-mucosal surface interface in complete denture wearers. J. Oral Rehabil. 1987, 14, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Assunção, W.G.; Barão, V.A.R.; Tabata, L.F.; De Sousa, E.A.C.; Gomes, A.; Delben, J.A. Comparison between complete denture and implant-retained overdenture: Effect of different mucosa thickness and resiliency on stress distribution. Gerodontology 2009, 26, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Suenaga, H.; Hogg, M.; Li, W.; Swain, M.; Li, Q. Determination of oral mucosal Poisson’s ratio and coefficient of friction from in-vivo contact pressure measurements. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Źmudzki, J.; Chladek, G.; Malara, P. Use of finite element analysis for the assessment of biomechanical factors related to pain sensation beneath complete dentures during mastication. J. Prosthet. Dent. 2018, 120, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Avci, M.; Aslan, Y. Measuring pressures under maxillary complete dentures during swallowing at various occlusal vertical dimensions. Part I: A new hydraulic pressure measuring system. J. Prosthet. Dent. 1991, 65, 661–664. [Google Scholar]
- Watson, C.J.; Abdul Wahab, M.D. The development of an inexpensive pressure transducer for use at the denture base-mucosal surface interface. Br. Dent. J. 1984, 156, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Chen, J.; Abu Hassan, M.I.; Li, Q.; Swain, M. Investigation of mucosa-induced residual ridge resorption under implant-retained overdentures and complete dentures in the mandible. Int. J. Oral Maxillofac. Implant. 2015, 30, 657–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, H.; Kobayashi, T.; Nomura, T.; Tanabe, N.; Takafuji, K.; Kihara, H.; Kondo, H. Oral mucosa pressure caused by mandibular implant overdenture with different types of attachments. J. Prosthodont. Res. 2020, 64, 145–151. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).