Simple Summary
We investigated how nest defense behavior in Saunders’s Gulls varies with nest density and predator type in South Korea. Using decoy experiments with a diurnal avian predator, a nocturnal mammalian predator, and a neutral co-nester, we found that gulls responded most rapidly and aggressively to magpies, less to raccoon dogs, and least to terns. Highly dense nest sites showed stronger collective defense with more mobbing individuals. Overall, Saunders’s Gulls flexibly adjusted their defense according to predator threat and social context, demonstrating the adaptive value of density-dependent collective defense in a colonially breeding seabird.
Abstract
Nest defense is a key component of avian reproductive success, yet its intensity and expression often depend on ecological and social contexts. We investigated the nest defense behaviors of Saunders’s Gulls (Saundersilarus saundersi) breeding in Incheon Bay of South Korea in 2022 in relation to nest density and perceived threats. Using decoy presentations of three heterospecifics, Oriental Magpie (Pica serica; diurnal avian nest predator), common raccoon dog (Nyctereutes procyonoides; nocturnal mammalian nest predator), and Little Tern (Sternula albifrons; neutral co-nester), we quantified latency to respond, bombing attack rate, and the number of mobbing individuals at high- and low-density nesting sites within a breeding colony. Mixed models revealed that latency to respond and attack rates varied strongly with stimulus type, with diurnal predator magpies eliciting the fastest and most intense responses, followed by nocturnal predator raccoon dogs and co-nester terns. Nest density influenced the number of mobbing individuals, which was significantly greater at high-density sites. Principal Component Analysis reduced the three behavioral metrics into a composite score, which correlated negatively with latency and positively with bombing attack rate and mobbing intensity. This score varied with both nest density and stimulus type. Our findings demonstrate that Saunders’s Gulls adjust their nest defense strategies according to both the social context and predator type, highlighting the importance of density-dependent collective nest defense in colonial breeders.
1. Introduction
Colonially breeding seabirds exhibit a suite of anti-predation strategies that are essential for minimizing the risk of nest loss, particularly since their nesting sites are often densely aggregated and spatially predictable [1]. In large colonies, the probability of early predator detection increases through the “many eyes effect”, followed by loud alarm calls that alert neighboring individuals and can collectively deter predators [2,3]. Synchronous behaviors—such as mass take-offs, coordinated flushing, and mobbing flights—further confuse or intimidate predators [4,5]. Some seabirds engage in direct mobbing attacks, including dive-bombing or physical harassment of intruders [6,7]. For example, coordinated mobbing and dive-bombing of the Black Skimmer (Rynchops niger) are most effective against avian predators, while alarm calling synchronizes group responses and recruits additional defenders [8]. Even within densely packed colonies, individual pairs defend small territories, resulting in one predator intrusion eliciting defense from multiple neighbors in European Herring Gull (Larus argentatus) and Great Black-backed Gull (L. marinus) colonies [9,10]. Recent research showed that colony-nesting gulls (Larus spp.) in western Norway suppressed the activity of Eurasian otters (Lutra lutra) during the breeding season, suggesting that colonial seabirds can directly influence predator behavior through aggressive nest defense [11]. Additionally, synchronous breeding may saturate a predator’s capacity to consume prey, thereby reducing the per-nest risk of predation as colony size increases. Moreover, individual variation in nest defense behavior can strongly influence reproductive outcomes within colonies. In Black-tailed Gulls (L. crassirostris), aggressive defenders lowered predation risk not only for their own nests but also for nearby non-aggressive neighbors [12]. Understanding how collective and individual defense strategies mitigate predation risk is essential for predicting colony stability and informing conservation management of threatened seabird populations.
In addition to these general strategies, predation pressure on seabird colonies can vary greatly depending on the time of day and the type of predator. Some predators are primarily diurnal, such as corvids and raptors, while others, including foxes and raccoon dogs, are mostly nocturnal or crepuscular, exposing nests to predation risks over a full 24-h cycle [13,14,15]. Studies on colonial gulls and terns have reported that nocturnal nest losses are often associated with mammalian predators, whereas diurnal attacks tend to be avian [13]. Moreover, different predator species target distinct prey stages: some specialize in consuming eggs, others prey upon chicks, and apex predators such as the White-tailed Eagle (Haliaeetus albicilla) may even capture adult birds [16,17,18]. Therefore, seabirds must adjust their defensive behavior depending on the predator’s foraging pattern and the vulnerability of different life stages within the colony.
The Saunders’s Gull (Saundersilarus saundersi) is a small, colonial, and globally vulnerable seabird distributed primarily in eastern China, with smaller breeding populations in Korea, and Japan [19,20]. In South Korea, the first breeding record was documented in 1998 at Incheon, and the species has since been designated as Vulnerable by the IUCN and protected under the Korean Ministry of Environment as an endangered wildlife species [21]. Currently, approximately 1500 breeding pairs are estimated to nest annually along the western coast of Korea [22], with the largest colonies concentrated in the reclaimed tidal flats of Incheon Bay. These reclaimed areas, formed through extensive coastal development projects in the 1990s, provide open and sparsely vegetated substrates that resemble the species’ natural breeding habitats. The flat terrain and proximity to tidal foraging grounds make such areas highly attractive for nesting gulls [21]. However, the artificial connection of these habitats to the mainland has simultaneously increased exposure to terrestrial predators and human disturbance. A diversity of mammalian and avian predators frequently invade the colonies, resulting in substantial egg and chick losses [21]. These pressures create ecological conditions characteristic of an ecological trap [23,24], in which birds are drawn to habitats that appear suitable but ultimately reduce reproductive success [25,26]. Our long-term monitoring conducted between 2011 and 2024 revealed that Saunders’s Gull colonies often persist for only two to three consecutive breeding seasons before relocating to new reclaimed sites, typically following severe nest predation events [21]. Such recurrent site shifts appear to function as an adaptive behavioral strategy to mitigate predation risk and maintain the stability of the national breeding population within rapidly changing coastal environments. Previous studies have reported that such breeding dispersal following heavy nest predation occurs frequently in reclaimed habitats [21]. However, breeding dispersal is not the focus of the present study and is referred to here only as ecological background information.
Beyond natural predators, human disturbance has emerged as a major factor influencing breeding success in colonial seabirds. Numerous studies have demonstrated that even infrequent human visitation can trigger flushing, heightened vigilance, or nest abandonment in gulls and terns [27]. Human approach distance is commonly quantified as the Flight Initiation Distance (FID), the distance at which a bird flees from an approaching human or intruder, serving as an indicator of perceived risk [28]. In densely populated colonies, however, seabirds may show shorter FIDs, as individuals rely on collective defence and social facilitation that reduce the perceived risk of approaching threats [29,30]. Nevertheless, excessive or repeated human presence can elevate physiological stress and indirectly increase predation risk by exposing unattended nests to opportunistic predators [31]. Taken together, these findings highlight that both natural predation and anthropogenic disturbance interactively shape behavioural strategies in colonial species. Therefore, understanding how Saunders’s Gulls respond to these pressures is essential for assessing colony persistence in highly modified coastal ecosystems.
The present study experimentally investigates the direct anti-predation strategies of Saunders’s Gulls, focusing on synchronous nest defense in relation to colony density and predator type. We tested the hypothesis that collective defense intensity increases with colony density through enhanced vigilance and cooperative mobbing—the so-called “many-eyes” and “group-attack” effects. We further predicted that gulls would exhibit predator-specific defensive responses, reacting more rapidly and aggressively toward threatening predators than toward neutral co-nesting species. To test these predictions, we conducted decoy experiments using both diurnal and nocturnal predator models and a neutral control species, and quantified multiple behavioral variables. By integrating these experimental results with long-term field observations, this study aims to elucidate how social context and predator recognition shape collective nest defense behavior in a colonially breeding seabird. The findings provide may insights into the adaptive significance of density-dependent defense and offer implications for the conservation of this endangered species in coastal ecosystems.
2. Materials and Methods
2.1. Study Site
Since the first domestic breeding colony of Saunders’s Gulls (Saundersilarus saundersi) was confirmed at the Sihwa Reclamation Area (37°15′29″ N, 126°37′53″ E) in 1998, breeding has persisted primarily within the Songdo reclamation complexes in Incheon, South Korea. Black-headed Gulls are known to relocate breeding sites when predation pressure exceeds a certain threshold [21]. The present study was conducted in Section 11-2 of the Songdo reclamation area in South Korea (37°23′13″ N, 126°38′35″ E) (see Figure 1), which comprises an extensive (approximately 153 ha) flat tidal-reclaimed tract directly connected to urban land. This open and low-lying terrain provided little natural cover, making the colonies easily accessible to potential predators. However, the sparse and low vegetation also offered broad visibility for breeding Saunders’s Gulls, allowing them to detect approaching predators at an early stage. The vegetation in the study area was sparse and primarily composed of halophytic species such as Korean seablite (Suaeda japonica), common glasswort (Salicornia europaea), sea plantain (Plantago maritima), and sea bindweed (Calystegia soldanella). Vegetation cover was generally below 20%, and extensive bare sand and mudflat areas dominated the central nesting sites [32]. Major avian predators include the Oriental Magpie (Pica pica) and Large-billed Crow (Corvus macrorhynchos), while mammalian predators such as the common raccoon dog (Nyctereutes procyonoides), Siberian weasel (Mustela sibirica), and feral dogs and cats (Canis and Felis spp.) were also observed within or around the breeding sites. Despite some snake and rodent predator species can potentially occur in our study sites, we did not detect any of them during our study period. Within the Saunders’s Gulls colonies, a small number of Little Terns (Sternula albifrons) and Eurasian Oystercatchers (Haematopus ostralegus) were also recorded, forming mixed-species nesting assemblages. Recreational human activities were not observed within or adjacent to the study area during the breeding season, ensuring minimal direct anthropogenic disturbance. The study area (Incheon) experiences a humid continental climate (Köppen Dwa type) under the influence of the East Asian monsoon, characterized by hot, humid summers and cold, dry winters [33]. According to data from the Korea Meteorological Administration (KMA), the 30-year (1991–2020) mean annual temperature in Incheon is 12.5 °C, with an average annual precipitation of 1220.8 mm. In 2022, the mean annual temperature was likewise 12.5 °C, while total annual precipitation reached 1620.5 mm, indicating a wetter-than-average year [34]. During the field survey (20–26 May 2022), weather conditions were stable and favorable for behavioral observations, with no rainfall and a mean wind speed of 2.7 m/s. The mean monthly temperature in May 2022 was 17.1 °C and total precipitation was 5.4 mm, reflecting typical pre-monsoon conditions. Sunrise and sunset times during the survey period ranged from 05:20 to 05:16 and from 19:37 to 19:43, respectively [34,35]. The mean tidal range in the study area, based on data from the Korea Hydrographic and Oceanographic Agency, approximately 9 m [36].
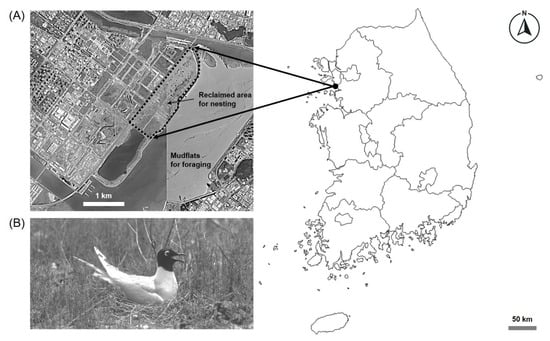
Figure 1.
Map of study site enclosed by a dashed line with focal species used to investigate the nest defense behavior of Saunders’s Gulls (Saundersilarus saundersi): (A) a breeding colony located in a reclaimed coastal area in Incheon Bay of South Korea; (B) an incubating Saunders’s Gull on its nest.
2.2. Study Species
The Saunders’s Gull (Saundersilarus saundersi) is a small, colonial, and ground-nesting seabird endemic to East Asia. Adults measure approximately 29–32 cm in body length, with a wingspan of 87–91 cm and a body mass of 170–220 g. During the breeding season, the entire head becomes black, contrasting sharply with the pale grey mantle and white underparts. The bill is black, the legs are reddish, and males and females are sexually monomorphic in appearance [37,38]. The global population of mature individuals is estimated at approximately 21,000–22,000 birds, distributed mainly along the eastern coast of China, the Korean Peninsula, and Japan. More than 10% of the world’s breeders (around 2900 individuals) occur in South Korea [19,35]. Owing to population declines associated with habitat loss and breeding-site disturbance, the species is listed as Vulnerable on the IUCN Red List and is legally protected as an endangered wildlife species under the Korean Ministry of Environment [38]. The breeding season extends from April to June. Saunders’s Gulls form dense colonies on flat, sparsely vegetated coastal reclamation areas or natural tidal flats located near foraging sites [39,40,41]. Their diet consists mainly of crabs and polychaete worms. Nests are built directly on the ground using dry plant stems, and the clutch size typically ranges from two to three eggs. both sexes share incubation duties for approximately 25–28 days [21]. The chicks are semi-precocial, leaving the nest 3–5 days after hatching and forming small crèches near the nesting territories [21,22]. Saunders’s Gulls show high breeding-site fidelity but tend to relocate to alternative sites in subsequent years when predation pressure or human disturbance intensifies [21]. Adults exhibit strong vigilance and alarm behaviors against avian and mammalian predators, often performing dive flights or bombing attacks as collective defense behaviors. Such coordinated mobbing actions are common among colonial seabirds and serve to reduce predation risk through group vigilance [11,42]. After the breeding season, individuals winter along the western and southern coasts of Korea, as well as in Hong Kong, southern Japan, Taiwan, and northern Vietnam [38].
2.3. Behavioral Data Collection
To examine Saunders’s Gull anti-predation strategies by predator type, we used two single plots (ca. 1.2 km apart) within the same colony differing in local breeding density: one high-density plot (37°21′57″ N, 126°40′59″ E) within Songdo Reclaimed Land Section 11-2 with ca. 531 nests·ha−1 and one low-density plot (37°22′22″ N, 126°41′36″ E) with ca. 86 nests·ha−1 in May of 2022. Here, nest density values for each plot were derived from imagery obtained during a drone-based population survey conducted on 19 May 2022, which was during the incubation period. Using three drones (Mavic 2, DJI, Shenzhen, China) flown at an altitude of 30 m and a constant speed of 10.1 m s−1 (i.e., previously tested a non-invasive protocol for Saunders’s Gulls [22,43]), we photographed the entirety of Section 11-2 and obtained 1807 images. To ensure seamless coverage, the photographs were acquired with intentional overlap, mosaicked into a single map, and then subdivided into 163 equal-sized image tiles (per-image footprint: 57 m × 57 m). We counted the number of nests in images encompassing the low- and high-density plots and calculated density accordingly [22]. To prevent visual and acoustic interference between the two focal plots during the experiments, we prioritized plots that were as far apart as possible. Because antipredator-behavior trials can disturb incubation, we confined the experiments to a short window (5 days; 20–26 May 2022) and scheduled them for the late incubation period (mid- to late May) based on our long-term survey data.
For antipredator behavioral assays (see Figure 2), we used taxidermic mounts of a common raccoon dog and an Oriental Magpie as nest predator stimuli, and a co-nesting Little Tern (Sternula albifrons) as a non-predatory control. Common raccoon dogs is a nocturnal predator that takes Saunders’s Gull eggs and chicks and can also injure adults. In contrast, the Oriental Magpie is a diurnal predator that primarily preys on eggs. We presented the three mounts to both the high- and low-density plots to test whether defensive responses differed by stimulus type. We then quantified three behavioral variables: response latency, number of mobbing individuals, and number of dive-bombing attacks. Because the mounts elicit strong reactions and one stimulus could carry over to the next, we controlled for order effects by implementing all six permutations (3! = 6) of the three-stimulus sequence. For each group, we established six presentation points—the five vertices of the pentagon plus the centroid—spaced 25 m apart. The order of stimuli and their assignment to points were drawn at random. To allow stabilization between trials, sessions alternated between groups, following the randomized schedule.
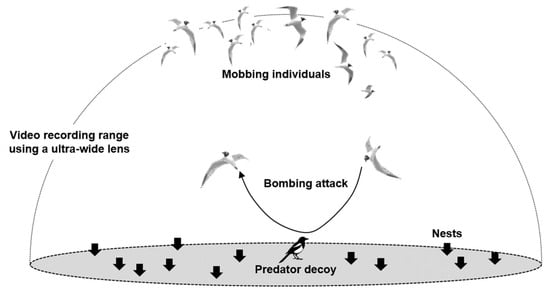
Figure 2.
Measurement of nest defense behaviors (latency time, number of bombing attacks, and number of mobbing individuals) in a breeding colony of Saunders’s Gulls (Saundersilarus saundersi). Observations were conducted using a smartphone recording function equipped with an ultrawide (180°) lens in the presence of predator and neutral decoys—Oriental Magpie (Pica serica), common raccoon dog (Nyctereutes procyonoides), and Little Tern (Sternula albifrons). Here, arrows indicate the direction of bombing attacks. The dashed-line area represents the observation range covered by the ultrawide lens.
For each specimen display for stimulus, a single experimental set proceeded as follows. To complete all trials, this sequence was repeated 33 times. First, at the designated presentation point, we placed the taxidermic mount on a turntable connected to a rope that could be pulled from a distance to rotate the platform and simulate predator movement. The mount and turntable were installed at the pre-marked point and the mount was concealed with a brown cloth similar in color to the ground-surface. A smartphone fitted with an ultrawide lens (Next, Seoul, Republic of Korea; 10 mm, 180° wide, 0.2×) was positioned adjacent to the mount to record the number of mobbing individuals, latency, and number of bombing attacks. Video recording was initiated prior to leaving the setup. Second, to avoid disturbing the gulls, the observer remained concealed inside a vehicle while exposing the stimulus. Two ropes were attached—one to the cover (for uncovering the mount) and one to the turntable—allowing remote removal of the cover and remote rotation from within the vehicle. Third, the cover was removed by pulling the rope, and the turntable rope was intermittently tugged for 5 min to simulate predator movement. From the recorded video, we quantified latency, number of mobbing individuals, and number of bombing attacks. Lastly, after each set, the observer approached the colony to re-cover the mount, removed the equipment from the immediate vicinity, and returned to the vehicle. We then waited until at least one individual had settled and the colony appeared stabilized near the presentation point before initiating the next trial. Conducting all trials under consistent daylight conditions minimized potential variability caused by light or visibility differences. Although the density contrast between the two plots was clear (approximately sevenfold difference in nest number), detailed spatial data of all active nests surrounding each stimulus point were not available. Therefore, we could not calculate the number of mobbing individuals relative to the local number of nests within a predefined radius. Nevertheless, the observation conditions (duration, distance, and viewing range) were kept consistent across all trials to ensure comparability between density treatments.
2.4. Statistical Analyses
We examined three measures of nest defense behavior—latency to respond (min), rate of bombing attacks (attacks·min−1), and number of mobbing individuals (individuals·min−1)—as a function of nest density (high vs. low) and stimulus type (Oriental Magpie as a diurnal nest predator, common raccoon dog as a nocturnal nest predator, and Little Tern as a neutral co-nester) using mixed models with experimental spot ID included as a random effect. Although formal normality tests were not conducted, inspection of model residuals and fitted values did not reveal any serious violations of normality or homoscedasticity. Given the moderate sample size (n = 33) and balanced experimental design, the Gaussian mixed model was considered robust to minor deviations from normality assumptions and was therefore retained to ensure stable and interpretable estimates. Given the limited sample size (n = 33), we retained only the experimental spot ID as the random effect to ensure model stability. Here, experimental timing (i.e., ranging from 08:00 to 16:00) and trial order (i.e., three decoy types) were excluded in the final statistical analyses, as preliminary analyses indicated that these factors had no significant effects on the response variables. Because detailed nest distribution data were unavailable, behavioral variables were analyzed as absolute measures per standardized trial rather than relative to the local number of nests. Although we did not systematically record environmental variables such as weather conditions, time of day, and breeding stage, we recognize that these factors may have influenced the birds’ defensive responses. To integrate these behaviors, we conducted a Principal Component Analysis (PCA; eigenvalue > 1) and extracted a composite principal component (PC) score. We then interpreted the PC score by examining its correlations with the original behavioral variables and tested its variation as a function of nest density, stimulus type, and their two-way interaction. All statistical analyses were performed in SPSS ver. 16.0 (SPSS, Chicago, IL, USA). We did not need to transform any variables to meet model assumptions. Behavioral measures are presented as means ± 1 SE.
3. Results
Defense behaviors of Saunders’s Gulls varied according to nest density (high vs. low) and stimulus type (decoys of Oriental Magpie, common raccoon dog, and Little Tern). First, latency to respond did not differ significantly between high-density (2.04 ± 0.33 min) and low-density sites (1.96 ± 0.34 min) (mixed model: F1,27 = 0.03, p = 0.87). However, latency differed markedly across stimulus types (F2,27 = 32.11, p < 0.001): responses occurred most rapidly to Oriental Magpies (0.21 ± 0.41 min), followed by common raccoon dogs (1.18 ± 0.41 min), and were slowest to Little Terns (4.60 ± 0.41 min). Second, the rate of bombing attacks (attacks per minute) did not differ significantly between high-density (7.88 ± 1.40) and low-density sites (4.79 ± 1.44) (F1,27 = 2.36, p = 0.14). In contrast, stimulus type strongly affected attack rates (F2,27 = 18.14, p < 0.001): Oriental Magpies elicited the highest rates (14.65 ± 1.74 times·min−1), followed by common raccoon dogs (3.95 ± 1.74 times·min−1), and Little Terns (0.40 ± 1.74 times·min−1). Third, the number of mobbing individuals was significantly greater at high-density sites (17.95 ± 2.06 individuals) compared with low-density sites (4.61 ± 2.12 individuals) (F1,27 = 20.40, p < 0.001), whereas this measure did not differ among stimulus types (F2,27 = 2.13, p = 0.14). To integrate these behavioral measures (latency, attack rate, mobbing individuals), we conducted a Principal Component Analysis (PCA; eigenvalue = 2.11). The first principal component explained 70.3% of the total variance, and the loadings for the three behavioral variables were −0.79 for latency, 0.92 for bombing attack rate, and 0.80 for the number of mobbing individuals. Both the Kaiser–Meyer–Olkin (KMO) measure (0.67) and Bartlett’s test of sphericity (p < 0.001) indicated that the data were suitable for PCA despite the small sample size. The first principal component (PC1) was defined as a “defense tendency index,” representing the shared variation among the three behavioral variables. This index was interpreted as an exploratory summary of correlated nest defense traits rather than as a causal variable. The PC1 was strongly correlated with each variable: negatively with latency (r = −0.79, p < 0.001), positively with attack rate (r = 0.92, p < 0.001), and positively with mobbing individuals (r = 0.80, p < 0.001). Nest defense behavior varied significantly with nest density, stimulus type, and their interaction (mixed model: density F1,27 = 6.92, p = 0.01; stimulus F2,27 = 17.90, p < 0.001; density × stimulus F2,27 = 1.32, p = 0.28; Figure 3). Specifically, gulls defended more quickly and intensely at high-density sites, and overall defense responses were strongest toward Oriental Magpies, intermediate toward common raccoon dogs, and weakest toward Little Terns.
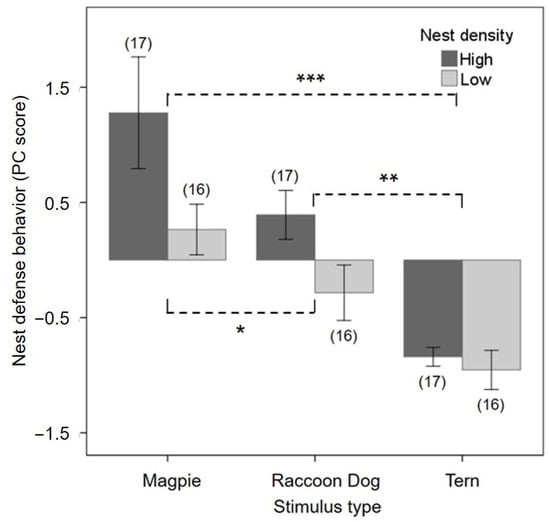
Figure 3.
Variation in nest defense behavior of Saunders’s Gulls as a function of nest density (high vs. low) and predator stimulus type (Oriental Magpie as a diurnal avian predator, common raccoon dog as a nocturnal mammalian predator, and Little Tern as a neutral co-nester). Bars represent mean ± 1 SE composite scores (i.e., degrees of nest defense) derived from latency time, bombing attack rate, and number of mobbing individuals using Principal Component Analysis. Asterisks above the bars indicate the significance of density-dependent pairwise differences among treatments (LSD post hoc test; * p < 0.05, ** p < 0.01, *** p < 0.001). Numbers in parentheses indicate sample sizes.
4. Discussion
Our findings demonstrate that nest defense behavior in Saunders’s Gulls is shaped by both social and ecological context, reflecting flexible behavioral strategies in response to perceived threats. Although response latency and attack rate did not differ significantly between high- and low-density colonies, gulls nesting at higher densities exhibited substantially more collective defense, as indicated by the greater number of mobbing individuals and overall higher composite defense scores. This suggests that social facilitation enhances vigilance and coordination among neighboring pairs, reinforcing the benefits of colonial breeding in deterring predators [1,2,3]. The pronounced variation in responses to different stimulus types further indicates fine-tuned predator recognition and threat assessment, with the most rapid and intense reactions directed toward the diurnal avian predator (Oriental Magpie), moderate responses toward the nocturnal mammalian predator (common raccoon dog), and minimal responses to the non-threatening co-nester (Little Tern). These results support the view that Saunders’s Gulls exhibit adaptive behavioral plasticity, modulating both individual and collective defense efforts according to the level of predation risk and the density of conspecifics [1,7]. Such context-dependent responses highlight the ecological and evolutionary significance of density-dependent collective defense in colonial seabirds and underscore the importance of maintaining suitable colony structure and habitat conditions for the conservation of this threatened species. In this study, the first principal component (PC1) derived from the PCA was defined as a “defense tendency index,” summarizing the shared variation among latency, attack rate, and the number of mobbing individuals. This exploratory index represents correlated behavioral traits rather than implying any causal relationship among variables. Therefore, the PCA results were interpreted as an integrated description of overall defense tendencies rather than as evidence of direct behavioral causation.
Our findings provide empirical support for the hypothesis that colonial breeding in seabirds functions as an effective anti-predation strategy by enhancing collective vigilance and coordinated nest defense [1,44]. In Saunders’s Gulls, individuals nesting at high-density sites exhibited markedly stronger collective responses—reflected in the greater number of mobbing individuals and higher composite defense scores—compared to those at low-density colonies. This density-dependent increase in defense intensity suggests that coloniality amplifies both predator detection and deterrence efficiency through social facilitation, a pattern consistent with studies of other colonial species such as Black-headed Gulls (Chroicocephalus ridibundus) and Common Terns (Sterna hirundo), where group size correlates positively with mobbing success and predator repulsion [2,6]. The enhanced defense observed in high-density gull colonies likely reduces per-nest predation risk via the dilution and confusion effects, making it adaptive even when individual aggression remains constant [1,4]. In contrast, the lower defense activity at sparse nesting sites may render isolated pairs more vulnerable to nest predators, underscoring the ecological trade-offs between breeding density, competition, and predation pressure. Collectively, these results highlight that coloniality in Saunders’s Gulls not only facilitates efficient predator recognition and rapid response but also strengthens the overall anti-predation function of social nesting, reinforcing its evolutionary persistence among sea-birds exposed to visually hunting, diurnal predators.
The present study provides clear evidence that Saunders’s Gulls discriminate be-tween avian and mammalian nest predators, and between diurnal and nocturnal threats, revealing fine-tuned nest predator recognition that likely evolved under differing predation pressures. Gulls exhibited the strongest and most rapid defense against the diurnal avian predator (Oriental Magpie), a common nest predator capable of visually locating eggs and chicks during daylight, while responses to the nocturnal mammalian predator (common raccoon dog) were slower and less intense. This distinction suggests that visual cues and daytime activity patterns strongly influence the gulls’ threat assessment and response strategy [7]. The markedly weaker reactions toward the neutral co-nester (Little Tern) further indicate that gulls can discriminate true predators from non-predatory species, minimizing unnecessary energetic costs of defense [45]. Such predator-specific recognition aligns with findings in other colonial seabirds: for example, Black-headed Gulls show heightened aggression toward corvids compared with mammalian predators [41], and Common Terns exhibit rapid, coordinated mobbing primarily against diurnal aerial predators such as crows and raptors, while remaining less responsive to nocturnal or ground-based threats [2]. Similarly, Arctic Terns (S. paradisaea) are known to direct intense mobbing toward visually conspicuous avian intruders during daylight, illustrating a shared reliance on vision-based predator recognition among colonial species [46]. Moreover, the stronger collective defense observed in high-density Saunders’s Gull colonies may enhance detection and deterrence of diurnal avian predators, reinforcing the adaptive value of social nesting in environments dominated by visually oriented nest threats. Together, these results highlight how both predator type and temporal activity patterns shape the evolution of context-dependent anti-predator behaviors in colonial seabirds.
Beyond immediate nest defense, breeding dispersal can function as an adaptive, in-direct anti-predation strategy in colonial seabirds. Movement between breeding seasons often follows severe nest predation or perceived risk, serving as a behavioral mechanism to avoid repeatedly unsafe sites [47,48]. Individuals may relocate to colonies that provide greater protection—such as those with more conspecifics for collective vigilance and mobbing—or rely on the prior breeding success of neighbors as “public information” when deciding whether to remain or disperse [47,49]. In this context, breeding dispersal in Saunders’s Gulls appears to operate as an adaptive response to high nest predation [21], which reported that colonies on reclaimed mudflats in Incheon frequently relocated following seasons of intense predation pressure. Such movements suggest that dispersal helps es-cape high-risk sites and enhance reproductive success—a pattern consistent with other colonial species such as Black-headed Gulls and Common Terns [50,51]. However, the effectiveness of this strategy in Saunders’s Gulls is constrained by the rapidly changing conditions of reclaimed habitats, where vegetation succession, human disturbance, and infrastructure development continually degrade nesting suitability. These reclaimed mudflats may act as ecological traps [25,26], attracting gulls to habitats that appear suitable but expose them to persistent predation risk and environmental instability. Thus, while breeding dispersal represents a potentially adaptive anti-predation mechanism, its efficacy diminishes in anthropogenic landscapes where habitat degradation outpaces behavioral adjustment, underscoring the need for integrated management of reclaimed coastal areas to prevent ecological traps and ensure the long-term viability of Saunders’s Gull breeding populations.
Although our findings provide novel insights into density-dependent and predator-specific nest defense behaviors of Saunders’s Gulls, several limitations with uncertainties should be acknowledged. The experimental design was restricted to two breeding plots (one high-density and one low-density), which may have confounded the effects of density with site-specific factors (e.g., microhabitat characteristics, local predator history, or human disturbance) [51,52]. Future studies should examine multiple colonies across a broader range of densities to treat density as a continuous ecological gradient and to better disentangle density effects from site-level variation [52,53]. The relatively small sample size and potential non-independence of repeated observations within colonies could have also reduced the statistical power and increased uncertainty [51]. Moreover, because the present study was conducted within a single breeding season, inter-annual variation in predator activity, colony composition, and individual site fidelity could not be assessed [53,54]. Additionally, environmental variables such as weather conditions, time of day, visibility, and breeding stage were not systematically recorded, which might have influenced the observed variation in defensive responses. Future studies should incorporate these covariates to improve model explanatory power and to distinguish intrinsic behavioral variation from environmentally driven responses. Long-term monitoring across multiple breeding cycles will be essential to confirm the consistency of these behavioral patterns and to strengthen the ecological basis for conservation planning in this endangered species [51,52,53,54]. This study was based on two density plots within a single colony, which limits the generalizability of our findings. Therefore, our results should be interpreted as reflecting within-colony patterns of density-dependent nest defense rather than colony-level or regional variation. Despite this limitation, the clear density contrast and consistent habitat conditions between plots provide confidence that the observed behavioral differences largely reflect effects of local breeding density. Future work incorporating replicated plots across multiple colonies and breeding seasons would help to verify the robustness and broader applicability of these patterns.
In South Korea, Saunders’s Gulls have shown remarkable adaptability by establishing breeding colonies on reclaimed tidal flats; however, this transition into artificial habitats has also introduced serious ecological challenges that threaten their long-term persistence [21,39,55]. One of the most critical consequences of mudflat reclamation is the influx of nest predators from the adjacent mainland ecosystem—such as corvids and raccoon dogs—which now have easy access to breeding colonies that were once naturally protected by tidal barriers [21]. This mainland-derived predation pressure, compounded by ongoing habitat modification, vegetation succession, and human disturbance, has resulted in reduced reproductive success, clutch size declines, and repeated colony abandonment [15,21,27]. Although wintering populations appear stable, the breeding population remains precarious due to these cumulative threats [53]. Future research should therefore prioritize understanding how reclaimed landscapes alter predator communities and spatial risk patterns, and evaluate the effectiveness of targeted predator management interventions—such as predator exclusion structures, buffer vegetation management, and strategic nest-site placement—to reduce predation pressure [55]. Long-term monitoring integrating ecological, behavioral, and spatial data will be essential to clarify how colony density, predator pressure, and habitat change interact to shape breeding performance. From a conservation perspective, protecting and restoring semi-natural coastal wetlands, reducing pollution, and implementing adaptive habitat management on reclaimed lands are vital steps toward mitigating the ecological traps created by reclamation and ensuring the sustainable breeding of Saunders’s Gull and other colonially nesting seabirds in rapidly changing coastal ecosystems.
5. Conclusions
Our study demonstrates that nest defense behavior in Saunders’s Gulls is shaped by both social and ecological context, highlighting flexible anti-predation strategies that integrate collective defense, predator recognition, and breeding dispersal. Gulls nesting at high-density colonies showed stronger collective responses, supporting the adaptive function of coloniality in enhancing vigilance and predator deterrence, while fine-tuned recognition of predator type—responding most aggressively to diurnal avian predators such as Oriental Magpie—illustrates the importance of visual cues and temporal patterns in threat assessment. High nest predation was also linked to breeding dispersal, as colonies relocated following predation events, suggesting that movement serves as an additional behavioral mechanism to mitigate predation risk. However, this strategy becomes less effective in reclaimed tidal flats, where human-driven habitat change, vegetation succession, and the influx of mainland predators (e.g., corvids and raccoon dogs) create ecological traps that undermine breeding success. To ensure the long-term persistence of this vulnerable species, conservation efforts should focus on managing nest predators, restoring semi-natural wetlands, and implementing adaptive habitat management and long-term monitoring to balance the ecological functions of colonial breeding with the challenges imposed by rapidly changing reclaimed environments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/birds6040061/s1.
Author Contributions
S.-J.L.: conceptualization, data curation, formal analysis, methodology, visualization, writing—original draft, writing—review & editing; B.-Y.H.: conceptualization, supervision, writing—review and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein; J.Y.: conceptualization, funding acquisition, project administration, resources, supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This material is based upon work supported by the National Institute of Ecology (NIE-B-2025-46).
Institutional Review Board Statement
Ethical permission for conducting this study was obtained from the National Institute of Ecology (NIEIACUC-2021-012).
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data and information relevant to this study are contained within the Supplementary File in this paper.
Acknowledgments
We thank two anonymous reviewers for providing helpful suggestions and improving our article. We would also like to express our sincere gratitude to J. M. Ha, S. Y. Lee, D. K. Yoo, C. W. Yoon, and J. K. Lee for their cooperation with drone filming and data analysis.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wittenberger, J.F.; Hunt, G.L., Jr. The adaptive significance of coloniality in birds. In Avian Biology; Farner, D.S., King, J.R., Parkes, K.C., Eds.; Academic Press: New York, NY, USA, 1985; Volume 8, pp. 1–78. [Google Scholar]
- Burger, J.; Gochfeld, M. The Common Tern: Its Breeding Biology and Social Behavior; Columbia University Press: New York, NY, USA, 1991. [Google Scholar]
- Beauchamp, G. What is the magnitude of the group-size effect on vigilance? Behav. Ecol. 2008, 19, 1361–1368. [Google Scholar] [CrossRef]
- Hamilton, W.D. Geometry for the selfish herd. J. Theor. Biol. 1971, 31, 295–311. [Google Scholar] [CrossRef]
- Quinn, J.L.; Cresswell, W. Testing domains of danger in the selfish herd: Sparrowhawks target centrally located redshanks in flocks. Proc. R. Soc. B Biol. Sci. 2006, 273, 2521–2526. [Google Scholar] [CrossRef]
- Andersson, M.; Wiklund, C.G. Clumping versus spacing out: Experiments on nest predation in fieldfares (Turdus pilaris). Anim. Behav. 1978, 26, 1207–1212. [Google Scholar] [CrossRef]
- Gochfeld, M. Antipredator behavior: Aggressive and distractive displays of shorebirds. In Shorebirds: Breeding Behavior and Populations; Burger, J., Olla, B.L., Eds.; Plenum Press: New York, NY, USA, 1984; pp. 289–377. [Google Scholar]
- Quinn, J.S. The Black Skimmer: Social Dynamics of a Colonial Species; Columbia University Press: New York, NY, USA, 1991; pp. 68–71. [Google Scholar]
- Burger, J. Territory size differences in relation to reproductive stage and type of intruder in Herring Gulls (Larus argentatus). Auk 1980, 97, 733–741. [Google Scholar]
- Butler, R.G.; Trivelpiece, W. Nest spacing, reproductive success, and behavior of the Great Black-backed Gull (Larus marinus). Auk 1981, 98, 99–107. [Google Scholar]
- Guidos, S.; van Dijk, J.; Systad, G.; Landa, A. Colony-nesting gulls restrict activity levels of a native top carnivore during the breeding season. Remote Sens. Ecol. Conserv. 2023, 9, 527–539. [Google Scholar] [CrossRef]
- Kazama, K.; Watanuki, Y. Individual differences in nest defense in the colonial breeding Black-tailed Gulls. Behav. Ecol. Sociobiol. 2010, 64, 1239–1246. [Google Scholar] [CrossRef]
- Huffeldt, N.P.; van Beest, F.M.; Kenyon, H.L.; Danielsen, J.; Guilford, T. Activity of predators in seabird colonies decreases during the darkest compared to the brightest phase of the diel cycle below, but not above, the Arctic Circle. Arct. Antarct. Alp. Res. 2024, 56, 2367262. [Google Scholar] [CrossRef]
- Toivonen, P.; Laaksonen, T.; Piironen, A.; Selonen, V. The habitat preferences of invasive raccoon dog imply elevated risks for wetland-associated prey species. Oecologia 2024, 206, 73–85. [Google Scholar] [CrossRef]
- Ratcliffe, N.; Schmitt, S.; Mayo, A.; Tratalos, J.; Drewitt, A. Colony habitat selection by Little Terns Sternula albifrons in East Anglia: Implications for coastal management. Seabird 2008, 21, 55–63. [Google Scholar] [CrossRef]
- Latorre, L.; Larrinaga, A.R.; Santamaría, L. Rats and seabirds: Effects of egg size on predation risk and the potential of conditioned taste aversion as a mitigation method. PLoS ONE 2013, 8, e76138. [Google Scholar] [CrossRef]
- Knudson, T.W.; Lovvorn, J.R.; Lawonn, M.J.; Corcoran, R.M.; Roby, D.D.; Piatt, J.F.; Pyle, W.H. Can oceanic prey effects on growth and time to fledging mediate terrestrial predator limitation of an at-risk seabird? Ecosphere 2020, 11, e03229. [Google Scholar] [CrossRef]
- Hipfner, J.M.; Blight, L.K.; Lowe, R.W.; Wilhelm, S.I.; Robertson, G.J.; Barrett, R.T.; Good, T.P. Unintended consequences: How the recovery of sea eagle Haliaeetus spp. populations in the Northern Hemisphere is affecting seabirds. Mar. Ornithol. 2012, 40, 39–52. [Google Scholar] [CrossRef]
- BirdLife International. Species Factsheet: Saundersilarus saundersi; The IUCN Red List of Threatened Species 2020. Available online: https://www.iucnredlist.org/species/22694436/132551327 (accessed on 30 October 2025).
- Burger, J.; Gochfeld, M.; Kirwan, G.M. Saunders’s Gull (Saundersilarus saundersi). In Handbook of the Birds of the World Alive; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Lynx Edicions: Barcelona, Spain, 2020; Available online: https://www.hbw.com/species/saunderss-gull-saundersilarus-saundersi (accessed on 30 October 2025).
- Yoon, H.J.; Joo, E.J.; Ha, D.S.; Nam, H.K.; Yoon, J. Does nest predation influence colony movements of Saunders’s Gulls (Saundersilarus saundersi) in a reclaimed land area? Zool. Sci. 2018, 35, 389–395. [Google Scholar] [CrossRef]
- Lee, S.J.; Yoo, D.K.; Yoon, C.; Choi, S.; Lee, J.; Yoon, J. A census of colonially breeding Saunders’s Gulls (Saundersilarus saundersi) using aerial imagery data from lightweight unmanned aerial vehicles. Korean J. Ornithol. 2022, 29, 144–148. [Google Scholar] [CrossRef]
- Dwernychuk, L.W.; Boag, D.A. How vegetative cover protects duck nests from egg predation. J. Wildl. Manag. 1972, 36, 955–958. [Google Scholar] [CrossRef]
- Gates, J.E.; Gysel, L.W. Avian nest dispersion and fledging success in field–forest ecotones. Ecology 1978, 59, 871–883. [Google Scholar] [CrossRef]
- Schlaepfer, M.A.; Runge, M.C.; Sherman, P.W. Ecological and evolutionary traps. Trends Ecol. Evol. 2002, 17, 474–480. [Google Scholar] [CrossRef]
- Robertson, B.A.; Rehage, J.S.; Sih, A. Ecological novelty and the emergence of evolutionary traps. Trends Ecol. Evol. 2013, 28, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Carney, K.M.; Sydeman, W.J. A review of human disturbance effects on nesting colonial waterbirds. Waterbirds 1999, 22, 68–79. [Google Scholar] [CrossRef]
- Blumstein, D.T. Flight-initiation distance in birds is dependent on intruder starting distance. J. Wildl. Manag. 2003, 67, 852–857. [Google Scholar] [CrossRef]
- Møller, A.P.; Tryjanowski, P. Direction of approach by predators and flight initiation distance of urban and rural populations of birds. Behav. Ecol. 2014, 25, 960–966. [Google Scholar] [CrossRef]
- Minias, P.; Gach, K.; Włodarczyk, R.; Bartos, M.; Drzewińska-Chańko, J.; Rembowski, M.; Janiszewski, T. Colony size as a predictor of breeding behaviour in a common waterbird. PLoS ONE 2020, 15, e0241602. [Google Scholar] [CrossRef]
- Steven, R.; Pickering, C.; Castley, J.G. A review of the impacts of nature-based recreation on birds. J. Environ. Manag. 2011, 92, 2287–2294. [Google Scholar] [CrossRef]
- Lee, M.; Kim, S.; Jung, H. Distribution patterns of halophytes in the coastal area in Korea. Sea (J. Korean Soc. Oceanogr.) 2019, 24, 139–159. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen–Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Korea Meteorological Administration (KMA). Annual Climate Statistics for the Incheon Region; Korea Meteorological Administration: Seoul, Republic of Korea, 2022; Available online: https://data.kma.go.kr (accessed on 29 October 2025).
- National Institute of Ecology (NIE). Conservation Research on the Population Trends and Factor Analyses in Endangered Species; National Institute of Ecology: Seocheon, Republic of Korea, 2022. [Google Scholar]
- Korea Hydrographic and Oceanographic Agency (KHOA). Tide Tables and Tidal Observation Data for Incheon Port; Korea Hydrographic and Oceanographic Agency: Busan, Republic of Korea; Available online: https://www.khoa.go.kr (accessed on 29 October 2025).
- National Institute of Ecology (NIE). Endangered Wildlife Species at a Glance; National Institute of Ecology: Seocheon, Republic of Korea, 2023. [Google Scholar]
- National Institute of Biological Resources (NIBR). Korea Biodiversity Information System (KBIS). Available online: https://kbr.go.kr (accessed on 30 October 2025).
- Kwon, Y.-S.; Chung, H. Breeding status and ecology of Saunders’s Gulls (Larus saundersi) in Songdo reclaimed land, west coast of Korea. Ocean Polar Res. 2009, 31, 233–242. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, Y.; Barter, M.; Lei, G. Saunders’s Gull: A new population estimate. Bird Conserv. Int. 2008, 18, 285–291. [Google Scholar] [CrossRef]
- Kruuk, H. Predators and Anti-Predator Behaviour of the Black-headed Gull (Larus ridibundus L.). Behav. Suppl. 1964, 11, 1–129. [Google Scholar]
- Carlson, N.V.; Griesser, M. Mobbing in animals: A thorough review and proposed future directions. Adv. Study Behav. 2022, 54, 1–41. [Google Scholar] [CrossRef]
- Choi, H.I.; Nam, H.K.; Yoon, J. Testing the potential of lightweight drones as a tool for monitoring the status of colonially breeding Saunders’s Gulls (Saundersilarus saundersi). Korean J. Ornithol. 2020, 27, 10–16. [Google Scholar] [CrossRef]
- Coulson, J.C. Colonial breeding in seabirds. In Biology of Marine Birds; Schreiber, E.A., Burger, J., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 87–113. [Google Scholar]
- Curio, E. The adaptive significance of avian mobbing I: Teleonomic hypotheses and predictions. Z. Tierpsychol. 1978, 48, 175–183. [Google Scholar] [CrossRef]
- Hatch, J.J. Predator–prey interactions in the Arctic Tern colony at Cape Cod. Wilson Bull. 1970, 82, 193–203. [Google Scholar]
- Boulinier, T.; Danchin, E. The use of conspecific reproductive success for breeding patch selection in terrestrial migratory species. Evol. Ecol. 1997, 11, 505–517. [Google Scholar] [CrossRef]
- Ponchon, A.; Chambert, T.; Lobato, E.; Tveraa, T.; Grémillet, D.; Boulinier, T. Breeding failure induces large-scale prospecting movements in the Black-legged Kittiwake. J. Exp. Mar. Biol. Ecol. 2015, 473, 138–145. [Google Scholar] [CrossRef]
- Danchin, E.; Wagner, R.H. The evolution of coloniality: The emergence of new perspectives. Trends Ecol. Evol. 1997, 12, 342–347. [Google Scholar] [CrossRef]
- Patterson, I.J. Timing and spacing of broods in the Black-headed Gull. IBIS 1965, 107, 433–459. [Google Scholar] [CrossRef]
- Palestis, B.G.; Hines, J.E. Adult survival and breeding dispersal of Common Terns (Sterna hirundo) in a declining population. Waterbirds 2015, 38, 221–228. [Google Scholar] [CrossRef]
- Savoca, M.S.; Avoca, M.; Nevitt, G.A.; Bonadonna, F. Nesting density influences reproductive success in Herring Gulls (Larus argentatus). Waterbirds 2011, 34, 436–441. [Google Scholar]
- McKellar, A.E. Patterns of inter- and intraspecific nest dispersion in colonies of gulls and grebes based on drone imagery. J. Field Ornithol. 2022, 93, 4. [Google Scholar] [CrossRef]
- Vidal, E.; Roche, P.K.; Bonnet, V.; Tatoni, T. Nest-density distribution patterns in a Yellow-legged Gull (Larus cachinnans) colony. Acta Oecol. 2001, 22, 235–243. [Google Scholar] [CrossRef]
- Yoon, J.; Park, J.; Chae, H.; Nam, H.K.; Yoon, H.J. Tide-associated incubation and foraging behaviour of Saunders’s Gulls (Larus saundersi). Ardea 2014, 101, 197–204. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).