Passive Acoustic Monitoring Provides Insights into Avian Use of Energycane Cropping Systems in Southern Florida
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
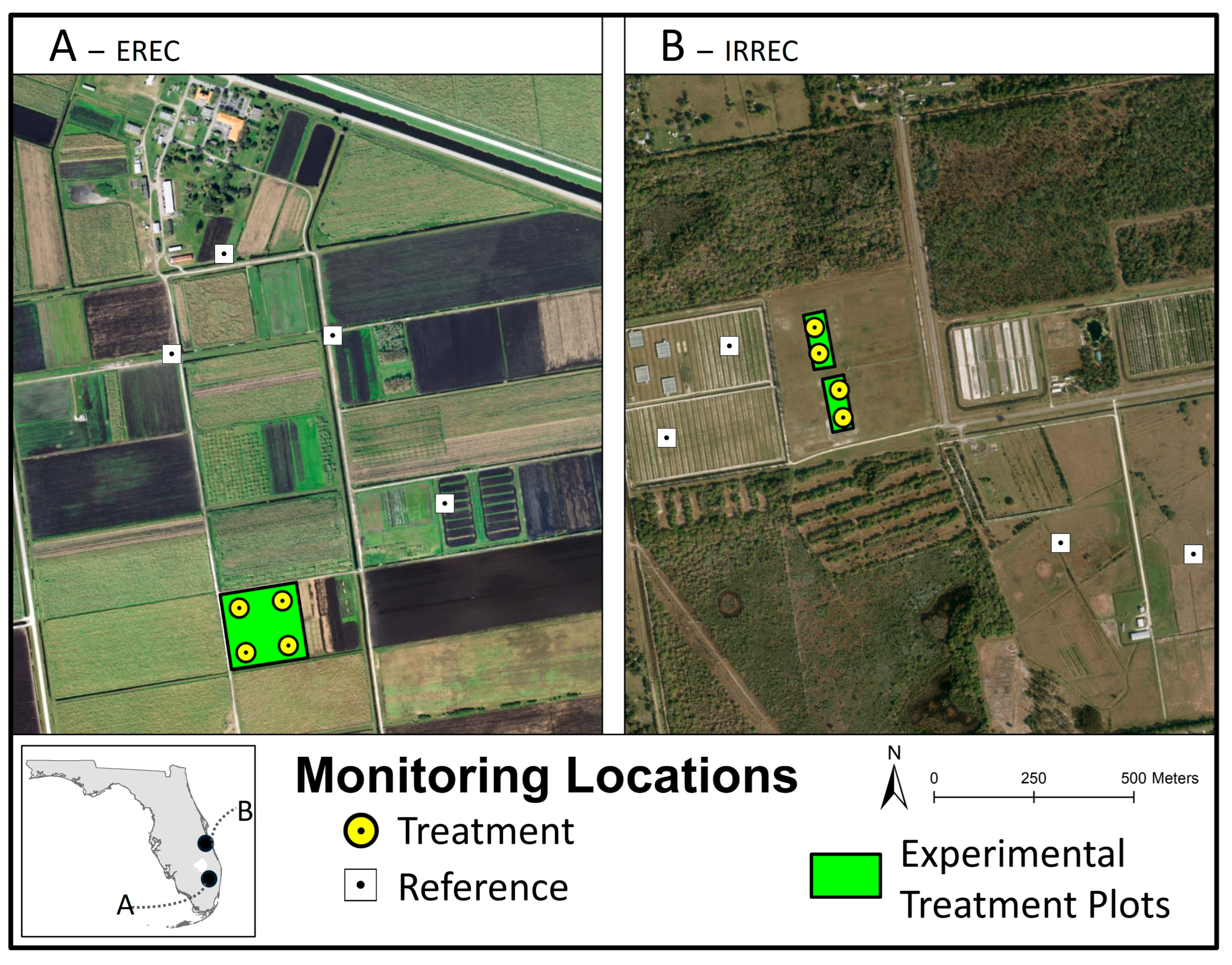
2.1. Study Area Overview
2.2. Study Site Location and Experiment Plot Layout
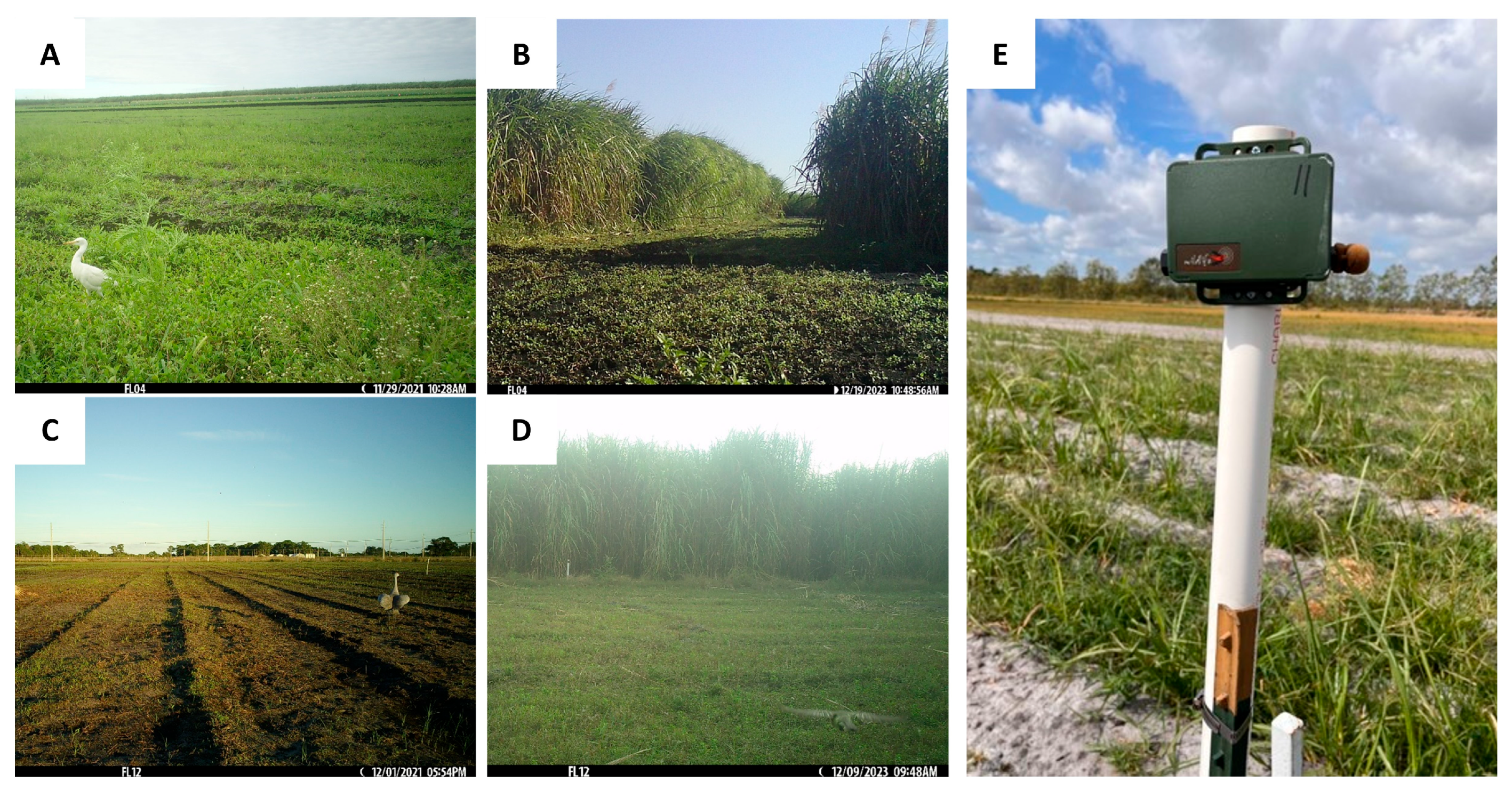
2.3. Acoustic Monitoring
2.4. Acoustic Data Processing
2.5. Statistical Methods
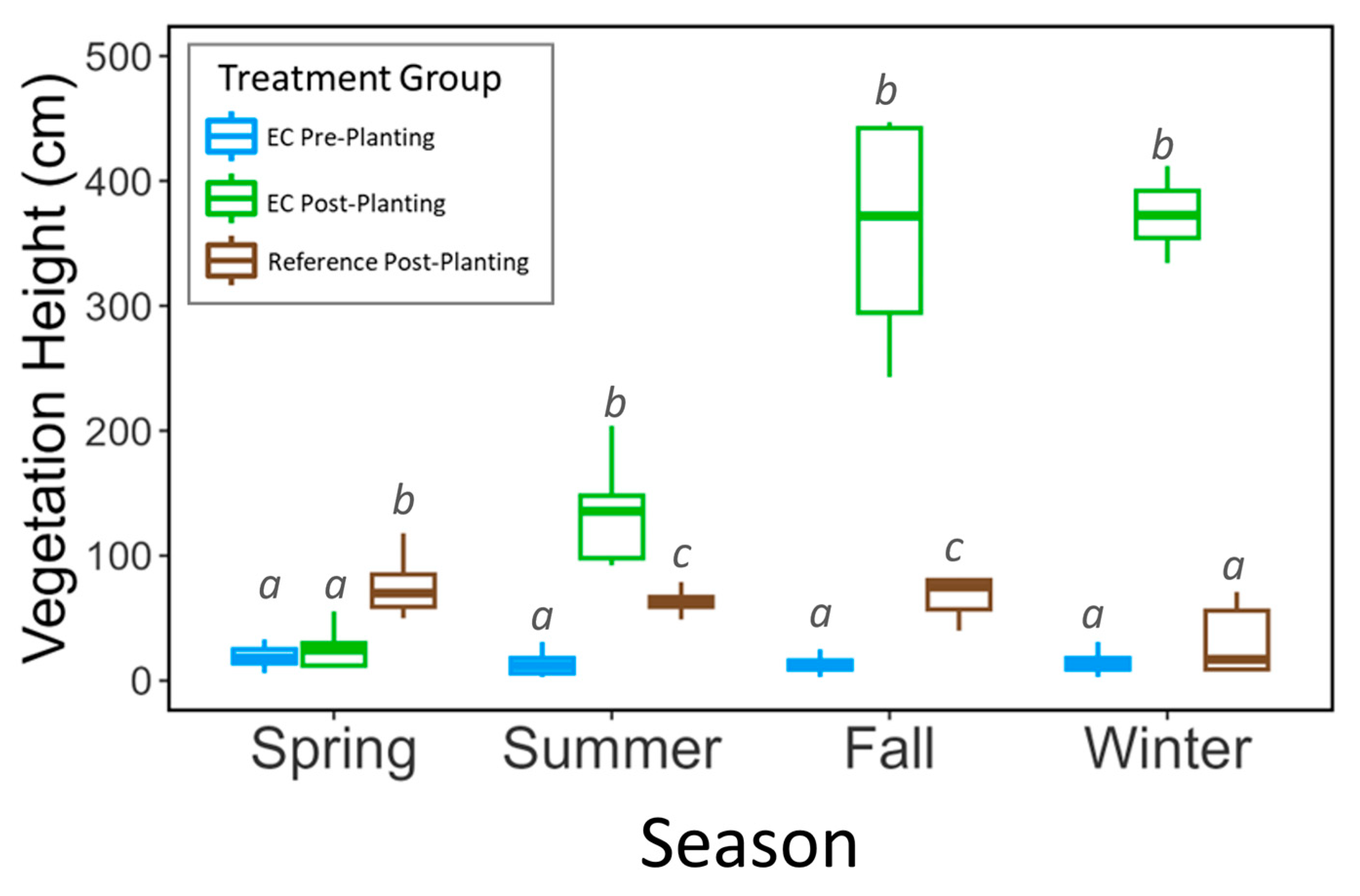
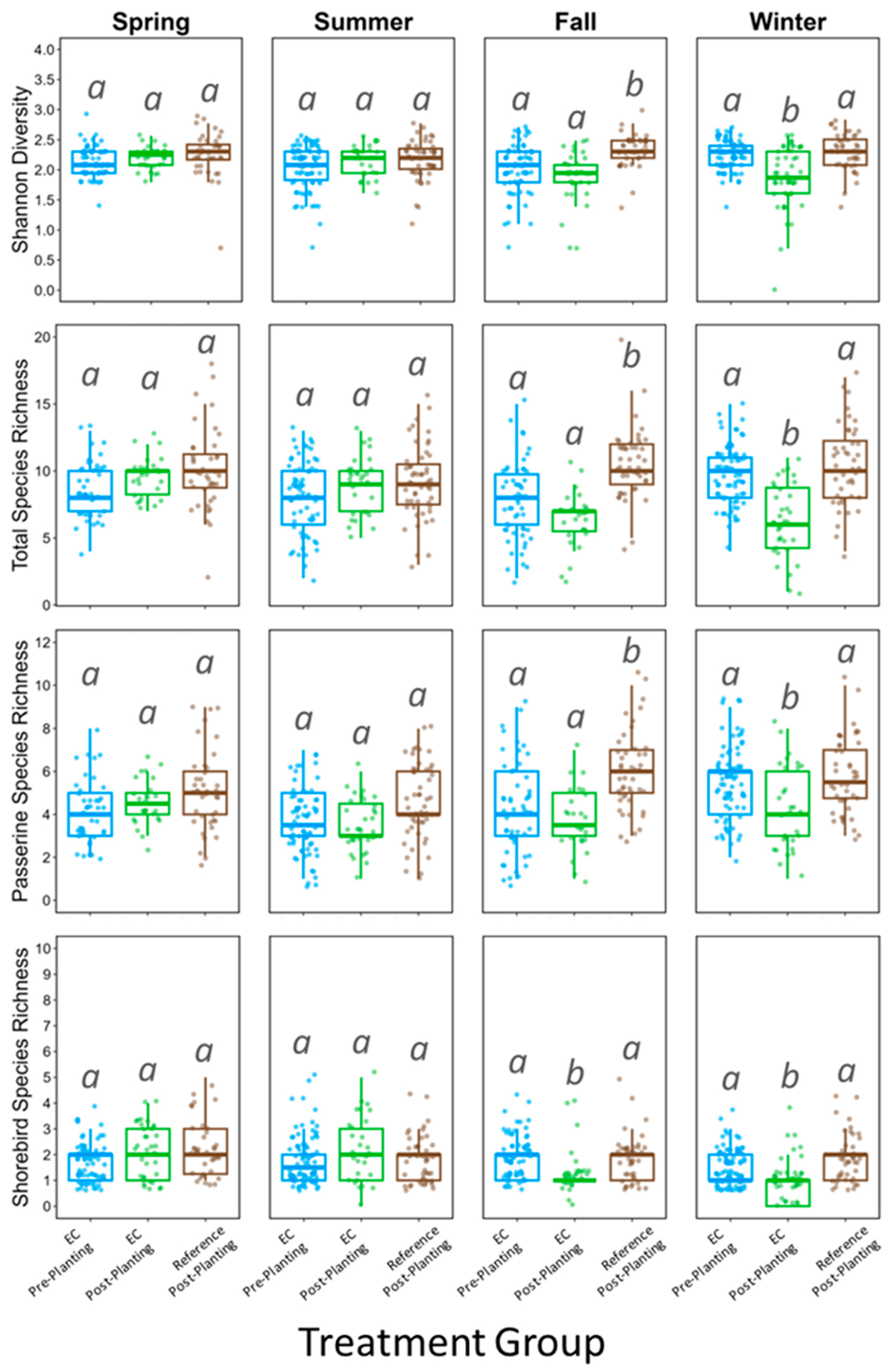
3. Results
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langholtz, M. 2023 Billion-Ton Report; Oak Ridge National Laboratory (ORNL): Oak Ridge, TN, USA, 2024. [CrossRef]
- Lee, D.K.; Aberle, E.; Anderson, E.K.; Anderson, W.; Baldwin, B.S.; Baltensperger, D.; Barrett, M.; Blumenthal, J.; Bonos, S.; Bouton, J.; et al. Biomass production of herbaceous energy crops in the United States: Field trial results and yield potential maps from the multiyear regional feedstock partnership. Glob. Change Biol. Bioenergy 2018, 10, 698–716. [Google Scholar] [CrossRef]
- U.S. Department of Energy (US DOE). Billion-Ton Update: Biomass Supply for a Bioenergy and Bioproducts Industry; (No. ORNL/TM-2011/224); US Department of Energy, Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2011. Available online: https://www1.eere.energy.gov/bioenergy/pdfs/billion_ton_update.pdf (accessed on 21 October 2025).
- Fedenko, J.R.; Erickson, J.E.; Woodard, K.R.; Sollenberger, L.E.; Vendramini, J.M.; Gilbert, R.A.; Helsel, Z.R.; Peter, G.F. Biomass production and composition of perennial grasses grown for bioenergy in a subtropical climate across Florida, USA. BioEnergy Res. 2013, 6, 1082–1093. [Google Scholar] [CrossRef]
- Sandhu, H.; Gilbert, R. Production of Biofuel Crops in Florida: Sugarcane/Energy Cane; The Institute of Food and Agricultural Sciences (IFAS), University of Florida: Gainesville, FL, USA, 2017. [Google Scholar] [CrossRef]
- Singerman, A.; Rogers, M.E. The economic challenges of dealing with citrus greening: The case of Florida. J. Integr. Pest Manag. 2020, 11, 3. [Google Scholar] [CrossRef]
- Blank, P.J.; Williams, C.L.; Sample, D.W.; Meehan, T.D.; Turner, M.G. Alternative scenarios of bioenergy crop production in an agricultural landscape and implications for bird communities. Ecol. Appl. 2016, 26, 42–54. [Google Scholar] [CrossRef]
- Beckmann, M.; Gerstner, K.; Akin-Fajiye, M.; Ceaușu, S.; Kambach, S.; Kinlock, N.L.; Phillips, H.R.P.; Verhagen, W.; Gurevitch, J.; Klotz, S.; et al. Conventional land-use intensification reduces species richness and increases production: A global meta-analysis. Glob. Change Biol. 2019, 25, 1941–1956. [Google Scholar] [CrossRef]
- Fargione, J.E.; Cooper, T.R.; Flaspohler, D.J.; Hill, J.; Lehman, C.; Tilman, D.; McCoy, T.; McLeod, S.; Nelson, E.J.; Oberhauser, K.S. Bioenergy and wildlife: Threats and opportunities for grassland conservation. BioScience 2009, 59, 767–777. [Google Scholar] [CrossRef]
- LaGory, K.E.; Cacho, J.F.; Zumpf, C.R.; Lee, D.; Feinstein, J.; Dematties, D.; Walston, L.J.; Namoi, N.; Negri, M.C. Bird species use of bioenergy croplands in Illinois, USA—Can advanced switchgrass cultivars provide suitable habitats for breeding grassland birds? Sustainability 2024, 16, 4807. [Google Scholar] [CrossRef]
- Kreig, J.A.; Lenhart, S.; Ponce, E.; Jager, H.I. Agent-based modeling to evaluate the effects of harvesting biomass and hunting on ring-necked pheasant (Phasianus colchicus) populations. Ecol. Model. 2024, 492, 110705. [Google Scholar] [CrossRef]
- Ambarlı, D.; Bilgin, C.C. Effects of landscape, land use and vegetation on bird community composition and diversity in Inner Anatolian steppes. Agric. Ecosyst. Environ. 2014, 182, 37–46. [Google Scholar] [CrossRef]
- Kahl, S.; Wood, C.M.; Eibl, M.; Klinck, H. BirdNET: A deep learning solution for avian diversity monitoring. Ecol. Inform. 2021, 61, 101236. [Google Scholar] [CrossRef]
- Bielski, L.; Cansler, C.A.; McGinn, K.; Peery, M.Z.; Wood, C.M. Can the hermit warbler (Setophaga occidentalis) serve as an old-forest indicator species in the Sierra Nevada? J. Field Ornithol. 2024, 95, 4. [Google Scholar] [CrossRef]
- Hack, B.; Cansler, C.A.; Peery, M.Z.; Wood, C.M. Fine-scale forest structure, not management regime, drives occupancy of a declining songbird, the olive-sided flycatcher, in the core of its range. Ornithol. Appl. 2024, 126, duad065. [Google Scholar] [CrossRef]
- Schuster, G.E.; Walston, L.J.; Little, A.R. Evaluation of an autonomous acoustic surveying technique for grassland bird communities in Nebraska. PLoS ONE 2024, 19, e0306580. [Google Scholar] [CrossRef]
- Volk, M.I.; Hoctor, T.S.; Nettles, B.B.; Hilsenbeck, R.; Putz, F.E.; Oetting, J. Florida land use and land cover change in the past 100 years. In Florida’s Climate: Changes, Variations, & Impacts; Chassignet, E.P., Jones, J.W., Misra, V., Obeysekera, J., Eds.; Florida Climate Institute: Gainesville, FL, USA, 2017; pp. 51–82. [Google Scholar] [CrossRef]
- Menges, E.S. Ecology and conservation of Florida scrub. In The Savanna, Barren, and Rock Outcrop Communities of North America; Anderson, R.C., Fralish, J.S., Baskin, J., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 7–22. [Google Scholar] [CrossRef]
- Bai, X.; Smidt, S.J.; Fan, Y.; Brophy, T.; Her, Y.G.; Manirakiza, N.; Li, Y.; Bhadha, J.H. Farming in shallow soils: Impacts of soil depth on crop growth in the Everglades Agricultural Area of Florida, USA. Field Crops Res. 2024, 316, 109523. [Google Scholar] [CrossRef]
- Baucum, L.E.; Rice, R.W.; Schueneman, T.J. An overview of Florida sugarcane. In University of Florida IFAS Extension Document SS-AGR-232; University of Florida: Gainesville, FL, USA, 2009. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture (USDA). 2024 State Agriculture Overview—Florida. 2024. Available online: https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=FLORIDA (accessed on 20 March 2025).
- Sandhu, H.S.; Gilbert, R.A.; Comstock, J.C.; Gordon, V.S.; Korndörfer, P.; Arundale, R.A.; El-Hout, N. Registration of ‘UFCP 82-1655’sugarcane. J. Plant Regist. 2016, 10, 22–27. [Google Scholar] [CrossRef]
- Matsuoka, S. Energy cane: A revolutionary clean energy crop for the transition to a sustainable energy system. Acad. Environ. Sci. Sustain. 2025, 2, 1–17. [Google Scholar] [CrossRef]
- Collins, J.M.; Rohli, R.V.; Paxton, C.H. Florida Weather and Climate: More than Just Sunshine; University Press of Florida: Gainesville, FL, USA, 2017. [Google Scholar]
- NOAA National Centers for Environmental Information (NCEI) (Year) Global Historical Climatology Network (GHCN)—Daily Observation Data. National Oceanic and Atmospheric Administration. 2025. Available online: https://www.ncei.noaa.gov/maps/daily (accessed on 21 October 2025).
- Wood, C.; Sullivan, B.; Iliff, M.; Fink, D.; Kelling, S. eBird: Engaging birders in science and conservation. PLoS Biol. 2011, 9, e1001220. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://r-project.org/ (accessed on 30 April 2025).
- Darras, K.; Batáry, P.; Furnas, B.; Celis-Murillo, A.; Van Wilgenburg, S.L.; Mulyani, Y.A.; Tscharntke, T. Comparing the sampling performance of sound recorders versus point counts in bird surveys: A meta-analysis. J. Appl. Ecol. 2018, 55, 2575–2586. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P. Vegan: Community Ecology Package. R Package Version 2.6-2. 2022. Available online: https://vegandevs.github.io/vegan/ (accessed on 21 October 2025).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means. AKA Least-Squares Means, R Package Version 1.8.1. 2019. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 21 October 2025).
- Dinno, A. Dunn.test: Dunn’s Test of Multiple Comparisons Using Rank Sums. 2024. Available online: https://cran.r-project.org/web/packages/dunn.test/index.html (accessed on 8 November 2025).
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘ggplot2.’ Create Elegant Data Visualisations Using the Grammar of Graphics Version 2:1–189. 2016. Available online: https://cran.r-project.org/web/packages/ggplot2/index.html (accessed on 21 October 2025).
- Pearlstine, E.V.; Mazzotti, F.J.; Rice, K.G.; Liner, A. Bird observations in five agricultural field types of the Everglades Agricultural Area in summer and fall. Fla. Field Nat. 2004, 32, 75–127. [Google Scholar]
- Pearlstine, E.V.; Mazzotti, F.J.; Kelly, M.H. Relative distribution and abundance of wintering raptors in agricultural and wetland landscapes of south Florida. J. Raptor Res. 2006, 40, 81–85. [Google Scholar] [CrossRef][Green Version]
- Miller, M.W.; Pearlstine, E.V.; Dorazio, R.M.; Mazzotti, F.J. Occupancy and abundance of wintering birds in a dynamic agricultural landscape. J. Wildl. Manag. 2011, 75, 836–847. [Google Scholar] [CrossRef]
- Townsend, S.E.; Pearlstine, E.V.; Mazzotti, F.J.; Deren, C.W. Wading birds, shorebirds, and waterfowl in rice fields within the Everglades Agricultural Area. Fla. Field Nat. 2006, 34, 9–20. [Google Scholar]
- Darras, K.; Pütz, P.; Fahrurrozi; Rembold, K.; Tscharntke, T. Measuring sound detection spaces for acoustic animal sampling and monitoring. Biol. Conserv. 2016, 201, 29–37. [Google Scholar] [CrossRef]
- Kross, S.M.; Bourbour, R.P.; Martinico, B.L. Agricultural land use, barn owl diet, and vertebrate pest control implications. Agric. Ecosyst. Environ. 2016, 223, 167–174. [Google Scholar] [CrossRef]
- Pérez-Granados, C. BirdNET: Applications, performance, pitfalls, and future opportunities. IBIS 2023, 165, 1068–1075. [Google Scholar] [CrossRef]
- Wood, C.M.; Kahl, S. Guidelines for appropriate use of BirdNET scores and other detector outputs. J. Ornithol. 2024, 165, 777–782. [Google Scholar] [CrossRef]
| Focal Species Name | Taxonomic Group | Total Detections (2021–2024) | Species-Specific Confidence Threshold (SSCT) a |
|---|---|---|---|
| Blue Jay (Cyanocitta cristata) | Passerine | 46,223 | 0.25 |
| Blue-gray Gnatcatcher (Polioptila caerulea) | Passerine | 1495 | 0.50 |
| Cedar Waxwing (Bombycilla cedrorum) | Passerine | 12,669 | 0.90 |
| Chimney Swift (Chaetura pelagica) | Passerine | 4374 | 0.80 |
| Common Yellowthroat (Geothlypis trichas) | Passerine | 86,338 | 0.80 |
| “Crow” (American [Corvus brachyrhynchos] and Fish [Corvus ossifragus]) | Passerine | 15,638 | 0.25 |
| Eastern Bluebird (Sialia sialis) | Passerine | 4184 | 0.90 |
| Eastern Meadowlark (Sturnella magna) | Passerine | 290,072 | 0.40 |
| Eastern Phoebe (Sayornis phoebe) | Passerine | 19,020 | 0.50 |
| “Grackle” (Boat-tailed [Quiscalus major] and Common [Quiscalus quiscula]) | Passerine | 285,975 | 0.25 |
| House Finch (Haemorhous mexicanus) | Passerine | 2442 | 0.80 |
| House Wren (Troglodytes aedon) | Passerine | 2055 | 0.50 |
| Loggerhead Shrike (Lanius ludovicianus) | Passerine | 45,294 | 0.90 |
| Northern Mockingbird (Mimus polyglottos) | Passerine | 43,654 | 0.50 |
| Palm Warbler (Setophaga palmarum) | Passerine | 108,771 | 0.50 |
| Purple Martin (Progne subis) | Passerine | 19,670 | 0.70 |
| Red-winged Blackbird (Agelaius phoeniceus) | Passerine | 72,086 | 0.25 |
| Savannah Sparrow (Passerculus sandwichensis) | Passerine | 50,400 | 0.40 |
| Swamp Sparrow (Melospiza georgiana) | Passerine | 5795 | 0.70 |
| Yellow-rumped Warbler (Setophaga coronata) | Passerine | 2029 | 0.90 |
| American Kestrel (Falco sparverius) | Owls & Raptors (Falconiformes) | 31,659 | 0.40 |
| Barn Owl (Tyto alba) | Owls & Raptors (Strigiformes) | 161,926 | 0.70 |
| Cooper’s Hawk (Astur cooperii) | Owls & Raptors (Accipitriformes) | 1407 | 0.90 |
| Northern Harrier (Circus hudsonius) | Owls & Raptors (Accipitriformes) | 8870 | 0.80 |
| Red-shouldered Hawk (Buteo lineatus) | Owls & Raptors (Accipitriformes) | 54,327 | 0.70 |
| Red-tailed Hawk (Buteo jamaicensis) | Owls & Raptors (Accipitriformes) | 2727 | 0.80 |
| Black-crowned Night-heron (Nycticorax nycticorax) | Shorebird (Pelecaniformes) | 8011 | 0.90 |
| Black-necked Stilt (Himantopus mexicanus) | Shorebird (Charadriiformes) | 26,705 | 0.80 |
| Caspian Tern (Hydroprogne caspia) | Shorebird (Charadriiformes) | 2391 | 0.80 |
| Common Gallinule (Gallinula galeata) | Shorebird (Gruiformes) | 10,211 | 0.80 |
| Great Blue Heron (Ardea herodias) | Shorebird (Pelecaniformes) | 10,312 | 0.90 |
| Great Egret (Ardea alba) | Shorebird (Pelecaniformes) | 2478 | 0.80 |
| Green Heron (Butorides virescens) | Shorebird (Pelecaniformes) | 5401 | 0.90 |
| Killdeer (Charadrius vociferus) | Shorebird (Charadriiformes) | 379,429 | 0.25 |
| Limpkin (Aramus guarauna) | Shorebird (Gruiformes) | 10,146 | 0.80 |
| “Yellowlegs” (Greater [Tringa melanoleuca] and Lesser [Tringa flavipes]) | Shorebird (Charadriiformes) | 26,705 | 0.80 |
| Chuck-will’s-widow (Antrostomus carolinensis) | Other (Caprimulgiformes) | 93,422 | 0.40 |
| Common Nighthawk (Chordeiles minor) | Other (Caprimulgiformes) | 2,039,821 | 0.30 |
| “Dove” (Common Ground [Columbina passerina] and Mourning [Zenaida macroura]) | Other (Columbiformes) | 28,484 | 0.60 |
| Northern Bobwhite (Colinus virginianus) | Other (Galliformes) | 64,511 | 0.50 |
| Metric | Taxonomic Group | Treatment Group | |
|---|---|---|---|
| χ2 | p | ||
| Spring | |||
| Species Diversity | Focal Species Diversity | 1.195 | 0.550 |
| Species Richness | Total Focal Species Richness | 3.931 | 0.140 |
| Passerines | 2.884 | 0.236 | |
| Shorebirds | 4.463 | 0.107 | |
| Summer | |||
| Species Diversity | Focal Species Diversity | 0.446 | 0.800 |
| Species Richness | Total Focal Species Richness | 2.600 | 0.272 |
| Passerines | 5.455 | 0.080 | |
| Shorebirds | 1.837 | 0.399 | |
| Fall | |||
| Species Diversity | Focal Species Diversity | 8.209 | 0.016 |
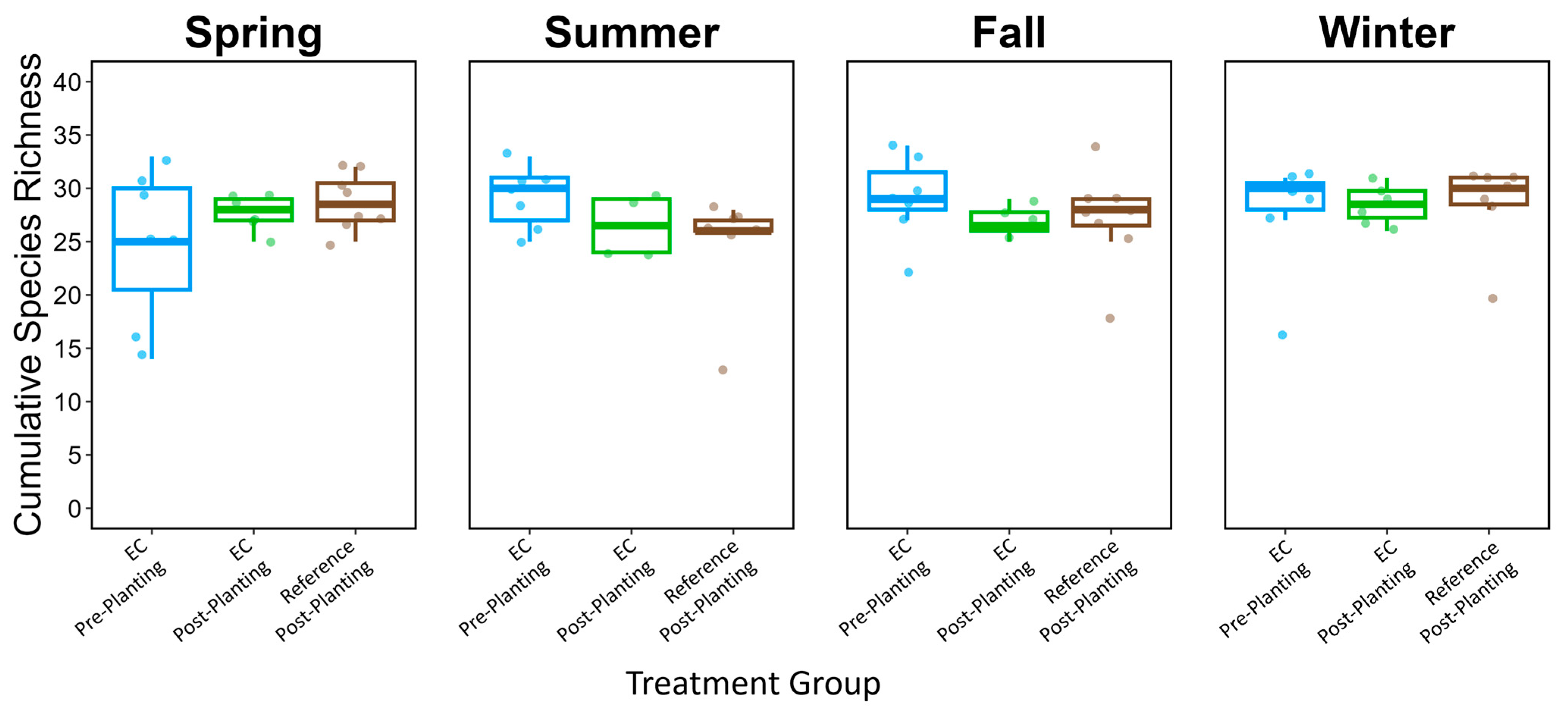
| Species Richness | Total Focal Species Richness | 14.091 | <0.001 |
| Passerines | 9.493 | 0.009 | |
| Shorebirds | 8.181 | 0.017 | |
| Winter | |||
| Species Diversity | Focal Species Diversity | 40.058 | <0.001 |
| Species Richness | Total Focal Species Richness | 5.922 | 0.050 |
| Passerines | 9.474 | 0.009 | |
| Shorebirds | 16.187 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walston, L.J.; Cacho, J.F.; Lesmes-Vesga, R.A.; Sandhu, H.; Zumpf, C.R.; Kasberg, B.; Feinstein, J.; Negri, M.C. Passive Acoustic Monitoring Provides Insights into Avian Use of Energycane Cropping Systems in Southern Florida. Birds 2025, 6, 60. https://doi.org/10.3390/birds6040060
Walston LJ, Cacho JF, Lesmes-Vesga RA, Sandhu H, Zumpf CR, Kasberg B, Feinstein J, Negri MC. Passive Acoustic Monitoring Provides Insights into Avian Use of Energycane Cropping Systems in Southern Florida. Birds. 2025; 6(4):60. https://doi.org/10.3390/birds6040060
Chicago/Turabian StyleWalston, Leroy J., Jules F. Cacho, Ricardo A. Lesmes-Vesga, Hardev Sandhu, Colleen R. Zumpf, Bradford Kasberg, Jeremy Feinstein, and Maria Cristina Negri. 2025. "Passive Acoustic Monitoring Provides Insights into Avian Use of Energycane Cropping Systems in Southern Florida" Birds 6, no. 4: 60. https://doi.org/10.3390/birds6040060
APA StyleWalston, L. J., Cacho, J. F., Lesmes-Vesga, R. A., Sandhu, H., Zumpf, C. R., Kasberg, B., Feinstein, J., & Negri, M. C. (2025). Passive Acoustic Monitoring Provides Insights into Avian Use of Energycane Cropping Systems in Southern Florida. Birds, 6(4), 60. https://doi.org/10.3390/birds6040060







