Simple Summary
The flock formation of bird species is a crucial behavioral process that enables them to colonize urban areas. However, the factors influencing the structure and composition of ground-feeding bird flocks have not yet been analyzed. Flock numbers were positively correlated with tree, lawn, and bare ground cover but negatively associated with raptor presence in parks. Flock species richness declined with increased noise and pedestrian traffic but rose in parks where raptors were present. The composition of species in flocks was linked to tree cover, noise, and the presence of raptors. While the Rock Dove (Columba livia) and the Rufous-bellied Thrush (Turdus rufiventris) were more abundant in parks with greater tree cover, the Eared Dove (Zenaida auriculata) and the Monk Parakeet (Myiopsitta monachus) showed increased abundance in more open parks. Zenaida auriculata and Columba livia experienced a decline in abundance in parks where raptors were present. Our findings indicate that resource availability and predation risk are crucial factors shaping flock formation in urban parks.
Abstract
The flock formation of bird species is a crucial behavioral process that enables them to colonize urban areas. However, the factors influencing the structure and composition of ground-feeding bird flocks have not yet been analyzed. This study aimed to relate flock characteristics, including size, number, species richness, and composition, to local and landscape factors in the urban parks of Buenos Aires City, Argentina. Surveys of flocks were conducted in 16 parks during the breeding season, covering both mono-specific and mixed-species flocks. Flock numbers were positively correlated with tree, lawn, and bare ground cover but negatively associated with raptor presence in the parks. Flock species richness declined with increased noise and pedestrian traffic but rose in parks where raptors were present. The composition of species in flocks was linked to tree cover, noise, and the presence of raptors. While the Rock Dove (Columba livia) and the Rufous-bellied Thrush (Turdus rufiventris) were more abundant in parks with greater tree cover, the Eared Dove (Zenaida auriculata) and the Monk Parakeet (Myiopsitta monachus) showed increased abundance in more open parks. Zenaida auriculata and Columba livia experienced a decline in abundance in parks where raptors were present. Our findings indicate that resource availability and predation risk are crucial factors shaping flock formation in urban parks.
1. Introduction
Bird flocks are social groups that can benefit their members, resulting in greater fitness and survival compared to solitary behaviors [1,2]. Foraging birds in groups might enhance predator detection due to more eyes scanning for threats and a dilution effect, as the likelihood of any individual being eaten is inversely related to group size [3,4]. Additionally, birds that form flocks are less vigilant for predators and spend more time foraging [5,6]. Furthermore, aggregations of birds can serve as indicators of food availability for other individuals [7]. These benefits apply to mono-specific and mixed-species flocks [4]. However, mixed-species flocks can offer additional advantages. For instance, individuals in mixed flocks may spend more time feeding than mono-specific flocks [8], or mixed flocks might include sentinel species that provide alarm calls in response to predator presence [9,10].
The assembly of mixed-species flocks has been described as structured or open membership [11]. Structured flocks have been described mostly in understory habitats of lowland evergreen forests, and flocks are characterized by a stable species composition and significant associations between them [12,13,14]. Open-membership flocks have been described for elevation gradients and open habitat types, and flocks have an unstable species composition and random associations between them [15,16,17]. Flock composition varies due to species dynamically joining and leaving the flocks as they enter or exit their territories [11], and flock richness is expected to vary accordingly to the bird richness of communities [16].
Anthropogenic impacts on natural habitats can affect bird flock structure—such as size, numbers, and species richness—and composition both directly by altering food availability and indirectly by reducing the population sizes of flocking species [18,19]. Habitat loss, fragmentation, and decreased habitat diversity have demonstrated negative impacts on flock size and richness, as well as significant changes in species composition in temperate and tropical forests [14,16,20,21,22,23]. Moreover, the changes in composition due to human disturbances exhibit distinct patterns in temperate and tropical regions [19]. In temperate areas, flock composition has displayed a gradual loss of species from the richest to poorer sites or a nested pattern [21,24]. Conversely, in tropical regions, variations in species composition among flocks are more closely related to species replacements or turnover [19,25].
These studies have primarily focused on insectivorous birds living in forests, while research examining terrestrial flocks of granivorous or insectivorous birds is significantly more limited [26,27,28]. This scarcity of studies on terrestrial flocks is even more surprising in urban areas, where flocking behavior appears to be a key trait that facilitates species colonization in cities [29,30,31,32].
Therefore, this study aims to analyze the relationship between ground-feeding flock structure, composition, and environmental variables in urban parks in Buenos Aires City. Food availability has positively influenced flock richness [28], so I anticipated greater flock richness in parks with more lawn and bare ground cover [33]. Larger and less isolated parks would increase flock size, numbers, and richness due to larger populations of flocking birds [34]. In addition, flock size and richness are expected to increase in parks with more raptors, thus increasing flock vigilance. Noise and pedestrian traffic are expected to decrease flock numbers, size, and abundance due to disrupted vocal communication and ongoing disassembly. Since Buenos Aires is a subtropical city, I anticipate a shift in species composition among flocks associated with species turnover.
2. Materials and Methods
2.1. Study Area
The study was conducted in the urban parks of Buenos Aires City (34°35′59″ S, 58°22′55″ W; 25 masl; 3,120,612 inhabitants). The climate type is classified as subtropical humid (Cfa, Köppen classification, [35]), with a mean annual temperature of 18.25 °C and a yearly precipitation of 1236.3 mm (National Meteorological Service). Buenos Aires is a coastal city surrounded by farmland, pastures, tree plantations, and small patches of natural and semi-natural landscapes (Figure 1). Originally, the landscape was composed of grasslands, xerophytic, and deltaic forests [36,37].
Figure 1.
Location of Buenos Aires City in Argentina (inset), and location of urban parks (black dots) in Buenos Aires City.
A total of 16 parks were selected, separated by at least 500 m (Figure 1). The tree species composition in urban parks is dominated by exotic species native to the Northern Hemisphere, such as the London Plane (Platanus acerifolia), Linden tree (Tilia viridis), and the White Mulberry (Morus alba). However, some species are native to northern Argentina, such as the Tipu tree (Tipuana tipu) and the Silk Floss tree (Ceiba speciose).
2.2. Flock Surveys
Each urban park was visited three times between December 2020 and February 2021, which coincides with the austral summer and breeding season of birds [38]. Bird observations were made during the first four hours of the morning on days without rain or strong winds. Each park was walked through defined pathways, generally following trails, surveying its entire area with the help of binoculars. The time employed in each park varied in direct relation to its area.
I defined a flock as three or more moving individuals nearby (within 10 m of the nearest individual) without indication that the birds were drawn to a concentration of food [15,39], which in parks was primarily provided by humans. I did not track flocks for longer than three minutes to prevent double counting of individuals within the same park and because flocks were often disturbed by humans or dogs [40]. While some flocks were seen in trees, this study focused exclusively on ground-foraging flocks.
In each park, I estimated the following four characteristics of the flocks: (1) flock number, which was the average number of flocks during the three visits; (2) flock richness, the average number of species in each flock during the three visits; (3) flock size, the average number of individual birds in each flock; and (4) mixed flock proportion, the proportion of flocks composed of two or more species compared to the total number of flocks.
2.3. Bird Species Surveys
Bird surveys were conducted in the parks to relate species abundances to flock formation. In each park, one or more fixed points with a 50 m radius were established. In parks smaller than 2 hectares, a point was placed at the center, while in larger parks, two or more points were established, separated by at least 200 m. Point surveys lasted five minutes and were carried out during the first four hours of the day from October to December 2020, coinciding with the bird’s breeding season [38]. Only individual birds foraging or perching in parks were recorded.
2.4. Environmental Variables
A total of nine environmental variables were measured (Table 1). Seven of these variables were collected at the local scale within each park (Table 1(a)). The park area (ha) was assessed using Google Earth Pro. The percent cover of trees, lawns, and bare ground was measured using one or more randomly located points. Each point featured four 20 m lines radiating from the center toward the cardinal directions. For every meter, the presence of each microhabitat cover was recorded. The percent cover was then calculated by dividing the amount of microhabitat presence by the total number of marks and multiplying by 100. Pedestrians and noise were measured simultaneously during bird species surveys conducted from October to December 2020. Pedestrians were counted as the number of people present during five-minute bird point count surveys. Noise levels were recorded in mean decibels 30 s before the bird surveys using the Sound Meter application [41] on a Galaxy S20 FE (Samsung Electronics, Suwon, Republic of Korea). Raptor presence was established from the bird surveys. The recorded species included the Harris’s Hawk (Parabuteo unicinctus) and the Southern Caracara (Caracara plancus).

Table 1.
Environmental variables of urban parks in Buenos Aires City, Argentina.
Landscape variables were those measured outside park boundaries (Table 1(b)). Isolation was assessed using Google Earth Pro and represented the minimum distance (km) to a park of 4 ha or larger. The surrounding building type for each park was a categorical variable indicating whether parks were primarily encircled (>50% covered) by houses or high-rise buildings (three or more stories). Data for this variable were collected through field observations.
2.5. Statistical Analysis
The relationship between the number of flocks occupied by each species and their densities in parks was evaluated using Spearman rank correlation analysis with the cor function in R (version 4.4.2) [42]. The association among species in flocks was examined with the cooccur function from the cooccur package [43]. The cooccur function determines whether the co-occurrence of two species is significantly large and greater than expected (positive association), significantly small and less than expected (negative association), or not significantly different and approximately equal to what was expected (random association) [43]. Species with low occurrence data were excluded from the analysis.
The association between flock characteristics and environmental variables was analyzed with generalized linear models using a Gaussian error structure. Model selection was assessed by backward elimination of non-significant variables (p > 0.05) from the full model using the anova function. Final models were compared with null models using a likelihood ratio test (LRT test) (p < 0.05). Model residuals distribution normality and heteroscedasticity were tested with the DHARMa package [44]. Multicollinearity of environmental variables was assessed with the performance package [45]. Final model results were plotted with the sjPlot package [46].
The differences in species composition among flocks can be attributed to a turnover of species individuals (balanced variation in abundance) or the gradual loss of species individuals between flocks (abundance gradients) [47]. The proportion of these two components was calculated from the total Bray–Curtis (BC) dissimilarity using the beta.multi.abund function from the betapart package [48]. The beta.multi.abund function computes the balanced variation in abundance and the abundance gradient components for multiple flocks. The association between flocking species and the environmental variables was assessed through distance-based Redundance Analysis (dbRDA). This ordination analysis used dissimilarity measures between flocks to relate ordination axes with environmental variables [49]. I used BC dissimilarity between flocks, which takes into account species abundances. dbRDA was run with the capscale function of the vegan package [50]. Models were obtained by backward variable selection and comparisons with null models using a likelihood ratio test (LRT test) (p < 0.05).
3. Results
A total of 1132 individuals of 24 species were detected in 167 flocks. Most of the species belong to the plant-seed diet guild (Table 2). The most common species forming flocks were the Eared Dove (Zenaida auriculata) and the Rock Dove (Columba livia) (Table 2), whereas the most abundant species in flocks were the Monk Parakeet (Myiopsitta monachus) and Columba livia. Columba livia and Zenaida auriculata showed the highest association in flocks (Table S1). Species propensity to flock was strongly positively related to their density in parks (rs = 0.79, N = 24; Figure 2). The mean percentage of mixed-species flocks was >80% (mean proportion = 0.85; Table 2). However, some species, such as the Grayish Baywing (Agelaiodes badius) and the Rufous-bellied Thrush (Turdus rufiventris), showed the lowest values of mixed flock formation.

Table 2.
List of species recorded in flocks, diet guild, number of flocks, mean density in flocks (individuals/flock), and proportion of mixed flocks for each species. Diet guilds are based on the classification by Wilman et al. [51]. VertFishScav refers to species that primarily feed on vertebrates and scavenge. * Exotic species.
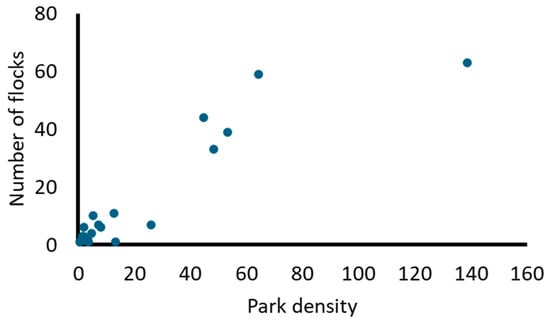
Figure 2.
Relationship between the number of flocks and the density of individuals in urban parks (individuals/park) for each species (N = 24).
The co-occurrence analysis was carried out on 23 species pairs. Only two pairs had a significant negative association (Figure S1), whereas the rest of the species had a random distribution in flocks.
Flock numbers (mean = 11.13, range = 3–30) were highly correlated with flock mixed-species numbers (Pearson correlation, r = 0.93). Therefore, only flock numbers were analyzed. Tree cover, bare cover, lawn cover, and raptor presence significantly explained flock number variation in urban parks (LRT = 34.64, p < 0.001, Table 3). Flock numbers increased with increasing cover of trees, bare ground, and lawns (Figure 3a–c). On the other hand, flock numbers decreased with raptor presence (Figure 3d).

Table 3.
Final models explaining the relationship between (a) flock numbers, (b) flock richness, (c) flock size, and (d) the proportion of mixed-species flocks with the explanatory variables in urban parks of Buenos Aires City, Argentina.
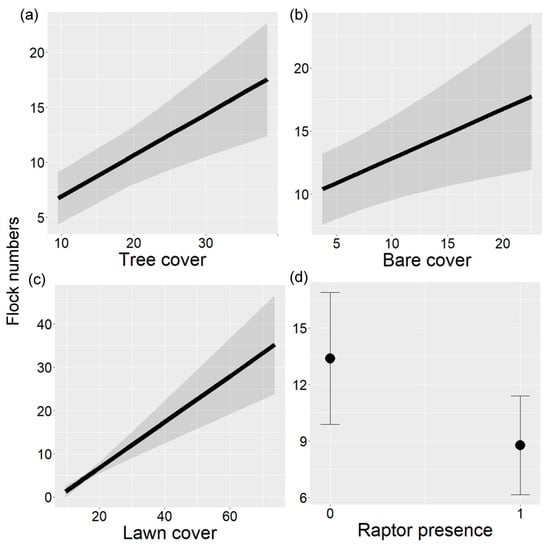
Figure 3.
Flock numbers in relation to (a) percent tree cover, (b) percent bare ground cover, (c) percent lawn cover, and (d) raptor presence in urban parks of Buenos Aires City, Argentina. In (a–c), the black line indicates the fitted line and gray bands indicate 95% confidence intervals. In (d), black points indicate the mean and vertical lines indicate 95% confidence intervals.
Flock richness (mean = 1.79, range = 1.00–2.42) varied according to noise, pedestrian traffic, and raptor presence (LRT = 0.89, p = 0.004; Table 3). Flock richness decreased with increasing noise and pedestrian traffic (Figure 4a,b), and increased with raptor presence (Figure 4c).
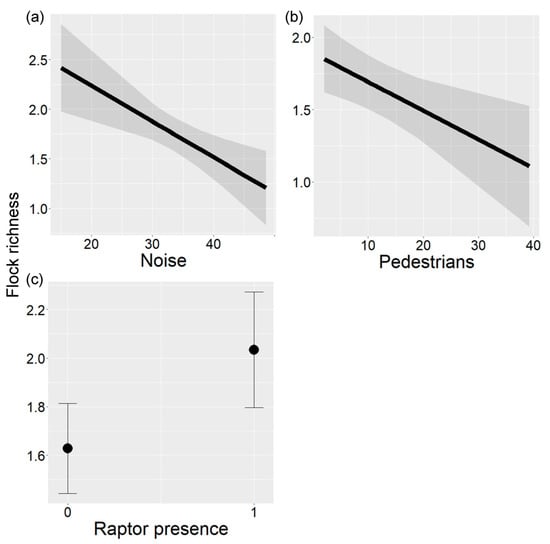
Figure 4.
Relationship between flock richness and (a) noise (db), (b) pedestrians, and (c) raptor presence in urban parks of Buenos Aires City, Argentina. In (a,b), the black line indicates the fitted line and gray bands indicate 95% confidence intervals. In (c), the black point indicates the mean and vertical lines indicate 95% confidence intervals.
Flock size (mean = 6.92, range = 3.67–10.50) and the proportion of mixed-species flocks (mean = 0.51, range = 0.00–0.75) did not vary significantly with the explanatory variables (flock size: LRT = 10.23, p = 0.162; mixed flock proportion: LRT = 1.04, p = 0.309; Table 3).
The multiple-flock dissimilarity was 0.99 (BC index), with 0.98 indicating a balanced variation in abundance between flocks. Thus, the primary pattern of species composition change among flocks was the turnover of individuals.
Flock composition varied significantly according to tree cover, noise, and raptor presence (LRT = 3.87, p < 0.001). Flocks composed of Zenaida auriculata and Columba livia were negatively related to raptor presence, whereas Turdus rufiventris was positively associated with raptor presence (Figure 5a). Myiopsitta monachus and the European Starling (Sturnus vulgaris) were positively associated with noisy parks (Figure 5a,b). Zenaida auriculata and Myiopsitta monachus were positively related to open parks, whereas Columba livia and Turdus rufiventris were positively associated with tree cover (Figure 5a).
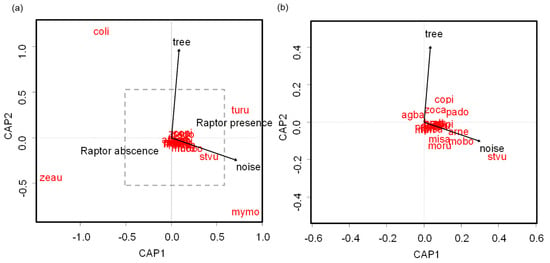
Figure 5.
Distance-based redundancy analysis showing (a) the relationship between the environmental variables and flock species composition in urban parks of Buenos Aires City, and (b) showing an expanded area delimited by the discontinuous line in (a). coli: Columba livia; zeau: Zenaida auriculata; copi: Columbina picui; mymo: Myiopsitta monachus; arne: Aratinga nenday; misa: Mimus saturninus; turu: Turdus rufiventris; zoca: Zonotrichia capensis; moru: Molothrus rufoaxillaris; mobo: Molothrus bonariensis; agba: Agelaioides badius; stvu: Sturnus vulgaris; pado: Passer domesticus.
4. Discussion
Species composition in flocks was linked to the species densities in parks, and species associations were mostly random. Therefore, flock formation supports an open membership pattern, where species gather into flocks based on their local densities. Additionally, the characteristics of flocks and their species composition were related to the local habitat features of parks.
4.1. Flock Structure
The associations between pairs of species were mainly random, with only two pairs exhibiting a negative association. Furthermore, the proportion of flocks occupied by each species was positively correlated with their densities in the parks. Additionally, our results indicated that the turnover of individuals from different species was the main pattern of compositional differences among flocks. These results agree with the idea of open membership [16,25], where species composition in flocks is determined by the species composition in the local community (see also [17,28]). The variable species composition of our flocks contrasts with other studies with more stable flock composition and the presence of nuclear species that contribute to the formation and/or cohesion of flocks [52,53]. For example, Maldonado-Coelho and Marini [14] found that understory flocks of the Atlantic Forest were stable due to the cohesion provided by Habia rubica.
Flock numbers and richness were linked to local environmental factors in urban parks. Flock numbers positively correlated with the percentage of tree cover, lawn area, and bare ground. Tree cover may protect birds, as they can seek refuge in the trees when predators or humans approach [6]. On the other hand, lawn and bare ground cover may be indicators of food availability for flocks [24]. In the study area, many ground species selected these substrates for foraging (LML, unpubl.) [33]. Flocks could help birds to find the most suitable sites for feeding [4]. Additionally, the number of flocks in parks was negatively correlated with the presence of raptors. Raptors, particularly those nesting in parks, could diminish the species densities that typically form flocks, ultimately reducing their numbers.
Flock richness was negatively related to noise and pedestrian traffic. On the one hand, noise can disrupt vocal communication among species in flocks, particularly the vocal alarm calls of vigilant individuals warning of raptor presence [9,54,55]. For instance, in the study area, a mixed-species flock consisting of Mimus saturninus, Passer domesticus, Molothrus bonariensis, and Turdus rufiventris took flight immediately after the vocal call of an individual Mimus saturninus perched in a nearby tree (see also [9]). Additionally, moving vehicles like cars or motorcycles on nearby streets can disturb flocks [56]. On the other hand, pedestrians and their dogs can disrupt the flocks. The energy lost from birds constantly chasing flies can exceed the energetic benefits of feeding in a group, making flocking less advantageous. Furthermore, noise and pedestrian traffic in parks have been linked to decreased species diversity in urban parks [57,58,59]. Consequently, reduced species diversity in parks may lead to fewer species joining flocks.
The presence of raptors in urban parks positively correlated with species richness in flocks. A greater number of species in flocks can improve predator detection strategies, as foraging species may vary in the time they allocate to vigilance and food searching [9,60].
Flock size and the proportion of mixed-species flocks did not vary with the analyzed environmental variables. These results contrast with those obtained by Fernández-Juricic [20], who found larger flock sizes in bigger and better-connected parks. However, Fernández-Juricic [20] focused on forest insectivorous birds, while our study concentrated primarily on granivorous and insectivorous ground foraging birds. Thus, ground-foraging birds may be less sensitive to green area size and isolation.
4.2. Flock Composition
Bird species composition of flocks was related to tree cover, noise, and raptor presence. Turdus rufiventris and Columba livia were more abundant in flocks located in parks with increased tree cover, whereas Myiopsitta monachus and Zenaida auriculata were in more open parks. The open structure of parks could give more visibility to the presence of raptors, pedestrians, or dogs. On the other hand, the positive relationship between Turdus rufiventris and Columba livia with tree cover might be linked to the food resources that trees offer. Turdus rufiventris may also utilize trees to evade predators or human disturbance.
Turdus rufiventris, Myiopsitta monachus, and Sturnus vulgaris were found to be more abundant in flocks in noisy parks. These species might have a high song pitch, which could enhance their vocal communication in such environments. On the other hand, Zenaida auriculata and Columba livia were more abundant in flocks found in parks without raptors. These species are likely significantly influenced by the predation risk posed by raptors, as several observations in the study area showed that Parabuteo unicinctus and Caracara plancus prey on them [61,62] (LML pers. obs.).
4.3. Limitations
Bird surveys and human disturbance were measured in spring, while flock surveys took place in summer. Thus, some temporal variation likely occurred in the explanatory variables between the seasons. For instance, pedestrian traffic and noise may decrease during summer due to less human activity associated with summer holidays. However, I do not believe these temporal dynamics would impact the spatial patterns of human disturbance or the flock structure among parks. Conversely, bird communities likely remain similar between spring and summer, as migratory bird species may stay in the study area.
5. Conclusions
The results showed that urban parks’ most common species forming ground flocks were abundant, suggesting that their local densities mainly drove flock propensity. On the other hand, flock number and richness were influenced by habitat variables and raptor presence, suggesting that resource availability and predation risk are critical factors molding flock formation. The species composition of flocks also varied according to habitat variables and raptor presence, suggesting that some species were more vulnerable to predation risk. In contrast, other species may adapt better to human disturbances, such as noise. Therefore, our results bring novel insights into the behavioral processes of urban bird communities. Flocking behavior in urban birds may improve their resource intake efficiency and avoid bird predation, thus enhancing their fitness.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/birds6020023/s1: Figure S1: Co-occurrence analysis showing the association between species in ground foraging flocks of urban parks in Buenos Aires City, Argentina. coli, Columba livia; mymo, Myiopsitta monacha; turu, Turdus rufiventris. Table S1: Co-occurrence matrix of species seen in five or more flocks. Co.li, Columba livia; Pa.pi, Patagioenas picazuro; Ze.au, Zenaida auriculata; Co.pi, Columbina picui; My.mo, Myiopsitta monachus; Fu.ru, Furnarius rufus; Mi.sa, Mimus saturninus; Tu.ru, Turdus rufiventris; Mo.bo: Molothrus bonariensis; Ag.ba, Agelaioides badius; St.vu, Sturnus vulgaris.
Funding
This research was funded by the Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación, PICT 2018-03871.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available upon request to the corresponding author.
Acknowledgments
The comments made by two anonymous reviewers improved a previous version of the manuscript.
Conflicts of Interest
The author has no relevant financial or non-financial interests to disclose.
References
- Jullien, M.; Clobert, J. The survival value of flocking in Neotropical birds: Reality or fiction? Ecology 2000, 81, 3416–3430. [Google Scholar] [CrossRef]
- Beauchamp, G. Flocking in birds increases annual adult survival in a global analysis. Oecologia 2021, 197, 387–394. [Google Scholar] [CrossRef]
- Pulliam, H.R. On the advantages of flocking. J. Theor. Biol. 1973, 38, 419–422. [Google Scholar] [CrossRef]
- Barnard, C.J.; Thompson, D.B.A. Gulls and Plovers: The Ecology and Behaviour of Mixed-Species Feeding Groups; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1985. [Google Scholar]
- Caraco, T.; Martindale, S.; Pulliam, H.R. Avian flocking in the presence of a predator. Nature 1980, 285, 400–401. [Google Scholar] [CrossRef]
- Barnard, C. Flock feeding and time budgets in the house sparrow (Passer domesticus L.). Anim. Behav. 1980, 28, 295–309. [Google Scholar] [CrossRef]
- Krebs, J.R. Colonial Nesting and Social Feeding as Strategies for Exploiting Food Resources in the Great Blue Heron (Ardea herodias). Behaviour 1974, 51, 99–134. [Google Scholar] [CrossRef]
- Rubenstein, D.I.; Barnett, R.J.; Ridgely, R.S.; Klopfer, P.H. Adaptive advantages of mixed-species feeding flocks among seed-eating finches in Costa Rica. Ibis 1977, 119, 10–21. [Google Scholar] [CrossRef]
- Ragusa-Netto, J. Vigilance towards raptors by nuclear species in bird mixed flocks in a Brazilian savannah. Stud. Neotrop. Fauna Environ. 2002, 37, 219–226. [Google Scholar] [CrossRef]
- Goodale, E.; Sridhar, H.; Sieving, K.E.; Bangal, P.; Colorado Z., G.J.; Farine, D.R.; Heymann, E.W.; Jones, H.H.; Krams, I.; Martínez, A.E.; et al. Mixed company: A framework for understanding the composition and organization of mixed-species animal groups. Biol. Rev. 2020, 95, 889–910. [Google Scholar] [CrossRef]
- Montaño-Centellas, F.A.; Muñoz, J.; Mangini, G.G.; Ausprey, I.J.; Newell, F.L.; Jones, H.H.; Fanjul, M.E.; Tinoco, B.A.; Colorado Z., G.J.; Cahill, J.R.A.; et al. Network structure of avian mixed-species flocks decays with elevation and latitude across the Andes. Philos. Trans. R. Soc. B 2023, 378, 20220099. [Google Scholar] [CrossRef]
- Munn, C.A.; Terborgh, J.W. Multi-Species Territoriality in Neotropical Foraging Flocks. Condor 1979, 81, 338–347. [Google Scholar] [CrossRef]
- Graves, G.R.; Gotelli, N.J. Assembly of avian mixed-species flocks in Amazonia. Proc. Natl. Acad. Sci. USA 1993, 90, 1388–1391. [Google Scholar] [CrossRef]
- Maldonado-Coelho, M.; Marini, M.Â. Mixed-species bird flocks from Brazilian Atlantic forest: The effects of forest fragmentation and seasonality on their size, richness and stability. Biol. Conserv. 2004, 116, 19–26. [Google Scholar] [CrossRef]
- Zarco, A.; Cueto, V.R. Winter Flock Structure in the Central Monte Desert, Argentina. Ardea 2017, 105, 89–97. [Google Scholar] [CrossRef]
- A Montaño-Centellas, F.; Jones, H.H. Temperature and vegetation complexity structure mixed-species flocks along a gradient of elevation in the tropical Andes. Auk 2021, 138, ukab027Auk. [Google Scholar] [CrossRef]
- Fanjul, M.E.; Echevarria, A.L.; Martínez, M.V. Relationship among vegetation structure and mixed-species flocks composition along the latitudinal gradient of the subtropical montane forest of the Yungas, Argentina. Philos. Trans. R. Soc. B 2023, 378, 20220107. [Google Scholar] [CrossRef] [PubMed]
- Goodale, E.; Ding, P.; Liu, X.; Martínez, A.; Si, X.; Walters, M.; Robinson, S.K. The structure of mixed-species bird flocks, and their response to anthropogenic disturbance, with special reference to East Asia. Avian Res. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Wu, J.; Goodale, E.; Li, W.; Zou, F.; Zhang, Q. Patterns in the global diversity of mixed-species bird flocks in relation to environmental factors. Biol. Divers. 2024, 1, 44–53. [Google Scholar] [CrossRef]
- Fernandez-Juricic, E. Forest fragmentation affects winter flock formation of an insectivorous guild. Ardea 2000, 88, 235–241. [Google Scholar]
- Tellería, J.L.; Virgós, E.; Carbonell, R.; Pérez-Tris, J.; Santos, T. Behavioural responses to changing landscapes: Flock structure and anti-predator strategies of tits wintering in fragmented forests. Oikos 2001, 95, 253–264. [Google Scholar] [CrossRef]
- Péron, G.; Crochet, P.-A. Edge effect and structure of mixed-species bird flocks in an Afrotropical lowland forest. J. Ornithol. 2009, 150, 585–599. [Google Scholar] [CrossRef]
- Hariharan, P.; Bangal, P.; Sridhar, H.; Shanker, K. Habitat use by mixed-species bird flocks in tropical forests of the Western Ghats, India. J. Trop. Ecol. 2022, 38, 393–400. [Google Scholar] [CrossRef]
- Fernández-Juricic, E. Nested patterns of species distribution and winter flock occurrence of insectivorous birds in a fragmented landscape. Écoscience 2002, 9, 450–485. [Google Scholar] [CrossRef]
- Jones, H.H.; Robinson, S.K. Patch size and vegetation structure drive changes to mixed-species flock diversity and composition across a gradient of fragment sizes in the Western Andes of Colombia. Condor 2020, 122, duaa006. [Google Scholar] [CrossRef]
- Kajiki, L.N.; Montaño-Centellas, F.; Mangini, G.; Colorado Z, G.J.; Fanjul, M.E. Ecology of mixed-species flocks of birds across gradients in the Neotropics. Rev. Bras. Ornitol. 2018, 26, 82–89. [Google Scholar] [CrossRef]
- Ferrari, A.; Motta-Junior, J.C. Mixed flocks in Gubernetes yetapa (Passeriformes: Tyrannidae) and Pseudoleistes guirahuro (Passeriformes: Icteridae) in grasslands of a Cerrado preserve, southeast Brazil. Atual. Ornitol. 2018, 201, 4–5. [Google Scholar]
- Zarco, A.; Cueto, V.; Sagario, M.; Marone, L. Effects of livestock grazing on flocks of seed-eating birds in the central Monte desert, Argentina. Can. J. Zool. 2019, 97, 606–611. [Google Scholar] [CrossRef]
- Kark, S.; Iwaniuk, A.; Schalimtzek, A.; Banker, E. Living in the city: Can anyone become an ‘urban exploiter’? J. Biogeogr. 2006, 34, 638–651. [Google Scholar] [CrossRef]
- Croci, S.; Butet, A.; Clergeau, P. Does urbanization filter birds on the basis of their biological traits? Condor 2008, 110, 223–240. [Google Scholar] [CrossRef]
- Leveau, L.M. Bird traits in urban–rural gradients: How many functional groups are there? J. Ornithol. 2013, 154, 655–662. [Google Scholar] [CrossRef]
- Sadam, A.; Khan, R.U.; Mahmood, S. Identifying Bird Traits that Enable them to Become Urban Exploiters in an Urban Area of Mardan, Pakistan. Pak. J. Zool. 2021, 53, 1813–1822. [Google Scholar] [CrossRef]
- Leveau, L.M. Microhabitat Selection by Ground-Foraging Birds in Urban Parks. Animals 2025, 15, 1155. [Google Scholar] [CrossRef] [PubMed]
- Beninde, J.; Veith, M.; Hochkirch, A. Biodiversity in cities needs space: A meta-analysis of factors determining intra-urban biodiversity variation. Ecol. Lett. 2015, 18, 581–592. [Google Scholar] [CrossRef]
- Köppen, W. Versuch einer Klassifikation der Klimate, vorzugsweise nach ihren Beziehungen zur Pflanzenwelt. Geogr. Zeitschrift 1900, 6, 593–611. [Google Scholar]
- Cabrera, A.L.; Willink, A. Biogeografía de América Latina; Programa Regional de Desarrollo Científico y Tecnológico: Washington, DC, USA, 1973; Volume 13, pp. 1–117. [Google Scholar]
- Oyarzabal, M.; Clavijo, J.; Oakley, L.; Biganzoli, F.; Tognetti, P.; Barberis, I.; Maturo, H.M.; Aragón, R.; Campanello, P.I.; Prado, D.; et al. Unidades de vegetación de la Argentina. Ecol. Austral 2018, 28, 40–63. [Google Scholar] [CrossRef]
- de la Peña, M.R. Nidos y reproducción de las aves argentinas; Serie Naturaleza, Conservación y Sociedad N° 8; Ediciones Biológica: Santa Fe, Argentina, 2013; p. 590. [Google Scholar]
- Ippi, S.; Trejo, A. Dinámica y estructura de bandadas mixtas de aves en un bosque de lenga (Nothofagus pumilio) del Noroeste de la Patagonia argentina. Ornitol. Neotrop. 2003, 14, 353–362. [Google Scholar]
- Fernández-Juricic, E.; Tellería, J. Effects of human disturbance on spatial and temporal feeding patterns of Blackbird Turdus merulain urban parks in Madrid, Spain. Bird Study 2000, 47, 13–21. [Google Scholar] [CrossRef]
- ToolsDev. Sound Meter-Decibel Meter and Noise Meter. 2016. Available online: https://play.google.com/store/apps/details?id=app.tools.soundmeter.decibel.noisedetector&hl=en_US (accessed on 10 December 2021).
- R Development Core Team. R: A Language and Environment for Statistical Computing, version 4.4.2; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Griffith, D.M.; Veech, J.A.; Marsh, C.J. Cooccur: Probabilistic Species Co-Occurrence Analysis in R. J. Stat. Softw. 2016, 69, 1–17. [Google Scholar] [CrossRef]
- Hartig, H.; Lohse, L.; de Souza, M. Package ‘DHARMa’, Residual Diagnostics for Hierarchical (Mul-ti-Level/Mixed) Regression Models. Version 0.4.7. 2025. Available online: https://cran.r-project.org/web/packages/DHARMa/DHARMa.pdf (accessed on 10 December 2021).
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. performance: An R package for assessment, comparison and testing of statistical models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Lüdecke, D. sjPlot: Data Visualization for Statistics in Social Science, R package version 2.8.17; The Comprehensive R Archive Network; 2018. Available online: https://cran.rstudio.com/ (accessed on 10 December 2021).
- Baselga, A. Partitioning abundance-based multiple-site dissimilarity into components: Balanced variation in abundance and abundance gradients. Methods Ecol. Evol. 2016, 8, 799–808. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, D.; Villeger, S.; De Bortoli, J.; Leprieur, F.; Logez, M.; Henriques-Silva, R. Pack-Age ‘Betapart’. 2023. Available online: https://cran.r-project.org/web/packages/betapart/betapart.pdf (accessed on 10 December 2021).
- Legendre, P.; Anderson, M.J. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-2. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 December 2021).
- Wilman, H.; Belmaker, J.; Simpson, J.; de la Rosa, C.; Rivadeneira, M.M.; Jetz, W. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals. Ecology 2014, 95, 2027. [Google Scholar] [CrossRef]
- Moynihan, M. The organization and probable evolution of some mixed species flocks of neotropical birds. Smithson. Misc. Collect. 1962, 143, 1–140. [Google Scholar]
- Mangini, G.G.; Rutt, C.L.; Sridhar, H.; Buitron, G.; Muñoz, J.; Robinson, S.K.; Montaño-Centellas, F.; Zarco, A.; Fanjul, M.E.; Fernández-Arellano, G.; et al. A classification scheme for mixed-species bird flocks. Philos. Trans. R. Soc. B 2023, 378. [Google Scholar] [CrossRef]
- Morse, D.H. Ecological Aspects of Some Mixed-Species Foraging Flocks of Birds. Ecol. Monogr. 1970, 40, 119–168. [Google Scholar] [CrossRef]
- Goodale, E.; Kotagama, S.W. Alarm calling in Sri Lankan mixed-species bird flocks. Auk 2005, 122, 108–120. [Google Scholar] [CrossRef]
- Yosef, R. Physical distances among individuals in flocks of greater flamingoes (Phoenicopterus ruber) are affected by human disturbance. Isr. J. Ecol. Evol. 1997, 43, 79–85. [Google Scholar]
- González-Oreja, J.A.; De La Fuente-Díaz-Ordaz, A.A.; Hernández-Santín, L.; Bonache-Regidor, C.; Buzo-Franco, D. Can human disturbance promote nestedness? Songbirds and noise in urban parks as a case study. Landsc. Urban Plan. 2012, 104, 9–18. [Google Scholar] [CrossRef]
- Perillo, A.; Mazzoni, L.G.; Passos, L.F.; Goulart, V.D.L.R.; Duca, C.; Young, R.J. Anthropogenic noise reduces bird species richness and diversity in urban parks. Ibis 2017, 159, 638–646. [Google Scholar] [CrossRef]
- Leveau, L.M.; Kopp, J. Bird color and taxonomic diversity are negatively related to human disturbance in urban parks. Web Ecol. 2024, 24, 1–10. [Google Scholar] [CrossRef]
- Thiollay, J.M. Frequency of mixed species flocking in tropical forest birds and correlates of predation risk: An intertropical comparison. J. Avian Biol. 1999, 30, 282–294. [Google Scholar] [CrossRef]
- De Lucca, E.R. Reproducción del Gavilán Mixto (Parabuteo unicinctus unicinctus) en la Ciudad de Buenos Aires y conurbano bonaerense, Argentina. Hist. Nat. 2023, 13, 75–93. [Google Scholar]
- De Lucca, E.R. Observations of a successful breeding group of Bay-Winged Hawk (Parabuteo unicinctus unicinctus) in the nerve center of Buenos Aires City, Argentina. Hist. Nat. 2023, 13, 225–230. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).