Simple Summary
Monitoring bird populations can provide crucial information about the health of ecosystems and alerts regarding emerging environmental problems. Bird species responses to habitat change differ depending on species-specific ecological traits and differences in modifications. The aim of this study was to evaluate the impacts of habitat conversion on bird species diversity and richness in the peri-urban landscape of the Mexico City Metropolitan Area in central Mexico. A total of 290 bird species belonging to 19 orders and 55 families were identified. The effect of distance to the nearest greenspace differed significantly among the three seasons. The results of this study show the importance of habitat heterogeneity and how bird richness and abundance vary in a highly urbanized environment. It found the highest diversity in waterbodies with heterogeneous environments, and significant decreases in avian diversity were recorded in the semi-urban and urban habitats. Conserving sites in peri-urban landscapes may be a viable conservation strategy for bird communities. To do this, emphasis must be placed on identifying and reducing anthropogenic stressors to protect and conserve bird diversity in the study area.
Abstract
Habitat loss, pollution, and climate change have a global impact on bird diversity, particularly in central Mexico, where human disturbances and unplanned urbanization can lead to the decline of this faunal group. In this study, the effects of season (rainy, warm–dry, or cool–dry) and environmental variables (size, perimeter, vegetation cover, built cover, distance to nearby greenspaces and distance to the closet natural vegetation patch) on the avian diversity at different sites located in a peri-urban landscape in the Metropolitan Area of Mexico City were determined. The study was conducted using the linear transect method to assess the diversity and composition of bird communities from November 2019 to March 2022, recording 290 total bird species. Zumpango Lagoon was the study site with the highest diversity (N = 209, H′ = 3.22) and evenness index (J′ = 0.76). Linear mixed models were used to determine the effects of season and environmental variables of the study sites on the avian diversity. The effect of distance to the nearest greenspace was significantly more positive during the rainy season than the two dry seasons. An ANOSIM test also showed that the avian community associated with water bodies differed significantly from the other communities (R = 0.16, p < 0.001). Despite some anthropogenic activities and human intrusion, sites with water bodies retain a high diversity of birds. This finding indicates the need for immediate conservation efforts to protect many resident breeding species and wintering migratory birds in the study area.
1. Introduction
Urbanization has numerous negative impacts on biodiversity, primarily through habitat loss and fragmentation, pollution, introduction of invasive species, alteration of natural cycles, resource availability, and distribution of different species [1,2]. These changes can lead to declining populations of native species, loss of genetic diversity, and alteration of ecological communities, underscoring the importance of incorporating conservation practices into urban development [1,2]. The accelerated urbanization process is one of the main threats to biodiversity and a major conservation challenge [3,4].
Urbanization has transformed landscapes worldwide, creating environments that significantly impact on the wildlife that inhabits them [1,2]. Among the species affected, birds have had to adapt to drastic changes in their ecosystems due to increasing urban expansion [5,6]. Birds are valuable bioindicators due to their diversity, sensitivity to environmental changes, ease of monitoring, and their role in various parts of the food chain [7,8]. Monitoring bird populations can provide crucial information about the health of ecosystems and an alert of emerging environmental problems [9,10]. Avian diversity and abundance vary, and both are influenced by the climate, and another environmental factors such as vegetation cover, and habitat heterogeneity [11,12] and thus serve as prominent indicators of ecosystem health. Environmental changes can have profound and varied impacts on birds, including altered behavior and migratory patterns to decrease long-term health and survival, often resulting in shifts in species composition and diversity in avian communities [13,14]. Therefore, understanding how birds respond to habitat loss can provide insights into the maintenance of ecosystem resilience [15]. While some bird species thrive in cities, many faces serious challenges that threaten their survival. The main consequences for birds that inhabit urban areas include habitat loss, exposure to pollution, changes in food availability, increased predation, and behavioral adaptations [16,17]. One of the most significant consequences of urbanization for birds is habitat loss and fragmentation. As cities expand, natural habitats such as forests, wetlands, and grasslands are replaced by buildings, roads, and infrastructure. This reduces the availability of suitable nesting sites and feeding areas, forcing birds to adapt to artificial structures like rooftops and bridges [5,17,18]. Additionally, habitat fragmentation isolates bird populations, making it harder for them to find mates and reducing genetic diversity, which can weaken species over time [16,17,18]. In this sense, bird species responses to habitat change differ depending on species-specific ecological traits and differences in modifications [19,20]. However, bird diversity can be affected by modifying the structure and function of urban space due to the continuous growth of the urban landscape [21,22]. Greenspaces and wetlands within the urban area or in the peri-urban area in a city are essential to maintain and promote urban biodiversity. They provide shelter and vital resources for wildlife, connect fragmented ecosystems, regulate climate and air quality, and provide numerous ecological and social benefits [23,24,25]. The conservation and creation of these spaces are crucial for the ecological balance and well-being of urban communities [26,27]. Within the urban ecosystem, wetlands serve as a critical link between natural resources management and agricultural practices [28]. Recent studies have found that the presence of wetlands in peri-urban areas helps mitigate urbanization effects on bird diversity by providing enhanced habitat and increased resource availability [29,30]. For this reason, identifying bird diversity between different habitats helps assess community structure and niche relationships, which are important for bird conservation. This is because different bird species have specific habitat preferences and ecological needs [29,30].
Urban habitats are refuges for many bird populations in cities and their surroundings; however, the urbanization process, like landscape changes, is a great threat to the bird population [31,32]. The aim of this study was to evaluate the effects of season (rainy, warm–dry, or cool–dry) and environmental variables (size, perimeter, vegetation cover, built cover, distance to nearby greenspaces and distance to the closet natural vegetation patch) on bird diversity in the peri-urban landscape of the Metropolitan Area of Mexico City. The study also examined, quantified and compared the avian diversity in a mosaic of different land cover types (e.g., urban, semi-urban, and rural areas). We hypothesized that the presence of more microhabitats (e.g., native vegetation remnants, wetlands and other habitats) and longer distance from the urban center would promote a higher species diversity and richness of bird assemblages than other habitats.
2. Materials and Methods
2.1. Study Area
The Mexico City Metropolitan Area (MCMA) is one of the largest megalopolises in the world [33]. This region contains 412 bird species [34] out of a total of 1151 species found in Mexico [35]. According to recent studies [23,25,36], the number of bird species in the MCMA has declined due to different factors, including global climate change, habitat loss, rapid urbanization, industrialization, and deforestation, which pose serious threats to avifauna globally, including within the MCMA [23,25,37]. There are many remaining greenspaces with native or non-native vegetation in the MCMA which are small and isolated remnants that vary widely in their habitat quality [19,20,24,25], depending largely on the degree of urbanization [38]. For most species, the destruction of habitats and the deterioration of habitat quality result in decreased population size, population fluctuations, reduced population viability, and low individual fitness [38,39,40].
The MCMA is located in the south-central region of Mexico (19°25′ N, 99°07′ W) (Figure 1), it is located in the Trans-Mexican Volcanic Belt physiographic province. Currently, it is one of the largest megalopolises in the world [33], covering more than 7954 km2. The rapid growth of the human population in the MCMA, which currently numbers over 22 million (>2559.8 hab/km2) [41], has led to the conversion of original habitats. The MCMA has a semi-humid temperate climate with a warm–dry season from March to May, cold–dry season from October to February and a rainy season from June to September [42] with a relatively narrow annual temperature range between 5 and 25 °C and precipitation between 600 and 1500 mm/year. The topography includes an extensive high plateau in the northern half of the city, where the urban area sits, surrounded by mountains and volcanoes that reach >3900 m of elevation, especially on the southern and western sides [43]. The urban area covers almost the whole northern half of Mexico City and is rapidly expanding, including through illegal, unplanned urban development. The southern half is occupied by mountains and ravines covered by extensive forests and grasslands, some of them highly fragmented by dwellings and agricultural areas. The intense loss of the original habitats in the area currently occupied by the MCMA has created a highly heterogeneous system with different land cover types, where only 34% of these remain [43].
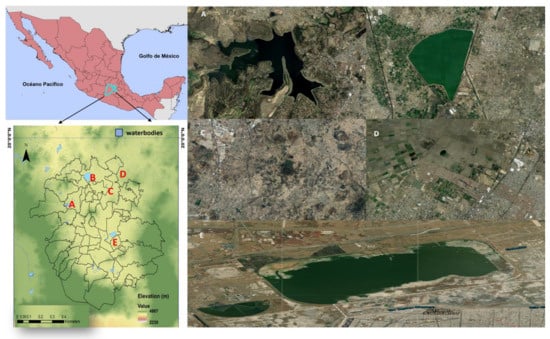
Figure 1.
Geographical location map showing the study sites in the Mexico City Metropolitan Area, Mexico: (A) Guadalupe Lake, (B) Zumpango Lagoon, (C) Cerro de Paula, (D) Sierra Hermosa, and (E) Nabor Carrillo Lake in the MCMA.
This study was conducted in contrasting habitats within the Valley of Mexico, with the specific purpose of generating quantitative information on bird diversity. The present study was conducted from November 2019 to March 2022 covering different seasons of the year (rainy, warm–dry and cold–dry seasons) in five study sites (Table 1, Figure 1) based on the anthropogenic landscape and habitat structures: (a) Nabor Carrillo Lake is a semi-urban and rural habitat with wetland surrounded by aquatic or hydrophilic vegetation, native and non-native trees (Eucalyptus sp., Schinus molle, Casuarina equisetifolia), halophytic grasslands and xeric scrub; (b) Zumpango Lagoon includes urban, semi-urban and rural habitats, with a wetland that is surrounded by urban areas, roads, industrial areas, scattered native and non-native woodland (Eucalyptus sp., Schinus molle, Casuarina equisetifolia, Salix sp.), xeric scrub (Prosopis juliflora, Opuntia sp., Mimosa biuncifera), induced grasslands for grazing and agricultural areas; (c) Guadalupe Lake is an urban and semi-urban habitat with wetland surrounded by urban areas (residential areas, residential houses, roads), remnants of native and non-native wooded vegetation (Eucalyptus sp., Schinus molle, Casuarina equisetifolia, Salix sp., Quercus sp., Juniperus sp., Pinus sp., Taxodium sp., Fraxinus sp.), agricultural areas and a golf course; (d) Sierra Hermosa Ecological, Tourist and Recreational State Park is an urban, semi-urban and rural habitat made up of two permanent and two semi-permanent water bodies surrounded by spars halophytic and reedy vegetation (tular), recreational areas, abandoned buildings, induced grasslands and induced wooded areas, and the vegetation is composed of a scarce presence of trees (Schinus molle, Eucalyptus sp., Cinnamomum canphora, Casuarina equisetifolia, Ficus sp., Castilla elastica, Jacaranda mimosifolia); the valley area is represented by induced grasslands in addition to being surrounded by urban areas (residential houses, roads, schools, international airport) and agricultural areas cultivating corn, barley, wheat and beans; and (e) Cerro de Paula (and surrounding areas) is a semi-urban and rural habitat with natural grasslands, scattered trees and live fences (Schinus molle, Eucalyptus sp.), with remnants of natural scrub and agricultural areas of corn, barley, Agave sp. and Opuntia sp.

Table 1.
Summary data of the study sites in Mexico City Metropolitan Area, Mexico.
For study sites, we analyzed various spatial features that could influence bird species richness within them: size, perimeter, urbanization degree (constructed and paved surfaces within the study site), built cover in the surroundings, vegetation cover (natural and non-native vegetation coverage within the study site), distance to nearest greenspace (i.e., distance from sampled study site to the nearest greenspace), and distance to the closet natural vegetation patch (i.e., distance from the sampled study site to the nearest protected area or to an area with natural vegetation greater than 1 km2) (see Supplementary Materials Table S1). These spatial features were estimated using digital maps from Google Earth Pro (© 2021), Google Earth Engine [44], and complemented with field work information. In each of these study sites, microhabitats were characterized by direct observation and divided into the following categories: agricultural land (Al) consisting of active or semi-abandoned corn, bean, barley, wheat, Agave sp. and Opuntia sp. crops; wetlands (W) defined by natural or artificial waterbodies with halophyte and tular vegetation; urban area (Ur), including residential houses surrounded by large and small trees, roads (streets and avenues), factories, and other urban infrastructure; grassland (Gl), characterized by natural or induced, short, small grassy and bushy vegetation with a maximum height ≤ 2 m; semi-desert xeric scrubs (Sm), consisting of native vegetation with trees ≤ 2 m (Prosopis juliflora, Opuntia sp., Mimosa biuncifera); and native and non-native vegetation remnants (Nr) defined by small patches of native (e.g., Pinus sp., Quercus sp., Juniperus sp.) or non-native trees (e.g., Schinus molle, Salyx babylonica, Eucalyptus sp., Casuarina equisetifolia).
2.2. Data Collection
Bird data were collected using the transect line method following Emlen [45,46], and surveys were conducted from 06:00 to 13:00 h. One day per month was spent at each site, and three linear transects were surveyed measuring ~4 km in length. Upon spotting any bird species, the number of individuals (abundance) of each bird species was counted and the microhabitat they were using during each survey was noted. Birds were observed using binoculars (Bushnell 10 × 45 m), and photographs were taken using digital cameras (Nikon D3500) for further identification. Taxonomic identification of observed birds was followed by Peterson and Chalif [47], Kaufmann [48], Dunn and Alderfer [49], and Sibley [50]. The bird taxonomy and nomenclature followed Chesser et al. [51].
The residency status of the enlisted birds was determined based on Howell and Webb [52] and personal observations. The residential status of the birds was categorized into resident breeders (the species can be found throughout the year in the area) and migratory (the species can be found throughout certain times of year in the area, including winter visitors, transient migrants, summer residents, non-breeding visitors, and vagrants). The vulnerability status of birds was classified based on Mexican national legislation NOM-059-Semarnat-2010 [53] and at the global level with the IUCN Red List of Species [54]. Bird species were grouped based on the major feeding guilds and complemented with González-Salazar et al. [55] into the following groups: carnivores, scavengers, insectivores, nectarivores, granivores, frugivores, and omnivores.
2.3. Statistical Analysis
Bird diversity was assessed in terms of species richness (overall, resident, and migrant species richness) and abundance, considering the total number of species and the number of individuals per species at each site. The species accumulation curve was plotted to evaluate whether the number of bird species sampled was representative in the study area. The total number of species in the study area was estimated using Jackknife first-order and Chao second-order estimators [56], since they are two of the estimators with the least bias. This was done using the ‘specpool’ function of the Vegan Package [57] in RStudio 2023.09.0 [58]. Species richness represents a measure of the variety of species, and this was calculated based on the number of different species present in a study site. Bird species diversity was calculated using the Shannon diversity index (H’) and evenness index (J’) at each site. Relative abundance (the abundance of a given bird species divided by the total number of individuals present at the study site) was also presented as a stacked bar diagram divided by habitat type for each study site.
To determine the effects of season (rainy, warm–dry, or cool–dry) and environmental variables of the study sites on the avian diversity variables (species richness, abundance, diversity index, and evenness index), a set of linear mixed models was constructed with each aspect of diversity as the response variable and the site as the random intercept. The perimeter, vegetation cover, and building cover were divided by the size (area) of each study site to make them independent of area size. Since there was multicollinearity among several of the environmental variables, these could not include them as explanatory variables in a single model; rather, there was a single environmental variable included in each model, then Akaike’s Information Criterion, adjusted for small sample size, was used (AICc) to determine which variable (if any) best explained each aspect of diversity. The explanatory variables were centered and scaled to a standard deviation of 0.5 to improve model convergence and interpretation using the arm package for R [59]. To minimize the total number of models generated, we first determined whether season had an important effect (95% confidence interval excluding zero), and if so, included it in the competing models. We also included a set of models with the interaction between season and the environmental variable (regardless of whether the main effect of season was important) to account for the possibility that the effect of the environmental variables differed among seasons. All model sets included the null model (random effect only). This resulted in a set of 16 competing models for each response variable (see Supplementary Materials Table S2). It was interpreted that the most supported model was the one with the lowest AICc score, or the simplest model was the one with equivalent support (within 2 AICc points) to avoid including uninformative parameters [60,61]. All models met the assumptions of normality and homoscedasticity of the residuals, except for those evaluating Abundance, which met the assumptions after log-transforming the response variable. Analyses were performed in R version 4.4.3 [62] using the RStudio interface [58]. Models were constructed using the lme4 [63] and lmerTest [64] packages; model assumptions were verified graphically for all models using the performance package [65]; pairwise comparisons among seasons were performed using the emmeans package [66]; and figures were produced using the ggeffects [67] and ggplot2 [68] packages.
The Bray–Curtis index was used to assess the similarity of the bird community among study sites. We also performed a cluster analysis with the Bray–Curtis index to observe the similarities among the different habitats [69], and this analysis was represented with a heatmap. Analysis of Similarities (ANOSIM) and a Similarity Percentages test (SIMPER) were performed to test significant differences between groups, and to identify which taxa are responsible for those differences amongst study sites, respectively. The ‘adonis’ and ‘simper’ functions were used from the vegan package of RStudio [58] for the ANOSIM analysis [57]. The non-parametric multidimensional scale (nMDS) test was performed to determine whether bird communities differed significantly among habitats using the permutation test. All statistical analyses were carried out using relevant statistical packages in PAST [70], Biodiversity Pro [71], JMP, v. 17 (available at https://www.jmp.com/), and JASP v.0.19 (available at https://jasp-stats.org/, accessed 14 September 2024). The ggplot2 package was used for plotting [72] in RStudio [58].
3. Results
During the study, 290 bird species belonging to 19 orders and 55 families were identified (Table A1 in Appendix A). The species accumulation curves illustrate the completeness of the survey conducted and the sufficiency of sampling efforts in study sites (Figure 2). The richness estimators predicted 300 to 305 species, which is very close to our observed species richness (N = 290).
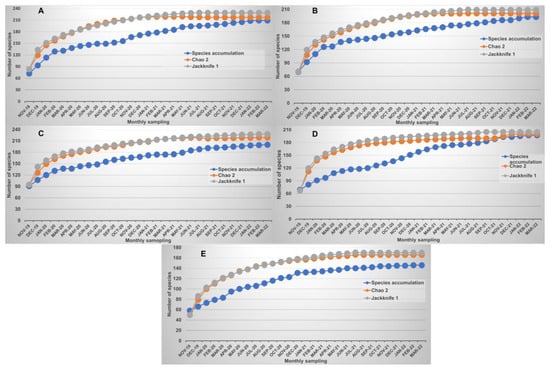
Figure 2.
Bird species accumulation curves of the study sites in the Mexico City Metropolitan Area, Mexico: Zumpango Lagoon (A), Nabor Carrillo Lake (B), Sierra Hermosa Ecological, Tourist and Recreational State Park (C), Guadalupe Lake (D), and Cerro de Paula (E).
In total, 809,337 individuals were recorded during the study period (Table 2). Species richness and abundance were both highest in Zumpango Lagoon (209 species, 11,408.1 ind/month) followed by Sierra Hermosa (199 species, 2669 ind/month), and the lowest in Cerro de Paula (146 species, 806.4 ind/month) (Figure 3). The Shannon diversity index (Hʹ) value was highest for Zumpango Lagoon (H′ = 3.22) and lowest for Cerro de Paula (H′ = 2.99) (Figure 3). Contrary to what was expected, the evenness of the species distribution was highest in Cerro de Paula (J′ = 0.82) and lowest in Zumpango Lagoon (J′ = 0.76), Guadalupe Lake (J′ = 0.76), and Nabor Carrillo Lake (J′ = 0.75) (Figure 3). The species’ richness differed significantly among sites (F4,139 = 35.18, p <0.001), as did abundance (F4,139 = 19.46, p < 0.001); as well as the Shannon diversity index (F4,139 = 3.01, p = 0.02) and evenness (F4,139 = 4.45, p =0.002) among sites, respectively. The residency status of the study sites revealed that 125 species (43.1% of the total birds) were residents and 140 were migrants (48.2%), and the remaining 25 were introduced species (8.6%); 209 species were terrestrial and 82 were aquatic. These proportions were similar across all the study sites. In terms of habitat, the native and non-native remnants had the highest species richness (n = 134 species), followed by wetlands (n = 110); both species richness and abundance varied among habitats. Regarding the feeding guild of bird species, it was found that insectivores dominated all study sites (n = 166 species) and all habitats. Granivores were the second most represented (n = 48), followed by carnivores (n = 43), except in the wetlands, where there were more omnivores (n = 28) than carnivores (n = 25). In all habitat types, frugivores, scavengers, and nectarivorous bird species were documented in comparatively lesser numbers.

Table 2.
Bird diversity values of the study sites in the Mexico City Metropolitan Area, Mexico.
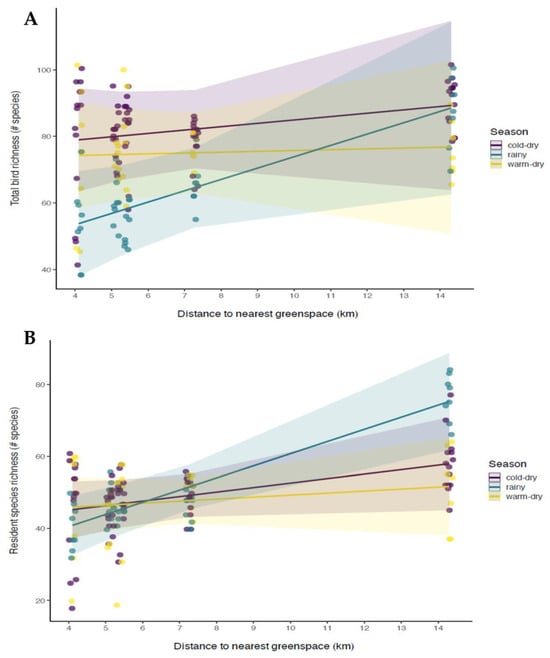
Figure 3.
Effect of the interaction of season and distance to the nearest greenspace on overall bird species richness (A) and resident species richness (B) in the study sites in Mexico City Metropolitan Area, Mexico. Points show partial residuals from monthly point counts in each of five greenspaces grouped by season. Points are slightly randomly displaced horizontally to improve clarity.
Model selection tables are provided in Supplementary Materials Table S2, as well as full results of the most supported model. For the models examining total richness, the most supported model contained the interaction of season and distance to the nearest greenspace, which outperformed the second-best model by >11 AICc points. Although the interaction showed that the effect of distance on richness was significantly more positive during the rainy season than during the two dry seasons (Figure 3A, Table A2 in Appendix A), none of these effects differed significantly from zero (cold–dry: β = 1.011, 95% CI= −3.70–5.72; warm–dry β = 0.248, 95% CI = −4.47–4.96; rainy: β = 3.404, 95% CI = −1.31–8.12). This trend was strengthened when considering only resident species richness; the most supported model (by >21 AICc points) contained the interaction of season and distance to the nearest greenspace, with a significantly stronger positive effect of distance during the rainy season than either of the two dry seasons (Table A2 in Appendix A). Here, there was a significant positive effect of distance during the rainy season (β = 3.372, 95% CI = 1.01–5.73), but no significant effect during the two dry seasons (cold–dry: β = 1.248, 95% CI = −1.10–3.60; warm–dry: β = 0.559, 95% CI = −1.81–2.92; Figure 3B). Considering only migrant species richness, there were four models within 2 AICc points; the simplest, and therefore best supported, was the model that included only the season. Migrant species richness was highest during the cold–dry season (estimated marginal mean [emm] = 33.0, 95% CI = 23.78–42.2), intermediate during the warm–dry season (emm = 27.3, 95% CI = 18.12–36.6), and lowest during the rainy season (emm = 13.0, 95% CI = 3.81–22.2; all pairwise comparisons were significant at p < 0.001; Figure 4A). For the Shannon diversity index, the most supported model contained the season with a similar trend; the two dry seasons had higher Shannon diversity index values than the rainy season (p < 0.0001 for rainy versus cold–dry and warm–dry; cold–dry versus warm–dry, p = 0.38; cold–dry: emm = 3.19, 95% CI = 3.07–3.31; warm–dry: emm = 3.25, 95% CI = 3.11–3.39; rainy: emm = 2.86, 95% CI = 2.73–3.00; Figure 4B). Finally, for both abundance and evenness, the most supported model was the null model (Table A2 in Appendix A).
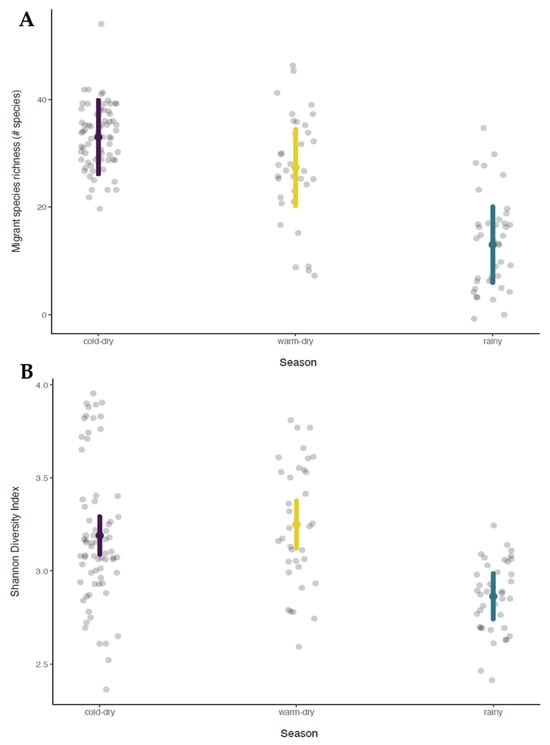
Figure 4.
Effect of season on migratory species richness (A) and Shannon diversity index (B) in the study sites in Mexico City Metropolitan Area, Mexico. Gray points show partial residuals from monthly point counts in each of five greenspaces grouped by season. Points are slightly randomly displaced horizontally to improve clarity.
According to the heatmap, the most similar sites in species composition were Zumpango Lagoon–Sierra Hermosa (0.82) and Zumpango Lagoon–Nabor Carrillo Lake (0.82), while the least similar sites to these were Nabor Carrillo Lake–Cerro de Paula (0.61) and Guadalupe Lake–Cerro de Paula (0.63) (Figure 5). The beta diversity pattern was assessed by the ANOSIM test, which indicated a significant difference in the avian communities (R = 0.1603; p = 0.0001) (Figure 6). Pairwise comparison showed significant differences between Zumpango Lagoon, Nabor Carrillo Lake, Sierra Hermosa, and Guadalupe Lake with Cerro de Paula (p = 0.0002), but no difference between Zumpango Lagoon, Nabor Carrillo Lake and Sierra Hermosa (p = 0.222). The nMDS results showed that bird communities were significantly different among study sites (stress = 1.1886, <0.3), indicating a good fit of the model (Figure 6).
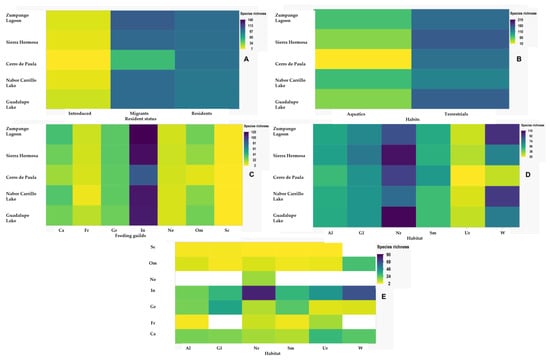
Figure 5.
Heatmaps of bird species richness among study sites in the Mexico City Metropolitan Area, Mexico: residency status (A), preference habits (B), feeding guilds (C), habitats (D), and feeding guilds by habitat (E).
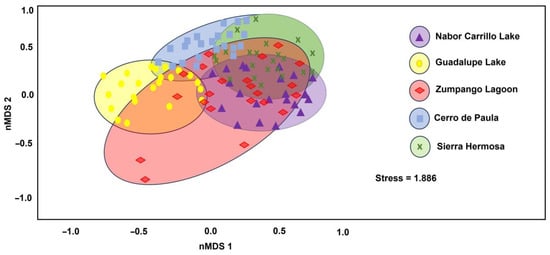
Figure 6.
Non-metric multidimensional (nMDS) plot shows separation of bird communities among study sites (transform = square-root; resemblance = Bray–Curtis similarity).
With respect to species composition among habitats, grasslands–agricultural lands (0.66) and grasslands–semi-desert xeric scrubs (0.58) clearly form a cluster, while the least similar sites were wetlands–native and non-native vegetation (0.06), wetlands–semi-desert xeric scrubs (0.09) and wetlands–urban areas (0.08), which formed different clusters (Figure 7).
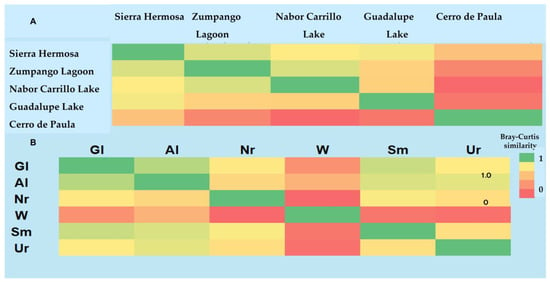
Figure 7.
Heatmap shows species similarities based on Bray–Curtis index for bird communities among study sites (A) and feeding guild by habitat (B): agricultural lands (Al), wetlands (W), urban areas (Ur), remnants of native and non-native vegetation (Nr), grasslands (Gl) and semi-desert scrubs (Sm).
Only one of the bird species was in a threat category of the IUCN Red List: Snowy Plover (Anarhynchus nivosus) is near threatened. The rest of all bird species were in the least concern category. However, 11 species were listed in the NOM-059 with regional level protected status: eight in special protection: Cooper’s Hawk (Accipiter cooperi), Sharp-shinned Hawk (A. striatus), Short-eared Owl (Asio flammeus), Broad-winged Hawk (Buteo platypterus), Swainson’s Hawk (B. swainsoni), Montezuma Quail (Cyrtonyx montezumae), Painted Bunting (Passerina ciris) and Least Grebe (Tachybaptus dominicus); and three as threatened: Mexican Duck (Anas diazi), American Bittern (Botaurus lentiginosus) and Snowy Plover (Anarhynchus nivosus).
4. Discussion
The results of this study showed no clear relationship between bird diversity and environmental variables of the study sites analyzed. The results found agree that sites located in the peri-urban landscape are more diverse than sites within the urban area [23,72,73]. Similarly, a study by Charre et al. [24] examined comparable variables and additional indices related to vegetation cover in the same region. Their findings indicated that bird species richness increased with greenspace size only during the breeding season, whereas during the migratory season, the richness of migratory birds was instead linked to the distance from natural vegetation. Similar, these results do not align with previous research, which typically associate bird species richness with greenspace size and vegetation cover in urban areas [74,75,76]. It is unsurprising that previous studies have consistently found greater biodiversity in larger greenspaces, as they typically provide a broader range of food resources, micro-habitats, and favorable conditions for reproduction, dispersion, and survival [77,78,79].
Of the environmental variables used in this study, only distance to the nearest greenspace differed significantly between the three seasons. The effect of distance to the nearest greenspace was significantly more positive during the rainy season than the two dry seasons and was significantly greater than zero only during the rainy season. However, previous studies reported weak relationships with spatial variables, suggesting that greenspace characteristics may not be the primary factors influencing species composition, as observed in other studies [80,81]. Instead, tree density may play a more influential role [21], and most studies have observed a significant relationship between size and bird diversity [74,82,83]. This discrepancy may be partly explained by greater habitat heterogeneity and the availability of water resources in larger landscapes [81,83,84]. An unexpected finding concerns dispersal—specifically, the distance to the nearest natural vegetation patch (or protected area)—which showed no significant effects. This suggests that the distance of greenspaces from natural vegetation does not limit bird dispersal and colonization [23,79]. Therefore, nearby greenspaces or vegetation patches offer shelter and food and may also serve as habitat corridors [76,85]. Other studies have indicated that bird species richness within a site study decline when surrounded by densely urbanized areas, leading to more uniform communities [85,86]. This highlights the ecological importance of peri-urban areas as shelters for maintaining bird diversity, even in an expanding city [23,72,73].
In Mexico, 1151 bird species have been described [35], while 412 species have been documented for the Valle of Mexico region according to Peterson and Navarro-Sigüenza [34]. Thus, in this study, we recorded 27.2% of the birds distributed in Mexico and around 70.3% of those described for the Valley of Mexico region. The sampling completeness was ~97%, highlighting that the bird species sampling was very adequate and efficient in the study area. Only migratory species richness differed significantly in pairwise comparisons across seasons (p < 0.01 in all cases), and the Shannon diversity index during the cold–dry and warm–dry season were similar to each other (p = 0.38) and were each significantly higher than the rainy season (p < 0.0001) based on estimated marginal means contrasts from a linear mixed model. Bird species richness, abundance and diversity were greater in heterogeneous environments [11,12] than in homogeneous environments [87] throughout the study period. This species richness pattern is consistent not only with our expectations, but also with the findings from previous studies in other areas close to the study area [88,89,90]. Spatial distribution of bird diversity is influenced by multiple environmental factors, including food resource diversity and availability [91,92]. Previous studies have confirmed that the presence of heterogeneous environments supports a high bird diversity, due exists higher food availability [12,59]. Similar patterns were observed in all study sites which mainly included native or non-native vegetation that supported a substantial abundance of birds [93]. In a heterogeneous landscape, the scattered anthropogenic habitats can provide additional food for birds that have higher environmental plasticity that can find food in both natural and human-dominated habitats [91].
Tu et al. [94] reported that species richness and evenness were different between natural and human-dominated habitats. Though a significant difference was observed in species richness among study sites, no observation of any difference in evenness was found. This may be because these study sites are rural settlements surrounded by agricultural areas, as with native and non-native trees and remnants of scrubland, and even minimal human disturbance significantly affected bird evenness in this site [95]. The sites with the greatest habitat heterogeneity had the maximum species richness throughout the year, although in general all sites had lower species richness and abundance during May and August. This could be related to the migratory period, and in the case of residents, the abundance and availability of food, increased anthropogenic activities (e.g., corn and prickly pear harvesting), which contribute to a lower diversity of species [11,12,92]. Bird diversity declines during the warmer month and is reduced to May to August, and this starts to increase from the beginning of September, with the arrival of migratory species, through March. This seasonal variation is similar to that reported in other studies [88,89,96]. Likewise, wetlands adjacent to peri-urban areas provided shelter and food for many migratory birds during colder months [23,88,89].
Migrant birds need to feed both during migration and on their wintering grounds; they therefore stop feeding at several sites along their migration route. For example, aquatic migrants move between different waterbodies until they reach a large one. Therefore, the conditions of these stopovers are critical for their survival during their stay, and this migratory species influx contributed to the increasing bird diversity during winter. It has been reported that water bodies with adjacent vegetation had greater species richness than water bodies with sparse vegetation [97] and that remnants of native and non-native vegetation are important bird habitats [93]. Water bodies contained the highest number of birds and abundance, and this revealed that this habitat significantly differed from other habitats in this study area. Bird diversity was higher in water bodies because of the large number of gathering colonial water birds, many of which were breeding, such as the American Coot (Fulica americana), Black-necked Still (Himantopus mexicanus), American avocet (Recurvirostra americana), Killdeer (Charadrius vociferus), Mexican Duck (Anas diazi), Ruddy Duck (Oxyura jamaicensis), Common Gallinule (Gallinula galeata), Pied-billed Grebe (Podilymbus podiceps), Eared Grebe (Podiceps nigricollis), Clarkʼs Grebe (Aechmophorus clarkii), Black-crowned Night Heron (Nycticorax nycticorax), Northern Shoveler (Spatula clypeata) and Blue-winged Teal (S. discors). Birds are abundant in these habitats because of some environmental characteristics of wetlands or waterbodies, like size, depth, water level, and plant species [73,98]. Several studies have shown that waterbird diversity is greatly influenced by the composition and structure of vegetation and microclimatic variables [73,99].
In many cases, overall bird richness and abundance have not been affected by land-use change (i.e., natural vegetation loss) on the landscape scale. This is because bird assemblages are composed of species that have different responses to environmental variables and anthropogenic impact [100,101]. Bird species from sensitive habitats may be replaced by those favored by human activity and habitat modification [32], resulting in the same overall richness and abundance. The results of this study argue against richness as the sole measure of diversity, as anthropized sites showed a relatively high species richness but more uneven distribution of abundance of individuals. In anthropized habitats there is stronger dominance of a smaller set of species that probably consume a larger proportion of available resources. These likely include generalist and non-native species like House Sparrow (Passer domesticus), Cattle Egret (Ardea ibis), House Finch (Haemorhous mexicanus), Great-tailed Grackle (Quiscalus mexicanus), among others. Meanwhile, low evenness and less dominance of certain species (those species associated with forests or endemic) were observed inside the urban avian community in comparison to the semi-urban and rural avian communities, where they were most frequently observed. Other studies have found similar results [31,102], in which the presence of some dominant (more relatively abundant) species is responsible for an uneven ecological community. Specifically, insectivore and granivore birds were more abundant in open areas with crops and grassland areas, due to the high availability of invertebrates and seeds. In addition to supporting more species, the remnants of natural habitat within peri-urban areas deserve better planning due to many species using them as shelter, feeding sites and breeding, as higher ecological diversity benefits species survival and human well-being [103,104]. Species can use transformed habitats to survive as ecosystems are destroyed and converted into human-dominated landscapes. However, these habitats may not be as valuable as the original ecosystems [31,91,95,102]. Bird diversity is influenced by habitat type and size around the world, but especially in countries with emerging economies, which tend to have rapid population growth and unplanned urban, agricultural, and industrial development.
Bird diversity can vary based on size [74,105], vegetation cover percentage, or overall habitat heterogeneity [21,23,24], suggesting that larger areas may attract a greater number of species. This aligns with previous studies showing that certain urban infrastructure components can have positive effects on bird species associated with highly urbanized areas [106,107], while species more sensitive to urbanization tend to seek refuge in peri-urban landscapes [25,108]. Alternatively, the lack of association between bird richness and local variables may also indicate the influence of other factors that were not considered, and that can be determining bird diversity in neotropical cities [18,109,110,111].
The peri-urban landscape also plays an important role for many species, especially migratory species [31,32] and could play an important role in improving connectivity in the urban landscape and with surrounding areas with natural vegetation [23,31,87]. Therefore, it is important to investigate the key factors affecting bird diversity in human-dominated areas, to propose actions to improve these environments, to maintain and conserve bird diversity. Accordingly, the preservation of pristine and restoration of degraded habitats and sustaining habitat heterogeneity and connectivity should be central to land use planning and management to reduce the impact of human disturbances on bird diversity.
5. Conclusions
Bird diversity is influenced by vegetation heterogeneity and structural complexity [21,112]; however, vegetation also plays a key role, as native vegetation can support more diverse bird communities [113]. Therefore, studying not only the size of urban greenspaces but also their configuration, vegetation structure, and management is essential for understanding their impact on urban bird diversity and populations [113,114].
For the long-term protection of bird communities, appropriate management programs must be designed and implemented. The results of this study reveal that cities can support a certain degree of avian diversity, but that diversity is far from that of non-urban sites. Therefore, measures are required to improve the urban landscape. The high diversity and species richness of birds in the study sites in MCMA emphasizes the importance of implementing conservation measures and that limiting human activity is important for bird conservation in order to help reduce threats to bird habitats and populations. The results showed that the highest diversity was recorded in water bodies with heterogeneous environments, while a significant decrease in avian diversity was observed in semi-urban and urban habitats. In addition, most bird species were found to have clustered dispersal patterns, especially aquatic migrants, suggesting the presence of threats and limited resources in some specific areas. Further quantification is needed to assess how anthropogenic stressors, and human intrusions impact the bird community throughout the study area. Conservation of wetlands close to the peri-urban area could be a viable strategy to preserve bird communities, as these ecosystems support and offer a variety of resources crucial for bird diversity.
Efforts are needed to mitigate the potential adverse effects of the various human interferences present in anthropogenic landscapes. Despite the great importance of conserving these habitats dominated by humans, efforts aimed at preserving native vegetation (scrublands, grasslands and native trees) should not be relaxed, because there were still many species that showed a higher specificity for these natural habitats, and some of them only persisted in these environments, being rarely found in habitats modified by humans. Furthermore, due to man-made landscape alterations and increasingly intense global changes, we recommend continuous monitoring of bird communities and their responses to ongoing environmental changes; to optimize management and conservation plans for these bird communities in central Mexico.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/birds6020018/s1, Table S1: Summarized environmental variables of the study sites in the MCMA, Mexico; and Table S2: Model selection that supported the interaction of bird diversity with season and environmental variables of study sites.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data are available upon request.
Acknowledgments
Thanks to Rogelio Bautista, Héctor Cayetano, and Oswaldo Gómez for their support in the field trips. To the staff of the Sierra Hermosa Ecological, Recreational and Tourist State Park, especially Monserrat Ramírez, Juan Valverde, Neptalí Piñón and Noé Cabañas for their logistical support and the facilities provided. To the ejidatarios of Tecámac, Nextlalpan, San Miguel Jaltocan, San Lucas Xolox, San Juan Pueblo Nuevo, Zumpango, Jaltenco, Texcoco, Santa Ana Tlachihualpa and San Juan Zitlaltepec for facilitating the field work on their lands.
Conflicts of Interest
The author declares no conflicts of interest.
Appendix A

Table A1.
Checklist of bird species of study sites in central Mexico.
Table A1.
Checklist of bird species of study sites in central Mexico.
| Species | Residency Status * | Habits † | Feeding Guilds ‡ | Habitats • | NOM-059 ◊ | IUCN ∆ |
|---|---|---|---|---|---|---|
| Accipiter cooperi | RES | TER | Ca | Gl, Al, Nr, W, Sm | Sp | LC |
| Accipiter striatus | RES | TER | Ca | Gl, Al, Nr, W, Sm | Sp | LC |
| Actitis macularia | MIG | AQ | In | W | LC | |
| Aechmophorus clarkii | RES | AQ | Ca | W | LC | |
| Aechmophorus occidentalis | RES | AQ | Ca | W | LC | |
| Aeronautes saxatalis | RES | TER | In | Gl, Sm, Nr | LC | |
| Agelaius phoeniceus | RES | TER | In, Gr | Gl, Al, W | LC | |
| Aimophila rufescens | RES | TER | Gr | Gl, Sm | LC | |
| Aimophila ruficeps | RES | TER | Gr | Gl, Sm | LC | |
| Amazona albifrons | EXO | TER | Fr | Gl, Ur, Nr | LC | |
| Amazona autumnalis | EXO | TER | Fr | Gl, Ur, Nr | LC | |
| Ammodramus savannarum | MIG | TER | Gr | Gl, Sm | LC | |
| Anas acuta | MIG | AQ | Om | W | LC | |
| Anas crecca | MIG | AQ | Om | W | LC | |
| Anas diazi | RES | AQ | Om | W | T | LC |
| Anas platyrhynchos (domestic) | EXO | AQ | Om | W | LC | |
| Anser albifrons (domestic) | EXO | AQ | Om | W | LC | |
| Anser anser (domestic) | EXO | AQ | Om | W | LC | |
| Anser cygnoides (domestic) | EXO | AQ | Om | W | LC | |
| Anthus rubescens | MIG | TER | In | Gl, Al | LC | |
| Aphelocoma ultramarina | RES | TER | Om | Nr | LC | |
| Archilochus alexandri | MIG | TER | Ne | Nr | LC | |
| Archilochus colubris | MIG | TER | Ne | Nr | LC | |
| Ardea alba | MIG | AQ | Ca, In | W | LC | |
| Ardea herodias | MIG | AQ | Ca, In | W | LC | |
| Ardea ibis | EXO | TER | In | Gl, Al, W | LC | |
| Asio flammeus | MIG | TER | Ca | Gl, Al | Sp | LC |
| Athene cunicularia | RES | TER | In | Gl, Al | LC | |
| Atthis heloisa | RES | TER | Ne | Nr | LC | |
| Aythya affinis | MIG | AQ | Om | W | LC | |
| Aythya americana | MIG | AQ | Om | W | LC | |
| Aythya collaris | MIG | AQ | Om | W | LC | |
| Aythya valisineria | MIG | AQ | Om | W | LC | |
| Bartramia longicauda | MIG | AQ | In | W | LC | |
| Basileuterus rufifrons | MIG | TER | In | Nr | LC | |
| Basilinna leucotis | RES | TER | Ne | Nr | LC | |
| Bombycilla cedrorum | MIG | TER | In, Fr | Nr | LC | |
| Botaurus lentiginosus | RES | AQ | In | W | T | LC |
| Bubo virginianus | RES | TER | Ca | Gl, Al, Nr | LC | |
| Buteo brachyurus | RES | TER | Ca | Gl, Al, Nr | LC | |
| Buteo jamaicensis | RES | TER | Ca | Gl, Al, Nr, Sm, Ur | LC | |
| Buteo lineatus | MIG | TER | Ca | Gl, Al, Nr, Sm | LC | |
| Buteo plagiatus | RES | TER | Ca | Gl, Al, Ur, Nr | LC | |
| Buteo platypterus | MIG | TER | Ca | Gl, Al, Ur, Nr | Sp | LC |
| Buteo swainsoni | MIG | TER | Ca | Gl, Al, Ur, Nr, Sm | Sp | LC |
| Butorides virescens | MIG | AQ | Ca, In | W | LC | |
| Cairina moschata (domestic) | EXO | AQ | Om | W | LC | |
| Calidris alba | MIG | AQ | In | W | LC | |
| Calidris alpina | MIG | AQ | In | W | LC | |
| Calidris bairdii | MIG | AQ | In | W | LC | |
| Calidris himantopus | MIG | AQ | In | W | LC | |
| Calidris mauri | MIG | AQ | In | W | LC | |
| Calidris melanotus | MIG | AQ | In | W | LC | |
| Calidris minutilla | MIG | AQ | In | W | LC | |
| Calidris pusilla | MIG | AQ | In | W | LC | |
| Calothorax lucifer | RES | TER | Ne | Nr | LC | |
| Camptostoma imberbe | RES | TER | In | Nr | LC | |
| Campylorhynchus brunneicapillus | RES | TER | In | Sm | LC | |
| Caracara cheriway | RES | TER | Ca, Sc | Gl, Al, Sm | LC | |
| Cardellina pusilla | MIG | TER | In | Nr | LC | |
| Cardinalis cardinalis | EXO | TER | Gr, Fr | Nr, Sm | LC | |
| Cathartes aura | RES | TER | Sc | Gl, Al, Nr, Ur, Sm | LC | |
| Catharus guttatus | MIG | TER | In | Nr | LC | |
| Catherpes mexicanus | RES | TER | In | Nr, Sm | LC | |
| Chaetura vauxi | RES | TER | In | Gl, Al, Sm | LC | |
| Anarhynchus nivosus | MIG | AQ | In | W | T | NT |
| Charadrius semipalmatus | MIG | AQ | In | W | LC | |
| Charadrius vociferus | RES | AQ | In | W, Al, Gl | LC | |
| Chlidonias niger | MIG | AQ | In | W | LC | |
| Chondestes grammacus | MIG | TER | Gr | Gl, Sm, Nr | LC | |
| Chordeiles acutipennis | RES | TER | In | Gl, Sm | LC | |
| Circus hudsonius | MIG | TER | Ca | Gl, Al, Sm, Nr | LC | |
| Cistothorus palustris | RES | TER | In | W | LC | |
| Cistothorus platensis | RES | TER | In | W | LC | |
| Coccyzus americanus | MIG | TER | In | Nr, Sm | LC | |
| Colaptes auratus | RES | TER | In | Nr | LC | |
| Colibri thalassinus | RES | TER | Ne | Nr | LC | |
| Colinus virginianus | RES | TER | Gr | Al, Sm | LC | |
| Columba livia | EXO | TER | Om | Ur, Al | LC | |
| Columbina inca | RES | TER | Gr | Ur, Gl, Al, Nr, Sm | LC | |
| Columbina passerina | RES | TER | Gr | Gl | LC | |
| Columbina tapalcoti | EXO | TER | Gr | Gl | LC | |
| Contopus cooperii | MIG | TER | In | Gl, Nr | LC | |
| Contopus pertinax | RES | TER | In | Gl, Nr | LC | |
| Contopus sordidulus | MIG | TER | In | Gl, Nr | LC | |
| Coragyps atratus | MIG | TER | Sc | Gl, Al | LC | |
| Corthylio calendula | MIG | TER | In | Nr | LC | |
| Corvus corax | RES | TER | Om | Gl, Al, Sm | LC | |
| Crotophaga sulcirostris | RES | TER | Gr, Fr | Gl, Al | LC | |
| Cyanocorax yncas | EXO | TER | Om | Nr, Sm | LC | |
| Cynanthus latirostris | RES | TER | Ne | Nr | LC | |
| Cyrtonyx montezumae | RES | TER | Gr | Sm, Al | Sp | LC |
| Dendrocygna autumnalis | RES | AQ | Om | W | LC | |
| Dendrocygna bicolor | RES | AQ | Om | W | LC | |
| Diglossa baritula | RES | TER | Ne | Nr | LC | |
| Dryobates scalaris | RES | TER | In | Nr, Sm, Al | LC | |
| Egretta caerulea | MIG | AQ | Ca, In | W | LC | |
| Egretta thula | MIG | AQ | Ca, In | W | LC | |
| Egretta tricolor | MIG | AQ | Ca, In | W | LC | |
| Elanus leucurus | RES | TER | Ca | Gl, Al | LC | |
| Empidonax fulvifrons | RES | TER | In | Nr | LC | |
| Empidonax hammondii | MIG | TER | In | Nr | LC | |
| Empidonax minimus | MIG | TER | In | Nr | LC | |
| Empidonax oberholseri | MIG | TER | In | Nr | LC | |
| Empidonax occidentalis | RES | TER | In | Nr | LC | |
| Empidonax traillii | MIG | TER | In | Nr | LC | |
| Empidonax wrightii | MIG | TER | In | Nr | LC | |
| Eremophila alpestris | RES | TER | Gr | Al | LC | |
| Eugenes fulgens | RES | TER | Ne | Nr | LC | |
| Euphagus cyanocephalus | RES | TER | Om | W, Al | LC | |
| Eupsittula canicularis | EXO | TER | Fr | Gl, Ur | LC | |
| Falco columbarius | MIG | TER | Ca | Gl, Al, Sm | LC | |
| Falco peregrinus | RES | TER | Ca | Gl, Al, Sm | LC | |
| Falco sparverius | MIG | TER | Ca, In | Gl, Al, Sm, Nr | LC | |
| Fulica americana | RES | AQ | Om | W | LC | |
| Gallinago delicata | MIG | AQ | In | W | LC | |
| Gallinula galeata | MIG | AQ | Om | W | LC | |
| Gelochelidon nilotica | MIG | AQ | Ca, In | W | LC | |
| Geococcyx californianus | RES | TER | Ca, In | Sm | LC | |
| Geothlypis tolmiei | MIG | TER | In | W | LC | |
| Geothlypis trichas | RES | TER | In | W | LC | |
| Glaucidium gnoma | RES | TER | In | Sm | LC | |
| Haemorhous mexicanus | RES | TER | Gr | Al, Ur, Nr, Gl, Sm | LC | |
| Himantopus mexicanus | MIG | AQ | In | W | LC | |
| Hirundo rustica | MIG | TER | In | Al, Sm, Gl, W, Nr, Ur | LC | |
| Hydroprogne caspia | MIG | AQ | Ca, In | W | LC | |
| Icteria virens | MIG | TER | In | Nr | LC | |
| Icterus abeillei | RES | TER | In, Fr | Nr | LC | |
| Icterus bullockii | RES | TER | In, Fr | Nr, Sm | LC | |
| Icterus cucullatus | MIG | TER | In, Fr | Nr | LC | |
| Icterus galbula | MIG | TER | In, Fr | Sm | LC | |
| Icterus parisorum | RES | TER | In, Fr | Sm | LC | |
| Icterus pustulatus | EXO | TER | In, Fr | Nr | LC | |
| Icterus spurius | MIG | TER | In | W, Al | LC | |
| Icterus wagleri | RES | TER | In, Fr | Nr, Sm | LC | |
| Junco phaeonotus | RES | TER | Gr | Gl, Sm | LC | |
| Lampornis clemenciae | RES | TER | Ne | Nr | LC | |
| Lanius ludovicianus | RES | TER | Ca, In | Gl, Al, Nr, Sm | LC | |
| Larus delawarensis | MIG | AQ | Ca, In | W | LC | |
| Leiothlypis ruficapilla | MIG | TER | In | Nr | LC | |
| Leiothlypis celata | MIG | TER | In | Nr | LC | |
| Leiothlypis peregrina | MIG | TER | In | Nr | LC | |
| Leiothlypis virginiae | MIG | TER | In | Nr | LC | |
| Leptotila verreauxi | RES | TER | Gr | Gl, Sm | LC | |
| Leucolia violiceps | RES | TER | Ne | Nr | LC | |
| Leucophaeus atricilla | MIG | AQ | Ca, In | W | LC | |
| Leucophaeus pipixcan | MIG | AQ | Ca, In | W | LC | |
| Limnodromus griseus | MIG | AQ | In | W | LC | |
| Limnodromus scolopaceus | MIG | AQ | In | W | LC | |
| Limosa haemastica | MIG | AQ | In | W | LC | |
| Mareca americana | MIG | AQ | Om | W | LC | |
| Mareca penelope | MIG | AQ | Om | W | LC | |
| Mareca strepera | MIG | AQ | Om | W | LC | |
| Megaceryle alcyon | MIG | TER | Ca | W | LC | |
| Melanerpes aurifrons | RES | TER | In | Nr | LC | |
| Melanerpes formicivorus | RES | TER | In | Nr | LC | |
| Melanotis caerulescens | RES | TER | Om | Nr | LC | |
| Melopsittacus undulatus | EXO | TER | Gr | Gl, Al, Ur | LC | |
| Melospiza lincolnii | RES | TER | Gr | Gl | LC | |
| Melospiza melodia | RES | TER | Gr | Gl, W | LC | |
| Melozone fusca | RES | TER | Gr | Gl, Nr, Ur, Al, Sm | LC | |
| Mimus polyglottos | RES | TER | In | Nr, Al, Ur, Sm | LC | |
| Mitrephanes phaeocercus | RES | TER | In | Nr | LC | |
| Mniotilta varia | MIG | TER | In | Nr | LC | |
| Molothrus aeneus | RES | TER | Gr | Gl, Al, W | LC | |
| Molothrus ater | RES | TER | Gr | Gl, Al, W | LC | |
| Myiarchus cinerascens | MIG | TER | In | Gl, Nr, Sm | LC | |
| Myiarchus crinitus | MIG | TER | In | Nr | LC | |
| Myiarchus nuttingi | RES | TER | In | Nr | LC | |
| Myioborus pictus | RES | TER | In | Nr | LC | |
| Myiopsitta monachus | EXO | TER | Fr | Nr, Ur | LC | |
| Nannopterum auritus | MIG | AQ | Ca | W | LC | |
| Nannopterum brasilianum | MIG | AQ | Ca | W | LC | |
| Numenius americanus | MIG | AQ | Om | W | LC | |
| Numida meleagris | EXO | TER | Gr | Gl | LC | |
| Nycticorax nycticorax | RES | AQ | Ca, In | W | LC | |
| Nyctinassa violacea | MIG | AQ | Ca, In | W | LC | |
| Oreothlypis superciliosa | MIG | TER | In | Nr | LC | |
| Oriturus superciliosus | RES | TER | In | Nr | LC | |
| Oxyura jamaicensis | RES | AQ | Om | W | LC | |
| Pandion haliaetus | MIG | TER | Ca | Al, W | LC | |
| Parabuteo unicinctus | EXO | TER | Ca | Gl, Al, Ur, Nr | LC | |
| Parkesia noveboracensis | MIG | TER | In | Nr | LC | |
| Passer domesticus | EXO | TER | Om | Ur, Al, Gl, Sm, W, Nr | LC | |
| Passerculus sandwichensis | RES | TER | Gr | Gl, Sm, W, Al | LC | |
| Passerina caerulea | RES | TER | In, Gr | Gl, Al, Nr, Sm | LC | |
| Passerina ciris | MIG | TER | Gr | Gl | Sp | LC |
| Passerina cyanea | MIG | TER | Gr | Gl, Al, Nr | LC | |
| Passerina versicolor | RES | TER | Gr | Gl, Al, Nr | LC | |
| Pavo cristatus | EXO | TER | Gr | Gl | LC | |
| Pelecanus erythrorynchus | MIG | AQ | Ca | W | LC | |
| Petrochelidon pyrrhonota | RES | TER | In | Al, W | LC | |
| Peucaea botterii | RES | TER | Gr | Gl, Sm | LC | |
| Peucedramus taeniatus | RES | TER | In | Nr | LC | |
| Phainopepla nitens | MIG | TER | In | Nr, Sm | LC | |
| Phalaropus lobatus | MIG | AQ | In | W | LC | |
| Phalaropus tricolor | MIG | AQ | In | W | LC | |
| Phasianus colchicus | EXO | TER | Gr | Gl | LC | |
| Pheucticus ludovicianus | RES | TER | In | Nr | LC | |
| Pheucticus melanocephalus | RES | TER | Gr | Nr | LC | |
| Phoenicopterus ruber | EXO | AQ | Om | W | LC | |
| Pipilo chlorurus | RES | TER | Gr | Sm | LC | |
| Pipilo maculatus | RES | TER | Gr | Sm | LC | |
| Piranga flava | RES | TER | In | Nr | LC | |
| Piranga ludoviciana | MIG | TER | In | Nr | LC | |
| Piranga rubra | MIG | TER | In | Nr | LC | |
| Plegadis chihi | RES | AQ | In | W | LC | |
| Pluvialis dominica | MIG | AQ | In | W | LC | |
| Pluvialis squatorola | MIG | AQ | In | W | LC | |
| Podiceps nigricollis | RES | AQ | In | W | LC | |
| Podylimbus podiceps | RES | AQ | In | W | LC | |
| Polioptila caerulea | MIG | TER | In | Nr, Gl, Sm | LC | |
| Pooecetes gramineus | MIG | TER | Gr | Gl, Al, Sm | LC | |
| Porphyrio martinicus | MIG | AQ | In | W | LC | |
| Porzana carolina | MIG | AQ | In | W | LC | |
| Progne chalybea | MIG | TER | In | Sm | LC | |
| Psaltriparus minimus | RES | TER | In | Nr | LC | |
| Ptiliogonys cinereus | RES | TER | In | Nr | LC | |
| Pyrocephalus rubinus | RES | TER | In | Gl, Al, W, Ur, Nr, Sm | LC | |
| Quiscalus mexicanus | EXO | TER | Om | Ur, Al, W, Nr, Sm, Gl | LC | |
| Rallus limicola | RES | AQ | In | W | LC | |
| Rallus tenuirostris | RES | AQ | In | W | NT | |
| Recurvirostra americana | RES | AQ | In | W | LC | |
| Riparia riparia | MIG | TER | In | W | LC | |
| Salpinctes obsoletus | RES | TER | In | Nr, Sm | LC | |
| Saucerottia beryllina | RES | TER | Ne | Nr | LC | |
| Sayornis nigricans | RES | TER | In | W | LC | |
| Sayornis phoebe | MIG | TER | In | Nr | LC | |
| Sayornis saya | MIG | TER | In | Gl, Sm, Al | LC | |
| Selasphorus platycercus | RES | TER | Ne | Nr | LC | |
| Selasphorus rufus | MIG | TER | Ne | Nr | LC | |
| Setophaga coronata | MIG | TER | In | Nr, Ur | LC | |
| Setophaga nigrescens | MIG | TER | In | Nr | LC | |
| Setophaga occidentalis | MIG | TER | In | Nr | LC | |
| Setophaga palmarum | MIG | TER | In | Nr | LC | |
| Setophaga petechia | MIG | TER | In | Nr | LC | |
| Setophaga pitiayumi | MIG | TER | In | Nr | LC | |
| Setophaga ruticilla | MIG | TER | In | Nr | LC | |
| Setophaga townsendi | MIG | TER | In | Nr | LC | |
| Setophaga virens | MIG | TER | In | Nr | LC | |
| Sicalis luteola | MIG | TER | Gr | Gl | LC | |
| Sitta canadensis | MIG | TER | In | Nr | LC | |
| Spatula clypeata | MIG | AQ | Om | W | LC | |
| Spatula cyanoptera | RES | AQ | Om | W | LC | |
| Spatula discors | MIG | AQ | Om | W | LC | |
| Sphyrapicus varius | RES | TER | In | Nr | LC | |
| Spinus pinus | MIG | TER | Gr | Nr, Sm | LC | |
| Spinus psaltria | RES | TER | Gr | Al, Nr, Gl, Ur, Sm | LC | |
| Spiza americana | MIG | TER | Gr | Gl | LC | |
| Spizella atrogularis | RES | TER | Gr | Gl, Sm | LC | |
| Spizella pallida | MIG | TER | Gr | Gl, Sm | LC | |
| Spizella passerina | RES | TER | Gr | Gl, Sm | LC | |
| Sporophila torqueola | RES | TER | Gr | W | LC | |
| Stelgidopteryx serripennis | RES | TER | In | Al, W, Ur, Sm, Gl, Nr | LC | |
| Sterna forsteri | MIG | AQ | Ca, In | W | LC | |
| Sterna hirundo | MIG | AQ | Ca, In | W | LC | |
| Streptopelia decaocto | EXO | TER | Om | Ur, Nr, Al | LC | |
| Sturnella magna | RES | TER | In | Al, Gl, Sm | LC | |
| Sturnus vulgaris | EXO | TER | Om | Ur, Al, Nr | LC | |
| Tachybaptus dominicus | MIG | AQ | Ca, In | W | Sp | LC |
| Tachycineta bicolor | MIG | TER | In | Al, W | LC | |
| Tachycineta thalassina | RES | TER | In | Al, W | LC | |
| Thryomanes bewickii | RES | TER | In | Nr, W, Sm, Ur | LC | |
| Toxostoma curvirostre | RES | TER | In | Nr, Al, Ur, Sm | LC | |
| Tringa flavipes | MIG | AQ | In | W | LC | |
| Tringa melanoleuca | MIG | AQ | In | W | LC | |
| Tringa semipalmata | MIG | AQ | In | W | LC | |
| Tringa solitaria | MIG | AQ | In | W | LC | |
| Troglodytes aedon | RES | TER | In | Nr, Sm | LC | |
| Turdus migratorius | RES | TER | In | Nr | LC | |
| Turdus rufopalliatus | RES | TER | In | Nr, Ur | LC | |
| Tyrannus forficatus | MIG | TER | In | Gl, Nr | LC | |
| Tyrannus melancholicus | RES | TER | In | Gl, Nr | LC | |
| Tyrannus tyrannus | MIG | TER | In | Gl | LC | |
| Tyrannus verticalis | MIG | TER | In | Gl, Nr, Sm | LC | |
| Tyrannus vociferans | RES | TER | In | Gl, Nr, Sm, Ur | LC | |
| Tyto furcata | RES | TER | Ca | Gl, Al, Nr, Ur | LC | |
| Vermivora chrysoptera | MIG | TER | In | Nr | LC | |
| Vireo bellii | MIG | TER | In | Nr | LC | |
| Vireo cassini | MIG | TER | In | Nr | LC | |
| Vireo gilvus | MIG | TER | In | Nr | LC | |
| Vireo griseus | MIG | TER | In | Nr | LC | |
| Vireo huttoni | RES | TER | In | Nr | LC | |
| Vireo plumbeus | MIG | TER | In | Nr | LC | |
| Vireo solitarius | MIG | TER | In | Nr | LC | |
| Volatinia jacarina | RES | TER | Gr | Gl | LC | |
| Xanthocephalus xanthocephalus | MIG | TER | Gr | Al, Gl, W | LC | |
| Zenaida asiatica | RES | TER | Gr, Fr | Gl, Ur, Al, Nr, Sm | LC | |
| Zenaida macroura | RES | TER | Gr, Fr | Gl, Al, Sm | LC | |
| Zonotrichia leucophrys | RES | TER | Gr | Gl | LC |
* Residency status: resident (RES), migrant (MIG). † Habits: terrestrial (TER), aquatic or semiaquatic (AQ). ‡ Feeding guilds: carnivore (Ca), insectivore (In), granivore (Gr), omnivore (Om), frugivore (Fr), nectarivore (Ne), scavenger (Sc). • Habitats: agricultural lands (Al), wetlands (W), urban areas (Ur), remnants of native and non-native vegetation (Nr), grasslands (Gr), semi-desert xeric scrubs (Sm). ◊ Vulnerability status in Mexican national legislation NOM-059-Semarnat-2010: special protection (Sp), threatened (T). ∆ Vulnerability status at global level (IUCN Red List): least concern (LC), near threatened (NT).

Table A2.
Results of most supported mixed models (CI = Confidence Interval, Dgs = Distance to nearest greenspace).
Table A2.
Results of most supported mixed models (CI = Confidence Interval, Dgs = Distance to nearest greenspace).
| Predictors | Estimates | 95% CI | p |
|---|---|---|---|
| Overall species richness | |||
| Intercept) | 74.81 | 48.88–100.75 | <0.001 |
| Dgs | 1.01 | −2.18–4.21 | 0.533 |
| Season [warm–dry] | −1.55 | −11.74–8.64 | 0.763 |
| Season [rainy] | −34.97 | −44.73–−25.22 | <0.001 |
| Dgs × Season [warm–dry] | −0.76 | −2.02–0.49 | 0.232 |
| Dgs × Season [rainy] | 2.39 | 1.19–3.59 | <0.001 |
| Random effects: Σ2Site = 167.3, Σ2Residual = 127.08 | |||
| Resident species richness | |||
| (Intercept) | 40.11 | 26.96–53.27 | <0.001 |
| Dgs | 1.25 | −0.37–2.87 | 0.130 |
| Season [warm–dry] | 3.56 | −4.30–11.42 | 0.372 |
| Season [rainy] | −13.07 | −20.59–−5.54 | 0.001 |
| Dgs × season [warm–dry] | −0.69 | −1.66–0.28 | 0.162 |
| Dgs × season [rainy] | 2.12 | 1.20–3.05 | <0.001 |
| Random effects: Σ2Site = 39.99, Σ2Residual = 75.57 | |||
| Migrant species richness | |||
| (Intercept) | 32.99 | 26.12–39.85 | <0.001 |
| Season [warm–dry] | −5.64 | −8.87–−2.42 | 0.001 |
| Season [rainy] | −19.96 | −23.05–−16.87 | <0.001 |
| Random effects: Σ2Site = 55.77, Σ2Residual = 62.17 | |||
| Shannon diversity index | |||
| (Intercept) | 3.19 | 3.09–3.29 | <0.001 |
| Season [warm–dry] | 0.06 | −0.07–0.19 | 0.378 |
| Season [rainy] | −0.33 | −0.45–−0.20 | <0.001 |
| Random effects: Σ2Site = 0.01, Σ2Residual = 0.10 | |||
| Log (Abundance) | |||
| (Intercept) | 7.96 | 7.22–8.71 | <0.001 |
| Random effects: Σ2Site = 0.68, Σ2Residual = 0.77 | |||
| Evenness | |||
| (Intercept) | 0.78 | 0.76–0.80 | <0.001 |
| Random effects: Σ2Site = 0.0004732, Σ2Residual = 0.0048856 | |||
References
- Li, G.; Fang, C.; Li, Y.; Wang, Z.; Sun, S.; He, S.; Qi, W.; Bao, C.; Ma, H.; Fan, Y.; et al. Global impacts of future urban expansion on terrestrial vertebrate diversity. Nat. Commun. 2022, 13, 1628. [Google Scholar] [CrossRef] [PubMed]
- Simkin, R.D.; Seto, K.C.; McDonald, R.I.; Jetz, W. Biodiversity impacts and conservation implications of urban land expansion projected to 2050. Proc. Natl. Acad. Sci. USA 2022, 119, e2117297119. [Google Scholar] [CrossRef] [PubMed]
- Clergeau, P.; Jokimäki, J.; Savard, J.-P.L. Are urban bird communities influenced by the bird diversity adjacent landscapes. J. Appl. Ecol. 2002, 38, 1122–1134. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; Cadenasso, M.L.; Grove, J.M.; Boone, C.G.; Groffman, P.M.; Irwin, E.; Kaushal, S.S.; Marshall, V.; McGrath, B.P.; Nilon, C.H.; et al. Urban ecological systems: Scientific foundations and a decade of progress. J. Environ. Man. 2011, 92, 331–362. [Google Scholar] [CrossRef]
- Marzluff, J.M. Worldwide Urbanization and its Effects on Birds. In Avian Ecology and Conservation in a Urbanizing World; Marzluff, J.M., Bowman, R., Donelly, R., Eds.; Springer: Boston, MA, USA, 2001. [Google Scholar]
- Santos, E.G.; Wiederhecker, H.C.; Pompermaier, V.T.; Gainsbury, A.M.; Schirmer, S.C.; Morais, C.V.F.; Fontenele, J.L.; Santana, M.C.d.M.; Marini, M.Â. Urbanization reduces diversity, simplifies community and filter bird species based on their functional traits in a tropical city. Sci. Total Environ. 2024, 935, 173379. [Google Scholar] [CrossRef]
- Larsen, S.; Sorace, A.; Mancini, L. Riparian bird communities as indicators of human impact along Mediterranean streams. Environ. Manag. 2010, 45, 261–273. [Google Scholar] [CrossRef]
- Maznikova, V.N.; Ormerod, S.J.; Gómez-Serrano, M.A. Birds as bioindicators of river pollution and beyond: Specific and general lessons from an apex predator. Ecol. Ind. 2024, 158, 11136. [Google Scholar] [CrossRef]
- Harisha, M.N.; Hosetti, B.B. Diversity and distribution of avifauna of Lakka Walli range forest, Bhadra Wildlife Sanctuary, western Ghat, India. Ecoprint 2009, 16, 21–27. [Google Scholar]
- Choudaj, K.; Wankhade, V. Reduction in avian diversity due to exotic tree plantations on the native savannas of Pune City, India. Trop. Ecol. 2021, 62, 499–507. [Google Scholar] [CrossRef]
- Anderle, M.; Brambilla, M.; Hilpold, A.; Matabishi, J.G.; Paniccia, C.; Rocchini, D.; Rossin, J.; Tasser, E.; Torresani, M.; Tappeiner, U.; et al. Habitat heterogeneity promotes bird diversity in agricultural landscapes: Insights from remote sensing data. Basic. Appl. Ecol. 2023, 70, 38–49. [Google Scholar] [CrossRef]
- Sultana, M.; Corlatti, L.; Storch, I. The interaction and habitat heterogeneity drives bird richness patterns in south Asian cities. Urban Ecosyst. 2021, 24, 335–344. [Google Scholar] [CrossRef]
- Chapman, K.A.; Reich, P.B. Land use and habitat gradients determine bird community diversity and abundance in suburban, rural and reserve landscapes of Minnesota, USA. Biol. Conserv. 2007, 135, 527–541. [Google Scholar] [CrossRef]
- Coetzee, B.W.T.; Chown, S.L. Land-use change promotes avian diversity at the expense of species with unique traits. Ecol. Evol. 2016, 6, 7610–7622. [Google Scholar] [CrossRef]
- Sousa, N.O.d.M.; Lopes, L.E.; Costa, L.M.; Motta-Junior, J.C.; de Freitas, G.H.S.; Dornas, T.; de Vasconcelos, M.F.; Nogueira, W.; Tolentino, V.C.d.M.; De-Carvalho, C.B.; et al. Adopting habitat-use to infer movement potential and sensitivity to human disturbance of birds in a Neotropical savannah. Biol. Conserv. 2021, 254, 108921. [Google Scholar] [CrossRef]
- Partecke, J.; Schwabl, I.; Gwinner, E. Stress and the city: Urbanization and its effects on the stress physiology in European blackbirds. Ecology 2006, 87, 1945–1952. [Google Scholar] [CrossRef]
- Kurucz, K.; Purger, J.J.; Batáry, P. Urbanization shapes bird communities and nest survival, but not their food quantify. Glob. Ecol. Conserv. 2021, 26, e01975. [Google Scholar] [CrossRef]
- Amaya-espinel, J.; Hostetler, M. The value of small forest fragments and urban tree canopy for Neotropical migrant birds during winter and migration seasons in Latin America countries: A systematic review. Landsc. Urban Plan. 2019, 190, 103592. [Google Scholar] [CrossRef]
- Asefa, A.; Davies, A.B.; McKechnie, A.E.; Kinahan, A.A.; van Rensburg, B.J. Effects of anthropogenic disturbance on bird diversity in Ethiopian montane forests. Condor 2017, 119, 416–430. [Google Scholar] [CrossRef]
- Matuoka, M.A.; Benchimol, M.; de Almeida-Rocha, J.M.; Morante-Filho, J.C. Effects of anthropogenic disturbances on bird functional diversity: A global meta-analysis. Ecol. Ind. 2020, 116, 106471. [Google Scholar] [CrossRef]
- Soifer, L.G.; Donovan, S.K.; Brentjens, E.T.; Bratt, H.R. Piecing together cities to support bird diversity: Development and forest edge density affect bird richness in urban environments. Landsc. Urban Plan. 2021, 213, 104122. [Google Scholar] [CrossRef]
- Pena, J.C.; Ovaskainen, O.; MacGregor-Fors, I.; Teixeira, C.P.; Ribeiro, M.C. The relationships between urbanization and bird functional traits across the streetscape. Landsc. Urban Plan. 2023, 232, 104685. [Google Scholar] [CrossRef]
- Ramírez-Albores, J.E.; Sánchez-González, L.A.; Pérez-Suárez, M.; Navarro-Sigüenza, A.G.; Franco-Maass, S. Greenspaces as shelters for the conservation of bird diversity in a big city. Urban Ecosyst. 2024, 27, 2047–2059. [Google Scholar] [CrossRef]
- Charre, G.M.; Zavala, A.; Néve, G.; Ponce-Mendoza, A.; Corcuera, P. Relationship between habitat traits and bird diversity and com position in selected urban green areas of Mexico City. Ornitol. Neotrop. 2013, 24, 275–293. [Google Scholar]
- MacGregor-Fors, I.; Escobar, F.; Rueda-Hernández, R.; Avendaño-Reyes, S.; Baena, M.L.; Bandala, V.M.; Chacón-Zapata, S.; Guillén-Servent, A.; González-García, F.; Lorea-Hernández, F.; et al. City green contributions: The role of urban greenspaces as reservoirs for biodiversity. Forest 2016, 7, 146. [Google Scholar] [CrossRef]
- Edeigba, B.; Ashinze, U.K.; Umoh, A.A.; Biu, P.W. Urban green spaces and their impact on environmental health: A global review. World J. Adv. Res. Rev. 2024, 21, 917–927. [Google Scholar] [CrossRef]
- Zhang, F.; Qian, H. A comprehensive review of the environmental benefit of urban green spaces. Environ. Res. 2024, 252, 118837. [Google Scholar] [CrossRef]
- Everard, M. Agricultural Management and Wetlands: An Overview. In The Wetland Book; Finlayson, C., Ed.; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Hoverman, J.T.; McKenzie, V.J.; Blaustein, A.R.; Richgels, K.L.D. Urbanization and Wetland communities: Applying metacommunity theory to understand the local and landscape effects. J. Appl. Ecol. 2013, 50, 34–42. [Google Scholar] [CrossRef]
- Alikhani, S.; Nummi, P.; Ojala, A. Urban Wetlands: A review on ecological and cultural values. Water 2021, 13, 3301. [Google Scholar] [CrossRef]
- Reis, E.; López-Ibarra, G.M.; Torres, R. Changes in bird species richness through different levels of urbanization: Implications for biodiversity conservation and garden design in central Brazil. Landsc. Urban Plan. 2012, 107, 31–42. [Google Scholar] [CrossRef]
- Xu, X.; Xie, Y.; Qi, K.; Luo, Z.; Wang, X. Detecting the response of bird communities and biodiversity to habitat loss and fragmentation due to urbanization. Sci. Total Environ. 2018, 624, 1561–1576. [Google Scholar] [CrossRef]
- United Nations. 2018 Revision of World Urbanization Prospects; United Nations: New York, NY, USA, 2018; Available online: https://population.un.org/wup/ (accessed on 1 December 2024).
- Peterson, A.T.; Navarro-Sigüenza, A.G. Hundred-year changes in the avifauna of the Valley of Mexico, Distrito Federal, Mexico. Huitzil 2006, 7, 4–14. [Google Scholar]
- Navarro-Sigüenza, A.G.; Rebón-Gallardo, M.F.; Gordillo-Martínez, A.; Peterson, A.T.; Berlanga, H.; Sánchez-González, L.A. Biodiversidad de aves. Rev. Mex. Biodivers. 2014, 85, S476–S495. [Google Scholar] [CrossRef]
- Pacheco-Muñoz, R.; Aguilar-Gómez, M.A.; Schondube, J.E. Overwintering in a megacity? Urban green areas and migratory birds in Mexico City. Urban For. Urban Green. 2022, 73, 127614. [Google Scholar] [CrossRef]
- Şekercioğlu, C.H.; Daily, G.C.; Ehrlich, P.R. Ecosystem consequences of bird declines. Proc. Natl. Acad. Sci. USA 2005, 101, 18042–18047. [Google Scholar] [CrossRef]
- Wilkie, D.S.; Bennett, E.L.; Peres, C.A.; Cunningham, A.A. The empty forest revisited. Ann. N. Y. Acad. Sci. 2011, 1223, 120–128. [Google Scholar]
- Reed, D.H.; Frankham, R. Correlation between fitness and genetic diversity. Conserv. Biol. 2003, 17, 230–237. [Google Scholar] [CrossRef]
- Melbourne, B.A.; Hastings, A. Extinction risk depends strongly on factors contributing to stochasticity. Nature 2008, 454, 100–103. [Google Scholar] [CrossRef]
- INEGI (Instituto Nacional de Geografía y Estadística). Censo de Población y Vivienda 2020; INEGI: Aguascalientes, México, 2020. [Google Scholar]
- Behzadi, F.; Wasti, A.; Rahat, S.H.; Tracy, J.N.; Ray, P.A. Analysis of the climate change signal in Mexico City given disagreeing data sources and scattered projections. J. Hydrol. 2020, 27, 100662. [Google Scholar] [CrossRef]
- Sorani, V.; Rodríguez, G.; Reygadas, D.D. Usos y Cobertura de Suelo. In La Biodiversidad de la Ciudad de Mexico; Conabio, Ed.; Conabio: Mexico City, Mexico, 2016; pp. 104–112. [Google Scholar]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyuschenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Emlen, J.T. Population densities of birds derived from transect counts. Auk 1971, 88, 323–342. [Google Scholar] [CrossRef]
- Emlen, J.T. Estimating breeding season bird densities from transect counts. Auk 1977, 94, 455–468. [Google Scholar]
- Peterson, R.T.; Chalif, E.L. Aves de México, Guía de Campo; Editorial Diana: México, 1989. [Google Scholar]
- Kauffman, K. Kaufmann Field Guide to Birds of North America; Houghton Mifflin Harcourt: Boston, MA, USA, 2005. [Google Scholar]
- Dunn, J.L.; Alderfer, J. Field Guide to the Birds of North America; National Geographic Society: Washington, DC, USA, 2008. [Google Scholar]
- Sibley, D.A. The Sibley Guide to Birds, 2nd ed.; Knopf: New York, NY, USA, 2014. [Google Scholar]
- Chesser, R.T.; Billerman, S.M.; Burns, K.J.; Cicero, C.; Dunn, J.L.; Hernández-Baños, B.E.; Jiménez, R.A.; Johnson, O.; Kratter, A.W.; Mason, N.A.; et al. Checklist of North. American Birds; American Ornithologists’ Union: Chicago, IL, USA, 2024; Available online: https://checklist.americanornithology.org/taxa/ (accessed on 22 March 2025).
- Howell, S.N.G.; Webb, S. A Guide to the Birds of Mexico and Northern Central America; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- NOM-059-ECOL-2010; Protección ambiental-especies nativas de México de flora y fauna silvestres categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-lista de especies en riesgo. Secretaria de Medio Ambiente y Recursos Naturales: Mexico City, Mexico, 2010.
- IUCN (International Union for Conservation of Nature). Red List of Threatened Species-International Union for Conservation of Nature. 2024. Available online: https://www.iucnredlist.org/ (accessed on 29 September 2024).
- González-Salazar, C.; Martínez-Meyer, E.; López-Santiago, G. A hierarchical classification of trophic guilds for North America birds and mammals. Rev. Mex. Biodivers. 2014, 85, 931–941. [Google Scholar] [CrossRef]
- Kindt, R.; Coe, R. Tree Diversity Analysis. In A Manual and Software for Common Statistical Methods and Biodiversity Studies; World Agroforestry Centre: Nairobi, Kenya, 2005. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package, Version 2.5-7; Research Gate: Berlin, Germany, 2020; Available online: https://CRAN.R-project.org/package=vegan (accessed on 21 September 2024).
- Allaire, J.J. RStudio: Integrated Development Environment for R; Posif Software, PBC: Boston, MA, USA, 2023. [Google Scholar]
- Gelman, A.; Su, Y.-S. arm: Data Analysis Using Regression and Multilevel/Hierarchical Models. Available online: https://cran.r-project.org/package=arm (accessed on 6 March 2025).
- Arnold, T.W. Uninformative parameters and model selection using Akaike’s Information Criterion. J. Wildl. Manag. 2010, 74, 1175–1178. [Google Scholar]
- Harrison, X.A.; Donaldson, L.; Correa-Cano, M.E.; Evans, J.; Fisher, D.N.; Goodwin, C.E.D.; Robinson, B.S.; Hodgson, D.J.; Inger, R. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 2018, 6, e4794. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.r-project.org/ (accessed on 6 March 2025).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. Available online: https://github.com/lme4/lme4/; http://lme4.r-forge.r-project.org/ (accessed on 6 March 2025).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar]
- Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. 2025. Available online: https://cran.r-project.org/package=emmeans (accessed on 6 March 2025).
- Lüdecke, D. ggeffects: Tidy Data Frames of Marginal Effects from Regression Models. J. Open Source Softw. 2018, 3, 722. [Google Scholar]
- Wickham, H. ggplot. Elegant Graphics for Date Analysis; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Everitt, B.S.; Landau, S.; Leese, M.; Stahl, D. Cluster Analysis, 5th ed.; Wiley and Sons: Chichester, UK, 2011. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological sta tistic software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- McAleece, N.; Gage, J.D.G.; Lambshead, P.J.D.; Paterson, G.L.J. BioDiversity Professional Statistics Analysis Software; The Scottish Association for Marine Science and the Natural History Museum: London, UK, 1997. [Google Scholar]
- Squalli, W.; Mansouri, I.; Douini, I.; Achiban, H.; Fadil, F.; Dakki, M.; Wink, M. Diversity of avian species in peri-urban landscapes surrounding Fez in Morocco: Species richness, breeding populations, and evaluation of menacing factors. Diversity 2022, 14, 945. [Google Scholar] [CrossRef]
- Karjee, R.; Palei, H.S.; Konwar, A.; Gogoi, A.; Mishra, R.K. Bird Assemblages in a Peri-Urban Landscape in Eastern India. Birds 2022, 3, 383–401. [Google Scholar] [CrossRef]
- Leveau, L.M.; Ruggiero, A.; Matthews, T.J.; Bellocq, M.I. A global consistent positive effect of urban green area size on bird richness. Avian Res. 2019, 10, 30. [Google Scholar] [CrossRef]
- Rico-Silva, J.F.; Cruz-Trujillo, E.J.; Colorado, G.J. Influence of environmental factor son bird diversity in greenspaces in an Amazonia city. Urban Ecosyst. 2020, 24, 365–374. [Google Scholar] [CrossRef]
- Zhao, Z.; Borzée, A.; Li, J.; Chen, S.; Shi, H.; Zhang, Y. Urban bird community assembly mechanisms and driving factors in University campuses in Nanjing, China. Animals 2023, 13, 673. [Google Scholar] [CrossRef]
- Carbó-Ramírez, P.; Zuria, I. The value of small urban greenspaces for birds in a Mexican city. Landsc. Urban Plan. 2011, 100, 213–222. [Google Scholar] [CrossRef]
- Enedino, T.R.; Loures-Ribeiro, A.; Santos, B.A. Protecting biodi versity in urbanizing regions: The role of urban reserves for the conservation of Brazilian Atlantic Forest birds. Perspect. Ecol. Conserv. 2018, 16, 17–23. [Google Scholar] [CrossRef]
- Dale, S. Urban bird community composition influenced by size of urban green spaces, presence of native forest, and urbanization. Urban Ecosyst. 2018, 21, 1–14. [Google Scholar] [CrossRef]
- Nava-Díaz, R.; Pineda-López, R.; Dorantes-Euan, A. Drives of functional composition of bird assemblages in green spaces of a neotropical city: A case study from Merida, Mexico. Trop. Conserv Sci. 2020, 13, 1940082920923896. [Google Scholar] [CrossRef]
- Korányi, D.; Gallé, R.; Donkó, B.; Chamberlain, D.E.; Batáry, P. Urbanization does not affect greenspace bird species richness in a mid-sized city. Urban Ecosyst 2021, 24, 789–800. [Google Scholar] [CrossRef]
- Morelli, F.; Mikula, P.; Benedetti, Y.; Bussière, R.; Tryjanowski, P. Cemeteries support avian diversity likewise urban parks in European cities: Assessing taxonomic, evolutionary and functional diversity. Urban For. Urban Green. 2018, 36, 90–99. [Google Scholar] [CrossRef]
- Leveau, L.M.; Bocelli, M.L.; Quesada-Acuña, S.G.; González-Lagos, C.; Tapia, P.G.; Dri, G.F.; Delgado, V.C.A.; Garitano-Zavala, Á.; Campos, J.; Benedetti, Y.; et al. Bird diversity-environment relationships in urban parks and cemeteries of the Neotropics driving breeding and non-breeding seasons. PeerJ 2022, 10, e14496. [Google Scholar] [CrossRef]
- Shih, W.Y. Bird diversity of greenspace in the densely devel oped city centre of Taipei. Urban Ecosyst. 2018, 21, 379–393. [Google Scholar]
- Richardson, J.; Lees, A.C.; Miller, E.T.; Marsden, S.J. Avian diversity and function across the world´s most population cities. Ecol. Lett. 2023, 26, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Jambheker, R.; Suryawanshi, K.; Nagendra, H. Relationship between lake area and distance from the city centre on lake-dependent resident and migratory birds in urban Bangalore, a tropical mega-city in southern India. J. Urban Ecol. 2021, 7, juab028. [Google Scholar] [CrossRef]
- Basile, M.; Storch, I.; Mikusiński, G. Abundance, species richness and diversity of forest bird assemblages—The relative importance of habitat structures and landscape context. Ecol. Indic. 2021, 133, 108402. [Google Scholar] [CrossRef]
- Ayala-Pérez, V.; Arce, N.; Carmona, R. Distribución espacio-temporal de aves acuáticas invernantes en la Ciénega de Tláhuac, planicie lacustre de Chalco, México. Rev. Mex. Biodiv 2013, 84, 327–337. [Google Scholar] [CrossRef]
- Berumen-Solórzano, A.; Maimone, M.R.; Villordo, J.A.; Olivera, C.I.; González-Oreja, J.A. Cambios temporales de la avifauna acuática en el sitio Ramsar “Presa de Valsequillo”, Puebla, México. Huitzil 2017, 18, 202–211. [Google Scholar] [CrossRef]
- Blasio-Quintana, C.; Pineda-López, R. Diversidad de aves en ambientes antrópicos en una localidad del semidesierto del centro de México. Huitzil 2020, 21, e572. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, G.; Ma, H.; Wu, Y.; Zhang, W.; Zhang, Y.; Li, C.; de Boer, W.F. Bird communities‘ responses to human modified landscapes in the southern Anhui Mountainous Area. Avian Res. 2022, 13, 100006. [Google Scholar] [CrossRef]
- Zhai, Z.; Liu, S.; Li, Z.; Ma, R.; Ge, X.; Feng, H.; Shi, Y.; Gu, C. The spatiotemporal distribution patterns and impact factors of bird species richness: A case study of urban built-up areas in Beijing, China. Ecol. Ind. 2024, 169, 112847. [Google Scholar] [CrossRef]
- Ikin, K.; Knight, E.; Lindenmayer, D.B.; Fischer, J.; Manning, A.D. The influence of native versus exotic streetscape vegetation on the spatial distribution of bird in suburbs and reserves. Divers. Distrib. 2013, 19, 294–306. [Google Scholar] [CrossRef]
- Tu, H.M.; Fan, M.W.; Ko, J.C.-J. Different habitat types affect bird richness and evenness. Sci. Rep. 2020, 10, 1221. [Google Scholar] [CrossRef]
- Kang, W.; Minor, E.S.; Park, C.-R.; Lee, D. Effects of habitat structure, human disturbance, and habitat connectivity on urban forest bird communities. Urban Ecosyst. 2015, 18, 857–870. [Google Scholar] [CrossRef]
- Cayetano-Rosas, H.; Ramírez, M.; Gómez-Garduño, J.O.; Piñón, N.; Bautista-Trejo, R.; Valverde, J.G.; Ramírez-Albores, J.E. Composición espacial y temporal de aves acuáticas y rapaces en humedales del centro de México. Cuad. de Investig. UNED 2023, 23, e4382. [Google Scholar] [CrossRef]
- Dvořáková, D.; Šipoš, J.; Suchomel, J. Impact of agricultural landscape structure on the patterns of bird species diversity at a regional scale. Avian Res. 2023, 14, 100147. [Google Scholar] [CrossRef]
- Pescador, M.; Díaz, S.; Peris, S. Abundances of waterbird species on lakes in Argentine Patagonia as a function of season, lake size and the presence of mink. Hydrobiologia 2012, 697, 111. [Google Scholar] [CrossRef]
- Xu, W.; Yu, J.; Huang, P.; Zheng, D.; Lin, Y.; Huang, Z.; Zhao, Y.; Dong, J.; Zhu, Z.; Fu, W. Relationship between Vegetation Habitats and Bird Communities in Urban Mountain Parks. Animals 2022, 12, 2470. [Google Scholar] [CrossRef]
- Alvarez-Alvarez, E.A.; Almazán-Núñez, R.C.; Corcuera, P.; González-García, F.; Brito-Millán, M.; Alvarado-Castro, V.M. Land use cover changes the bird distribution and functional groups at the local and landscape level in a Mexican shaded-coffee agroforestry system. Agric. Ecosyst. Environ. 2022, 330, 107882. [Google Scholar] [CrossRef]
- Mugatha, S.M.; Ogutu, J.O.; Piepho, H.-P.; Maitima, J.M. Bird species richness and diversity responses to land use change in the Lake Victoria Basin, Kenya. Sci. Rep. 2024, 14, 1711. [Google Scholar] [CrossRef]
- Ortega-Álvarez, R.; MacGregor-Fors, I. Living in the big city: Effects of urban land-use on bird community structure, diversity, and composition. Landsc. Urban Plan. 2009, 90, 189–195. [Google Scholar] [CrossRef]
- Muñoz-Sáez, A.; Pérez-Quezada, J.F.; Estades, C.F. Agricultural landscapes as hábitat for birds in central Chile. Rev. Chil. Hist. Nat. 2017, 90, 3. [Google Scholar] [CrossRef]
- Robinson RA, Wilson JD, Crick HQP The importance of arable habitats for farmland birds in grassland landscapes. J. Appl. Ecol. 2001, 38, 1059–1069.
- Thompson, R.; Tamayo, M.; Singurŏsson, S. Urban bird diver sity: Does abundance and richness vary unexpectedly with green space attributes? J. Urban Ecol. 2022, 8, 017. [Google Scholar]
- MacGregor-Fors, I.; Morales-Pérez, L.; Schondube, J.E. Does size really matter? Species-area relationships in human settlements. Divers. Distrib. 2011, 17, 112–121. [Google Scholar] [CrossRef]
- MacGregor-Fors, I.; Ortega-Álvarez, R. Fading from the forest: Bird community shifts related to urban park site-specific and landscape traits. Urban For. Urban Green 2011, 10, 239–243. [Google Scholar] [CrossRef]
- Pal, M.; Pop, P.; Mahapatra, A.; Bhagat, R.; Hore, U. Diversity and structure of bird assemblages along urban-rural gradient in Kolkata, India. Urban For. Urban Green 2019, 38, 84–96. [Google Scholar] [CrossRef]
- Oliver, A.J.; Hong-Wa, C.; Devonshire, J.; Olea, K.R.; Rivas, G.F.; Gahl, M.K. Avifauna richness enhanced in large, isolated urban parks. Landsc. Urban Plan. 2011, 102, 215–225. [Google Scholar] [CrossRef]
- Barbosa, K.V.C.; Rodewald, A.D.; Ribeiro, M.C.; Jahn, A.E. Noise level and water distance drive resident and migratory bird species richness within a Neotropical megacity. Landsc. Urban Plan. 2020, 197, 103769. [Google Scholar] [CrossRef]
- da Silva, B.F.; Pena, J.C.; Viana-Junior, A.B.; Vergne, M.; Pizo, M.A. Noise and tree species richness modulate the bird community inhabiting small public urban green spaces of a Neotropical city. Urban Ecosyst. 2020, 24, 71–81. [Google Scholar] [CrossRef]
- Matthies, S.A.; Rüter, S.; Schearschmidt, F.; Prasse, R. Determi nants of species richness within and across taxonomic groups in urban green spaces. Urban Ecosyst. 2017, 20, 897–909. [Google Scholar] [CrossRef]
- Holtmann, L.; Philipp, K.; Becke, C.; Fartmann, T. Effects of habitat and landscape quality on amphibian assemblages of urban stormwater ponds. Urban Ecosyst. 2017, 20, 1249–1259. [Google Scholar] [CrossRef]
- Dyson, K. Conserving native trees increases native bird diversity and community composition on commercial office developments. J. Urban Ecol. 2020, 6, juaa033. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).