Abstract
Animals use specific behaviors and skills to overcome challenges and access resources. Environmental enrichment is provided to animals in human care to both promote species-appropriate behaviors and reduce undesired behaviors. Feather pecking in birds is an undesired behavior without a clear cause. The Saint Louis Zoo houses three pairs of young Bali mynas (Leucopsar rothschildi) who pluck neck feathers from conspecifics. To reduce this behavior, animal care staff presented the birds with seven enrichment items from four categories, presenting each item twice. The enrichment included a modifiable, progressively challenging bamboo tube device at multiple levels of difficulty. While plucking was not affected by any enrichment item, we observed significant increases in locomotion and decreases in autopreening, allogrooming, and head bobbing. Leafy greens produced the greatest changes when compared to other enrichment types. Overall engagement with the progressively challenging enrichment increased with the change from the first to the second level of difficulty, and interaction with the device was highest for the third and most difficult version. These increases suggest that no habituation to the progressively challenging device occurred, while a possible neophobic effect declined with multiple uses and increased familiarity.
1. Introduction
Animals in the wild are constantly presented with dynamic landscapes filled with challenges [1]. Surviving these environments relies on using specific behaviors and skills to garner rewards, i.e., food, shelter, and access to mates [2]. In human care, when presented with the option to effortlessly obtain free resources or to instead use skills and work for a reduced resource gain, livestock, non-human primates, giraffes, and carnivores have been observed performing the latter, suggesting that attempting challenges may be inherently rewarding. Langbein [3] observed that goats (Capra hircus) will work to solve a cognitive task awarding water when they could receive a similar or greater quantity from a simpler device. Grizzly bears (Ursus arctos horribilis) were noted by McGowan [4] to spend time uncovering and interacting with concealed food, even when free food was available. In addition, concealed food once uncovered was not always devoured, which suggested another motivation besides resource gain alone. Sasson-Yenor and Powell [5] found that all giraffes (Giraffa camelopardalis) in their study exhibited contrafreeloading behavior to obtain grain, though individuals varied in their propensity to engage in contrafreeloading. Animals bereft of opportunities to use skills may perform fewer species-appropriate behaviors, manifest undesired and stereotypic behaviors, and otherwise display signs of poor welfare [6].
Habitats for animals in human care are relatively static, with routine, predictable diets fed at scheduled times. Human caretakers must assume the responsibility of providing opportunities for animals to use their skills [7]. Furnishing animals with environmental enrichment can increase species-appropriate behaviors, reduce unwanted behaviors [8,9,10,11,12], and otherwise improve well-being [13].
The Association for Zoos and Aquariums (AZA) requires member zoological facilities to have documented environmental enrichment programs. Their exact implementation is decided within individual facilities [14], but AZA guidelines recommend that the use of enrichment should be behavior-based, incorporate goal planning, and have ways to measure successful implementation [15]. Common enrichment goals are to change behavioral budgets by increasing species-appropriate behaviors and decreasing undesired behaviors that can be indicative of poor welfare [16,17]. Enrichment items in the programs are usually grouped into categories, though these vary between facilities. Common categories include sensory, food-based, manipulative, cognitive, structural, and social enrichment [18], with exact definitions, items, and implementation dependent on the facility.
Cognitive enrichment methods requiring problem solving can be mastered over time, resulting in less engagement during subsequent encounters. However, if cognitive enrichment is too difficult relative to the skills of the animal to which it is introduced, then the recipient can become frustrated; if it is too simple, they become bored or habituated [19,20,21]. Adding new enrichment can combat this problem of habituation, so novel items are introduced as part of most enrichment programs. Novelty elicits an acute response in animals, whether investigatory or aversive [22,23,24], though frequent reintroductions of a given item results again in habituation [25,26,27]. While novel items can drive increased activity, especially exploration and foraging, neophobic individuals prefer consistency and familiar enrichment; this aversion manifests in moving away from and creating distance from novel objects, more abnormal behaviors, and comparatively lower interaction with new items [28].
To maintain engagement while minimizing both habituation and possible aversive neophobic behaviors, we propose the use of progressively challenging enrichment; that is, an initially simple but easily modified piece of enrichment that is made subsequently more difficult upon continued uses in order to increase specific skill mastery and maintain engagement while keeping the object familiar enough to accommodate neophobic individuals. The Saint Louis Zoo has previously implemented this paradigm using PVC pipes of different lengths to stimulate species-appropriate foraging and feeding behaviors in sloth bears (Melursus ursinus) [29], and by offering swamp monkeys (Allenopithecus nigroviridis) vertically hanging enrichment tubes pierced with an increasing number of rods that needed to be removed to gain access to food rewards [30]. In the case of the swamp monkeys, we noticed that total engagement with any difficulty level dropped after two uses but was renewed when difficulty—as measured according to the number of rods used and the opacity of the tube—was increased.
Carnivores and primates are the most well-represented groups in publications of environmental enrichment [31,32,33,34,35,36,37]. Studies on the class Aves focus on food-based enrichment [38] and on larger or well-known birds, such as hornbills and psittacines [39,40,41,42,43]. In this paper, we instead examined a critically endangered species whose behavior has not been well studied due to their small population numbers. Endemic to the Indonesian island Bali, Bali mynas (Leucopsar rothschildi) face many threats, including overhunting, lack of government protection, and the exotic wildlife trade. Part of the family Sturnidae, the Bali myna shares numerous characteristics with the starlings from which this family gets its name [44,45]; in particular, Bali mynas are frugivorous and insectivorous, gleaning from foliage or the ground, like other mynas and starlings [46]. A social bird, they prefer to live in large groups in grasslands and open forests. During mating season, Bali mynas produce a high-pitched call to attract mates, and accompany this action with a distinct up and down “head bobbing” motion.
Saint Louis Zoo bird keeper staff noticed alloplucking in their pairs of Bali mynas that went beyond normal preening and left bare patches on the necks of both males and females. Feather pecking (FP) in birds describes self- or conspecific-initiated undesirable behaviors that result in the removal (and sometimes consumption) of feathers, as well as occasional injuries [47]. Excessive feather pecking is well documented between conspecifics in domestic chickens and as self-inflicted injury in psittacines; these behaviors have caused skin tears, tissue damage, and hypothermia due to loss of insulation [48,49], as well as other physiological effects beyond the removal of feathers, such as increased corticosterone levels [50]. The causes of FP are not well understood, though some possibilities include a genetic origin [51], diet [52], and the consequences of human raising [53]. Environmental enrichment has been shown to cause a statistically significant (but mathematically small) reduction in this behavior [54,55]. Saint Louis Zoo’s keepers wanted to investigate whether offering certain categories of environmental enrichment would decrease this behavior in Bali mynas.
Herein, we have described the responses of six Bali mynas to a variety of enrichment items in order to measure their reaction to enrichment and any differences between categories, especially in regard to the goal of reducing conspecific plucking. In addition, a progressively challenging enrichment (PCE) device was developed and introduced to the birds at three levels of challenge to determine whether subsequent introductions of this otherwise novel device resulted in changes in engagement. The main function of PCE was to hold consistent engagement between difficulty levels. Within each difficulty level, we expected relatively higher engagement with the PCE device during first presentations, followed by decreased interaction when presented additional times at that difficulty. Increasing the difficulty after a few uses will hopefully reengage the birds and maintain engagement with the item over a longer period.
2. Materials and Methods
2.1. Study Animals and Habitats
Three 1.1 pairs of sibling Bali mynas between 0.75 and 1 year old residing in an indoor off-display area of the Saint Louis Zoo Bird House were filmed using a Nite Owl 4-channel 1080p DVR system between April and June 2019. Individual birds were identified by colored metal leg bands. Each pair was housed in separate mesh-walled habitats in a room kept at 23.9 °C. Habitats were arranged in a row such that each shared at least one wall with another. One pair was adjacent to habitats containing Guam kingfishers (Todiramphus cinnamominus) and a solitary golden white-eye (Cleptornis marchei). Birds could see and hear other birds in the room, as well as interact with objects in adjacent habitats that were near the mesh walls. All three habitats were identical in size, measuring 0.91 m wide × 3.05 m long × 1.91 m high. The front left corner of each was furnished with a 0.23 m × 0.43 m wire platform set 1.07 m from the floor, on which a shallow food dish and other small items could be placed. At least three habitat-length branches (intended for perching) were anchored across two walls, though the exact number and placement of branches varied. Each habitat had one Nite Owl camera mounted in a front upper corner, positioned such that birds could not perch on it, and at an angle that could record the wire platform and all branches but not the habitat floor. Cameras were introduced two weeks before data collection to habituate birds to their presence. Video recording was programmed to occur daily from 04:00 to 20:00.
Three keepers were assigned to this room, but only one would work the myna habitats per day. Morning husbandry consisted of visual assessment and removal of old food starting at 08:00, followed by feeding between 09:30 and 10:30, depending on day. Daily diets for each pair consisted of 1/2 cup of Mazuri Soft Bill Diet pellets, 1/2 cup of fruit mix (green peas, pigeon peas, apple, pear, blueberries, carrots, corn, beets), 10 mealworms, and a dusting of Reptivite and calcium carbonate, served in a shallow dish placed on the feeding platform. All enrichment was placed no later than 11:00, and any food-based enrichment was given in addition to the diet above. Habitats were spot cleaned at least once per week.
2.2. Enrichment and Filming
Birds were filmed from 25 April to 19 June 2019. Only videos from days in which cameras functioned for all study pairs were considered for analysis. Keepers at the Saint Louis Zoo offered enrichment from a premade calendar of assigned categories, though the specific item given from the category varied at the keepers’ discretion. Fourteen unique modes of enrichment across 5 categories were offered. Days with sensory-only enrichment (sound playback, misting, etc.) were not used for analysis due to how interaction with enrichment was measured for this study, as there were no physical items in this category with which the birds could physically interact. Similarly, we excluded sensory, taste-based food items (e.g., reconstituted nectar) that were mixed with the animals’ diet, because interactions with these pieces could not be differentiated from eating their regular diet. Only enrichment items that were offered at least twice were included in data analysis, and only the first two uses of any enrichment were used for analysis. Following these criteria, only 7 items were considered for analysis. Three of these were difficulty levels for a PCE device. Items were reclassified from their original keeper designations to criteria described in Table 1.

Table 1.
Final enrichment category descriptions.
The PCE item was a suet feeder set on its side, such that four bamboo tubes of lengths within +/−0.32 cm of each other could be attached at the four corners via a notch in the bamboo. Just above the notch in each bamboo tube, a wax worm was placed on a solid interior node to stimulate the natural gleaning behavior of the mynas. Difficulty was represented by the length of the bamboo and the presence of a substrate (Table 2). After the PCE item was offered three times at a particular difficulty, the keepers increased the difficulty on its next use (Figure 1).

Table 2.
Progressively challenging enrichment item difficulty levels.
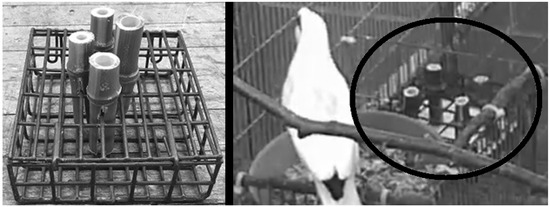
Figure 1.
Examples of PCE item. Left: Prototype model, showing tubes in center cut to different lengths. Widths were standardized before use in project. Right: Footage from this project featuring PCE item (circled in black) placed on feeding platform. Tubes in corners are cut to length for difficulty level 2.
Exact placement of the enrichment items varied between habitats and days. Hanging items were generally placed close to the middle of the habitat, near the perches. Items without hanging apparatuses were often placed on the feeding shelf, within 15 cm of the food dish. Items attached via other means (e.g., skewers) were attached directly to the perches, mesh walls, and the mesh ceiling. Final selections for items and the dates used for analysis are both described in Table 3.

Table 3.
Enrichment items selected for analysis with dates filmed.
This project was reviewed and approved internally by the Saint Louis Zoo’s Research Committee. Saint Louis Zoo animals receive environmental enrichment as part of their standard care. Aside from the PCE device, all items given to the animals had been used prior to this project, and the PCE device was reviewed and approved by animal keeper staff. There were no other changes in management.
2.3. Behaviors
Only those periods 30 min before (“pre-” period) and after (“post-” period) the placement of daily enrichment were observed, in order to gauge mynas’ acute reactions. An ethogram was developed following input from Saint Louis Zoo bird keepers and initial observations outside of this study. Data were collected using 1-0 time interval sampling (with 30 s intervals), as well as continuous sampling [56] exclusively for the pluck event behavior (Table 4). All observers were tested for reliability by scoring behaviors from an example Bali myna video that was otherwise not used in the study. Answers were compared against a key derived by consensus from the responses of this study’s primary author and three trained volunteers. For potential observers to receive permission to collect data, agreement between their scores and the consensus key had to be 80% or higher.

Table 4.
Ethogram of behaviors sampled in Bali mynas.
2.4. Statistical Analysis
All analysis was performed using NCSS 2020 Statistical Software, version 20.0.3. All behaviors scored through 1-0 sampling were converted into daily proportions based on the number of non-out-of-sight scans in which they were scored, and then divided into pre-enrichment addition and post-enrichment addition periods. The behavior pluck was converted into an hourly rate.
Multiple measurements were taken on the same subjects. Each subject had the same number of measurements taken at the same times, requiring repeated-measures analysis, which was performed using linear mixed models. Tests for normality were performed on all behavioral responses using Martinez–Iglewicz and D’Agostino Skewness methods, but were rejected for autopreen, allogroom, and pluck rate data, and all data except for locomotion were right-skewed. Behavior values for all responses were subsequently log-transformed with formula [log(x + 1)] to establish normality. In addition, Schielzeth et al. [57] and Arnau et al. [58] have suggested that mixed model analyses are robust against violations of assumptions, including skewness. Factors for enrichment, either by category or item, had unequal variances.
For all statistical tests, we used bird ID as the subject factor and log-transformed proportions of behaviors as the response variables. Time was a fixed factor, and the relationship between time and the response variables was not linear. Preliminary model fitting was performed by comparing AIC and adjusting factors until lowest value could be attained. This procedure revealed that sex was never a significant factor and worsened models’ fits, so it was removed from further testing. Based on initial research objectives and hypotheses, three final models were constructed, as described below. For all tests, α = 0.05.
To determine how behaviors changed before and after enrichment introduction, we used the observation period (pre- or post-enrichment presentation) as a fixed within factor. Because the time range for when food and enrichment were placed could, but did not always, overlap (with no specific pattern), food was not always in the habitat in the pre- period. Feeding and enrichment interaction were therefore not part of this test, because they could not be scored before the enrichment was added. A diagonal pattern with random effects (random subjects term) was used for the within-subject variance-covariance matrix, except for head bob data, where analysis resulted in a variance component estimate that was equal to zero. To complete the analysis of head bob data, a diagonal pattern without random effects was used instead for the within-subject variance-covariance matrix.
To test for differences in behaviors based on the enrichment category (manipulative, food-based, greens, PCE), we only used data from after the enrichment was added (post-observation period), since animals cannot be influenced by enrichment until it is present. Enrichment category was a fixed within factor modelled with unequal variances. A diagonal pattern with random effects (random subjects term) was again used for the within-subject variance-covariance matrix, except for feeding and head bob data; in these cases, analysis resulted in a variance component estimate that was equal to zero for the former and negative for the latter. To complete analysis for these two responses, a diagonal pattern without random effects was used for the within-subject variance-covariance matrix instead.
We tested the effect of specific items and first or second introduction with a 2-way test using the fixed within factors of enrichment item (bells, food in hanging cups, greens, the hollow rubber dog toy “holey moley” sphere, and puzzle levels 1, 2, and 3) and the round of use for that item (first or second). The enrichment item factor was modelled with unequal variances. A diagonal pattern with random effects (random subjects term) was used for the within-subject variance-covariance matrix for all responses.
3. Results
Pre-/post- tests revealed that there were significant behavioral differences before and after enrichment was introduced for locomotion (F1,161 = 4.82, p = 0.030), autopreen (F1,161 = 8.57, p = 0.004), allogroom (F1,161 = 5.82, p = 0.0017), and head bob (F1,166 = 18.62, p < 0.001), but not for pluck. Post hoc tests with Bonferroni correction revealed that the direction of the difference varied based on behavior. Locomotion was higher in the post- period, but autopreen, allogroom, and head bob were scored at a higher proportion in the pre- period (Figure 2).
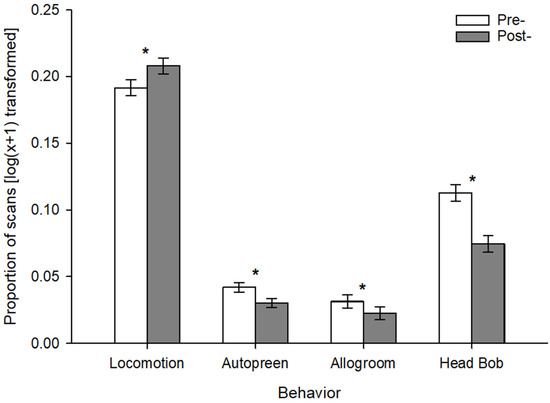
Figure 2.
Comparison between proportions of behaviors scored before and after (pre- and post-, respectively) addition of enrichment. Asterisks indicate significant differences between pre- and post- periods for that behavior.
Tests of post- period data for each enrichment category (greens, manipulative, food-based, PCE) revealed differences in behavior for locomotion (F3,31.3 = 52.40, p < 0.001), enrichment interaction (F3,26.9 = 4.71, p = 0.009), feeding (F3,33.4 = 17.64, p < 0.001), autopreen (F3,30.2 = 10.49, p < 0.001), and head bob (F3,75 = 19.77, p < 0.001). Post hoc tests with a Bonferroni correction revealed that the direction of the difference varied depending on the behavior examined (Figure 3). Days with greens saw significantly more locomotion and enrichment interaction but significantly less autopreening, head bobbing, and feeding on the regular diet in the food dish. The use of the progressively challenging enrichment resulted in significantly more locomotion than when the manipulative and food-based enrichments were offered, but less than when greens were used. Similarly, the proportion of head bobbing behaviors seen when using PCE was significantly higher than with greens, but manipulative enrichment saw the largest amount of head bobbing, followed by food-based enrichment. Manipulative and food-based enrichments had no other significant differences in behavioral results between each other. There were no significant differences across enrichment categories for either the allogrooming or plucking behaviors.
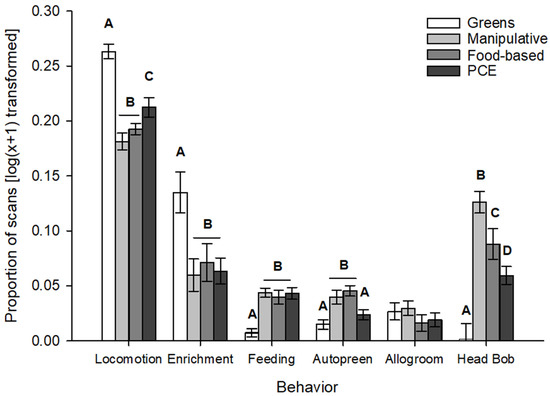
Figure 3.
Differences in behaviors based on enrichment category in post-enrichment addition periods. Letters indicate groupings of categories with significantly different responses for the listed behavior.
Tests based on specific items (bells, cups, greens, “holey moley”, puzzle levels 1–3) revealed significant differences (Figure 4) for the behaviors of locomotion (F6,21.9 = 32.27, p < 0.001), enrichment interaction (F6,65 = 19.63, p < 0.001), feeding (F6,23.9 = 16.44, p < 0.001), autopreen (F6,24 = 8.02, p < 0.001), allogroom (F6,24 = 2.60, p = 0.044), and head bob (F6,23.4 = 114.18, p < 0.001), but no differences were found for pluck. Differences based on presentation number (regardless of item used) were only significant for the behaviors of enrichment interaction (F1,65 = 13.88, p < 0.001) and head bob (F1,36.4 = 28.00, p < 0.001), with more head bobs observed during the first presentation of any enrichment item, and more enrichment interactions observed during second use of any enrichment item. Pluck behaviors never showed any significant differences in response between factors.
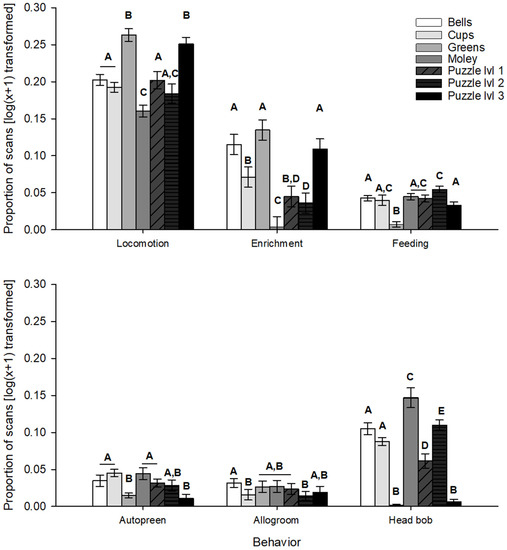
Figure 4.
Differences in behaviors based on enrichment item in post-enrichment addition periods. Letters indicate groupings of categories with significantly different responses for the listed behavior.
The enrichment item and round number (first, second) interaction was significant for enrichment interaction (F6,65 = 5.218, p < 0.001), feeding (F6,23.9 = 20.50, p < 0.001), autopreen (F6,24 = 5.157, p = 0.002), and head bob (F6,23.4 = 14.80, p < 0.001), but not for pluck. Figure 5 shows the results of post hoc tests of this interaction.
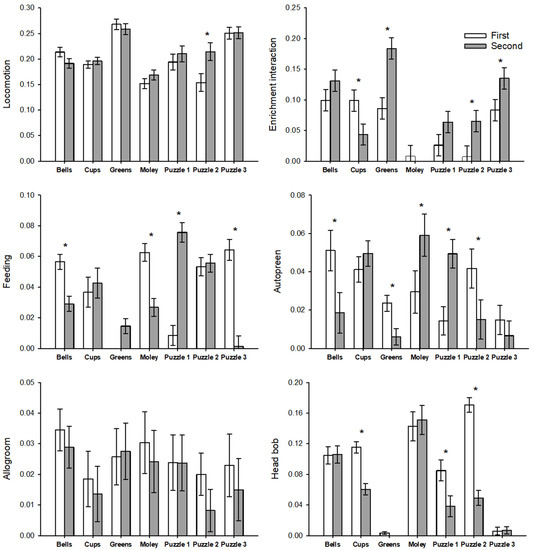
Figure 5.
Differences in behaviors based on the interaction effect of a given enrichment item and the presentation round of that item in the post-enrichment addition period. Behaviors are listed on the y-axis, with units of proportion of scans [log(x + 1) transformed]. Asterisks indicate significant differences between the first and second presentation of that item.
4. Discussion
Neither enrichment category or specific item offered nor number of times an item was used influenced pluck rate; consequently, the goal of pluck reduction was unmet. What motivates FP behavior is currently not well studied in mynas. Although thought to be a redirection of food-related pecking, a recent exploration of FP in domestic fowl showed inconsistency in the link between feather pecking, activity rates, and different types of foraging material [59]. This result complicates prior findings that feather pecking is compensating for natural foraging behavior. Further studies have examined environmental stresses, and Shi [60] found reduced levels of feather pecking in hens during the laying period when treated with red-colored and lower-intensity lights. Schwarzer [61] described a positive correlation between bird density and FP, in addition to reduced FP in birds with access to free-range areas. The Saint Louis Zoo mynas were kept in pairs at densities much lower than those described by Schwarzer. While all Saint Louis mynas performed behaviors that met the criteria for FP, we never observed that feathers plucked from conspecifics were ingested or used as nesting material. Further research would help provide insight into these undesirable behaviors.
Acute responses to enrichment were observed in the pre-/post- test of other behaviors, characterized by significantly more locomotion and less grooming and head bob behaviors. Birds clearly focused attention on enrichment and did not spend as much time on social and self-maintenance behaviors. Increases in activity that included locomotion and foraging as acute responses to added enrichment items have been seen in other birds, including rheas (Rhea Americana) [62], and psittacines [63,64]. These changes were interpreted positively and suggested that use of enrichment was beneficial for these animals. Miglioli’s study showed that blue-and-yellow macaws (Ara ararauna) interacted with food-based enrichment more often than with just physical objects, such as additional perches, bamboo, pine cones, cardboard boxes, and similar items without food. While we found no difference in enrichment interaction between our food-based and manipulative categories, our results showed that behaviors on days when greens were offered were significantly different from days with enrichment from other categories. When greens were presented to mynas, we recorded more locomotion and enrichment interaction, but less autopreening, head bobs, and feeding on their usual diet from the bowl. PCE was different from food-based and manipulative enrichment categories, with PCE presentations characterized by more locomotion and autopreening, but less head bobbing. Enrichment items were usually placed singly, whereas several green leaves were placed around the habitat. These greens were easily manipulated, and birds were observed pulling, tearing, and throwing the leaves to the ground, but none of them appeared to ingest leaves. Bali mynas are arboreal insectivores who glean for insects, searching under leaves for caterpillars and similar food items [65,66]. The greens may have stimulated foraging by best simulating opportunities that these birds’ wild counterparts might experience, allowing mynas to perform species-appropriate behaviors, and driving interaction and movement in the habitat.
Autopreening, allogrooming, and head bob occurred less often once enrichment was introduced, but there were differences between them in the post enrichment period. Allogrooming post-enrichment introduction was the same for all enrichment categories, while autopreening, though lower than in the pre- period, was higher post-addition of the manipulative and food-based enrichments than it was with greens and PCE. Similarly, head bobs were most often observed when manipulative and food-based enrichments were offered, but seen at lower proportions for PCE, and at the lowest proportions for greens. These two categories, greens and PCE, elicited stronger locomotion responses; in particular, birds separated to fly toward individual green leaves and spent more time apart.
Although we expected more engagement during the first presentation of each PCE level and less in subsequent ones, we have found the observed opposite pattern of less engagement at first to be compelling, and ultimately considered the PCE item to be a qualified success for two reasons. First, the PCE was interacted with no less frequently than the other types of enrichment offered—that is, it was at least as engaging as the other items. Second, PCE use increased between the first and second presentations within difficulty levels and increased overall from first to third difficulty levels; these changes suggested, as we had hoped, that animals did not habituate to the PCE, especially as more difficulty was added. For all levels of PCE difficulty, the first presentation had lower enrichment interaction than the second presentation, and the third level of difficulty of this device was interacted with significantly more than the first two levels. This increasing engagement could represent an initial, transient neophobia from the birds towards this unique item designed for the study; all other enrichment items had been previously offered. Neophobic animals take longer to approach new objects, and interact less frequently with them [22], but increased familiarity can increase use. A study of neophobia in corvids [67] found that subjects approached novel objects more quickly after multiple rounds of presentations. Our original prediction was based on work conducted on two mammal species, but a more recent examination of Bali myna neophobia [68] found that these birds were slower to respond to novel objects or novel food, and that interactions with novel objects (as measured by pecking frequency) were also reduced compared to familiar enrichments. The Miller corvid study also posited that subjects were slower to approach items that did not obviously contain food. While we did not measure latency to approach, enrichment interaction in our study for the PCE category was no different than it was for manipulative and food-based enrichments. It is possible that the mealworms in our PCE items were not apparent in initial introductions, resulting in less interest from the mynas at first. In addition to building skills over time, the fact that PCE is a modified version of one style of enrichment that is made iteratively more difficult (instead of the introduction of many completely new devices) may also help neophobic species. In particular, the repeated nature of PCE seems to, in this case, have alleviated some of this neophobia, particularly by the third difficulty level, though admittedly the “magic number” of presentations required to balance against neophobia and habituation is unknown. A study of how the presentation of two types of increasingly complex enrichment devices affected enrichment use and stereotypic behavior in sun bears (Helarctos malayanus) had mixed results [69]. While giving either device to the bears decreased stereotypic behavior compared to the baseline, one item’s subsequent introductions with increased complexity continued to lower the mean duration of both stereotypic behavior and enrichment use; the opposite was true for the other item. We recommend additional investigations using other styles of PCE and across different taxa to better appreciate this style of modifiable enrichment device.
5. Conclusions
- ⮚
- Bali mynas had an acute reaction to the addition of environmental enrichment to their habitats, characterized by interactions with the enrichment, as well as significant differences in locomotion, and a reduction in autopreening, allogrooming, and head bobbing. Partner plucking, however, was unaffected by enrichment.
- ⮚
- Different items elicited stronger or weaker responses, with the offerings of naturalistic greens having reactions that were the most different from the other enrichment types. Greens strongly stimulated locomotion and interaction with the enrichment, reminiscent of natural foliage gleaning behavior.
- ⮚
- Engagement with progressively challenging enrichment increased with each successive presentation of a given difficulty level and increased significantly between the first and third difficulty level, whereas interaction decreased or saw no change during the second presentation of familiar manipulative and food-based enrichment items (e.g., cups, balls, “holey moley”).
Author Contributions
Conceptualization, E.B. and M.E.; Formal analysis, E.B.; Investigation, E.B.; Methodology, E.B.; Project administration, D.M.P.; Resources, M.E.; Supervision, D.M.P.; Visualization, E.B.; Writing—original draft, E.B. and A.P.; Writing—review and editing, E.B., A.P., M.E. and D.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data are available upon request to corresponding author.
Acknowledgments
The authors wish to thank the entire Bird House keeper staff at the Saint Louis Zoo. In addition, we recognize, with gratitude, the efforts of Sarah Slayton, who performed the initial review of the videos before data collection, as well as the team of animal behavior research interns who scored and recorded the data. Credit is also due to both Ashley Franklin and Ashley Edes for their comments on portions of this manuscript. JoEllen Toler provided the Bali myna photograph for the graphical abstract.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Farina, A.; Belgrano, A. The eco-field hypothesis: Toward a cognitive landscape. Landsc. Ecol. 2006, 21, 5–17. [Google Scholar] [CrossRef]
- Špinka, M.; Wemelsfelder, F. Environmental challenge and animal agency. In Animal Welfare, 3rd ed.; Appleby, M.C., Olsson, I.A.S., Galindo, F., Eds.; CAB International: Wallingford, UK, 2011; pp. 39–55. [Google Scholar] [CrossRef]
- Langbein, J.; Siebert, K.; Nürnberg, G. On the use of an automated learning device by group-housed dwarf goats: Do goats seek cognitive challenges? Appl. Anim. Behav. Sci. 2009, 120, 150–158. [Google Scholar] [CrossRef]
- McGowan, R.T.S.; Robbins, C.T.; Alldredge, J.R.; Newberry, R.C. Contrafreeloading in grizzly bears: Implications for captive foraging enrichment. Zoo Biol. 2009, 29, 484–502. [Google Scholar] [CrossRef] [PubMed]
- Sasson-Yenor, J.; Powell, D.M. Assessment of contrafreeloading preferences in giraffe (Giraffa Camelopardalis). Zoo Biol. 2019, 38, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Mason, G.; Clubb, R.; Latham, N.; Vickery, S. Why and how should we use environmental enrichment to tackle stereotypic behaviour? Appl. Anim. Behav. Sci. 2007, 102, 163–188. [Google Scholar] [CrossRef]
- Meehan, C.L.; Garner, J.P.; Mench, J.A. Environmental enrichment and development of cage stereotypy in orange-winged Amazon parrots (Amazona amazonica). Dev. Psychobiol. 2004, 44, 209–218. [Google Scholar] [CrossRef]
- Clegg, I.L.K.; Domingues, M.; Ström, E.; Berggren, L. Cognitive foraging enrichment (but not non-cognitive enrichment) improved several longer-term welfare indicators in bottlenose dolphins. Animals 2023, 13, 238. [Google Scholar] [CrossRef]
- Gronqvist, G.; Kingston-Jones, M.; May, A.; Lehmann, J. The effects of three types of environmental enrichment on the behaviour of captive Javan gibbons (Hylobates moloch). Appl. Anim. Behav. Sci. 2013, 147, 214–223. [Google Scholar] [CrossRef]
- Hamilton, J.; Fuller, G.; Allard, S. Evaluation of the impact of behavioral opportunities on four zoo-housed aardvarks (Orycteropus afer). Animals 2020, 10, 1433. [Google Scholar] [CrossRef]
- Shepherdson, D.J. Environmental enrichment: Past, present and future. Int. Zoo Yearb. 2003, 38, 118–124. [Google Scholar] [CrossRef]
- Swaisgood, R.R.; Shepherdson, D.J. Scientific approaches to enrichment and stereotypies in zoo animals: What’s been done and where should we go next? Zoo Biol. 2005, 24, 499–518. [Google Scholar] [CrossRef]
- Douglas, C.; Bateson, M.; Walsh, C.; Bédué, A.; Edwards, S.A. Environmental enrichment induces optimistic cognitive biases in pigs. Appl. Anim. Behav. Sci. 2012, 139, 65–73. [Google Scholar] [CrossRef]
- Rodríguez-López, R. Environmental enrichment for parrot species: Are we squawking up the wrong tree? Appl. Anim. Behav. Sci. 2016, 180, 1–10. [Google Scholar] [CrossRef]
- Association of Zoos and Aquariums Accreditation Standards & Related Policies 2023. Available online: https://assets.speakcdn.com/assets/2332/aza-accreditation-standards.pdf (accessed on 13 January 2023).
- Grimberg-Henrici, C.G.E.; Vermaak, P.; Bolhuis, J.E.; Nordquist, R.E.; van der Staay, F.J. Effects of environmental enrichment on cognitive performance of pigs in a spatial holeboard discrimination task. Anim. Cogn. 2016, 19, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Newberry, R.C. Environmental enrichment: Increasing the biological relevance of captive environments. Appl. Anim. Behav. Sci. 1995, 44, 229–243. [Google Scholar] [CrossRef]
- Hoy, J.M.; Murray, P.J.; Tribe, A. Thirty years later: Enrichment practices for captive mammals. Zoo Biol. 2009, 29, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Burn, C.C. Bestial boredom: A biological perspective on animal boredom and suggestions for its scientific investigation. Anim. Behav. 2017, 130, 141–151. [Google Scholar] [CrossRef]
- Clark, F.E.; Smith, L.J. Effect of a cognitive challenge device containing food and non-food rewards on chimpanzee well-being: Chimpanzee cognitive challenge. Am. J. Primatol. 2013, 75, 807–816. [Google Scholar] [CrossRef]
- Meagher, R.K.; Mason, G.J. Environmental enrichment reduces signs of boredom in caged mink. PLoS ONE 2012, 7, e49180. [Google Scholar] [CrossRef]
- Greenberg, R.; Mettke-hofmann, C. Ecological aspects of neophobia and neophilia in Birds. In Current Ornithology; vol Nolan, V., Thompson, C.F., Eds.; Springer: Boston, MA, USA, 2001; pp. 119–178. [Google Scholar] [CrossRef]
- Meehan, C.L.; Mench, J.A. The challenge of challenge: Can problem solving opportunities enhance animal welfare? Appl. Anim. Behav. Sci. 2007, 102, 246–261. [Google Scholar] [CrossRef]
- Oesterwind, S.; Nürnberg, G.; Puppe, B.; Langbein, J. Impact of structural and cognitive enrichment on the learning performance, behavior and physiology of dwarf goats (Capra aegagrus hircus). Appl. Anim. Behav. Sci. 2016, 177, 34–41. [Google Scholar] [CrossRef]
- Keen, H.A.; Nelson, O.L.; Robbins, C.T.; Evans, M.; Shepherdson, D.J.; Newberry, R.C. Validation of a novel cognitive bias task based on difference in quantity of reinforcement for assessing environmental enrichment. Anim. Cogn. 2014, 17, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Meade, T.M.; Hutchinson, E.; Krall, C.; Watson, J. Use of an aquarium as a novel enrichment item for singly housed rhesus macaques (Macaca mulatta). J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 472–477. [Google Scholar] [PubMed]
- Tarou, L.R.; Bashaw, M.J. Maximizing the effectiveness of environmental enrichment: Suggestions from the experimental analysis of behavior. Appl. Anim. Behav. Sci. 2007, 102, 189–204. [Google Scholar] [CrossRef]
- Podturkin, A.A. In search of the optimal enrichment program for zoo-housed animals. Zoo Biol. 2021, 40, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Tully, E.; Felsher, C. A progressively challenging enrichment project for a sloth bear (Melursus ursinus) at the Saint Louis Zoo. In Proceedings of the 40th National Conference of the American Association of Zoo Keepers, Inc., Greensboro, NC, USA, 24 September 2013. [Google Scholar]
- Hoppe, P.; Baskir, E. Swamp monkeys solve kerplunk: A progressively challenging enrichment experiment. Anim. Keep. Forum 2016, 43, 312–316. [Google Scholar]
- Boinski, S.; Swing, S.P.; Gross, T.S.; Davis, J.K. Environmental enrichment of brown capuchins (Cebus apella): Behavioral and plasma and fecal cortisol measures of effectiveness. Am. J. Primatol. 1999, 48, 49–68. [Google Scholar] [CrossRef]
- Caselli, M.; Messeri, P.; Dessì-Fulgheri, F.; Bandoli, F. Enriching zoo-housed ring-tailed lemurs (Lemur catta): Assessing the influence of three types of environmental enrichment on behavior. Animals 2022, 12, 2836. [Google Scholar] [CrossRef]
- Laméris, D.W.; Verspeek, J.; Depoortere, A.; Plessers, L.; Salas, M. Effects of enclosure and environmental enrichment on the behaviour of ring-tailed lemurs (Lemur catta). J. Zool. Bot. Gard. 2021, 2, 164–173. [Google Scholar] [CrossRef]
- Renner, M.J.; Lussier, J.P. Environmental enrichment for the captive spectacled bear (Tremarctos ornatus). Pharmacol. Biochem. Behav. 2002, 73, 279–283. [Google Scholar] [CrossRef]
- Rooney, M.B.; Sleeman, J. Effects of selected behavioral enrichment devices on behavior of western lowland gorillas (Gorilla gorilla gorilla). J. Appl. Anim. Welf. Sci. 1998, 1, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.I.; Ensenyat, C.; Serrat, S.; Maté, C. Introducing a semi-naturalistic exhibit as structural enrichment for two brown bears (Ursus arctos). Does this ensure their captive well-being? J. Appl. Anim. Welf. Sci. 2006, 9, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Szokalski, M.S.; Litchfield, C.A.; Foster, W.K. Enrichment for captive tigers (Panthera tigris): Current knowledge and future directions. Appl. Anim. Behav. Sci. 2012, 139, 1–9. [Google Scholar] [CrossRef]
- Rose, P.E.; Brereton, J.E.; Rowden, L.J.; de Figueiredo, R.L.; Riley, L.M. What’s new from the zoo? An analysis of ten years of zoo-themed research output. Palgrave Commun. 2019, 5, 128. [Google Scholar] [CrossRef]
- Azevedo, C.S.; Caldeira, J.R.; Faggioli, Â.B.; Cipreste, C.F. Effects of different environmental enrichment items on the behavior of the endangered Lear’s Macaw (Anodorhynchus leari, Psittacidae) at Belo Horizonte Zoo, Brazil. Rev. Bras. Ornitol. 2016, 24, 204–210. [Google Scholar] [CrossRef]
- Brereton, J.E.; Myhill, M.N.G.; Shora, J.A. Investigating the effect of enrichment on the behavior of zoo-housed southern ground hornbills. J. Zool. Bot. Gard. 2021, 2, 600–609. [Google Scholar] [CrossRef]
- Coulton, L.E.; Waran, N.K.; Young, R.J. Effects of foraging enrichment on the behavior of parrots. Anim. Welf. 1997, 6, 357–363. [Google Scholar] [CrossRef]
- Fangmeier, M.L.; Burns, A.L.; Melfi, V.A.; Meade, J. Foraging enrichment alleviates oral repetitive behaviors in captive red-tailed black cockatoos (Calyptorhynchus banksii). Zoo Biol. 2020, 39, 3–12. [Google Scholar] [CrossRef]
- Field, D.A.; Thomas, R. Environmental enrichment for psittacines at Edinburgh Zoo. Int. Zoo Yearb. 2000, 37, 232–237. [Google Scholar] [CrossRef]
- Jepson, P.R. Saving a species threatened by trade: A network study of Bali starling Leucopsar rothschildi conservation. Oryx 2016, 50, 480–488. [Google Scholar] [CrossRef]
- Zuccon, D.; Pasquet, E.; Ericson, P.G.P. Phylogenetic relationships among Palearctic-Oriental starlings and mynas (genera Sturnus and Acridotheres: Sturnidae). Zool. Scr. 2008, 37, 469–481. [Google Scholar] [CrossRef]
- Higgins, P.J.; Peter, J.M.; Cowling, S.J. (Eds.) Handbook of Australian, New Zealand & Antarctic Birds; Volume Oxford University Press: Melbourne, Australia, 2006. [Google Scholar]
- Nikolov, S.; Kanakov, D. Influencing factors leading to damaging behavior—Feather pecking and cannibalism in game birds. Trakia J. Sci. 2020, 18, 377–387. [Google Scholar] [CrossRef]
- Kinkaid, H.Y.M.; Mills, D.S.; Nichols, S.G.; Meagher, R.K.; Mason, G.J. Feather-damaging behaviour in companion parrots: An initial analysis of potential demographic risk factors. Avian Biol. Res. 2013, 6, 289–296. [Google Scholar] [CrossRef]
- van Zeeland, Y.R.A.; Spruit, B.M.; Rodenburg, T.B.; Riedstra, B.; van Hierden, Y.M.; Buitenhuis, B.; Korte, S.M.; Lumeij, J.T. Feather damaging behaviour in parrots: A review with consideration of comparative aspects. Appl. Anim. Behav. Sci. 2009, 121, 75–95. [Google Scholar] [CrossRef]
- Costa, P.; Macchi, E.; Valle, E.; De Marco, M.; Nucera, D.M.; Gasco, L.; Schiavone, A. An association between feather damaging behavior and corticosterone metabolite excretion in captive African grey parrots (Psittacus erithacus). PeerJ 2016, 4, e2462. [Google Scholar] [CrossRef]
- Heinsius, J.; van Staaveren, N.; Kwon, I.Y.; Li, A.; Kjaer, J.B.; Harlander-Matauschek, A. Chickens selected for feather pecking can inhibit prepotent motor responses in a Go/No-Go task. Sci. Rep. 2020, 10, 6485. [Google Scholar] [CrossRef]
- Mens, A.J.W.; van Krimpen, M.M.; Kwakkel, R.P. Nutritional approaches to reduce or prevent feather pecking in laying hens: Any potential to intervene during rearing? World’s Poult. Sci. J. 2020, 76, 591–610. [Google Scholar] [CrossRef]
- Costa, P.; Macchi, E.; Tomassone, L.; Ricceri, F.; Bollo, E.; Scaglione, F.E.; Tarantola, M.; De Marco, M.; Prola, L.; Bergero, D.; et al. Feather picking in pet parrots: Sensitive species, risk factor and ethological evidence. Ital. J. Anim. Sci. 2016, 15, 473–480. [Google Scholar] [CrossRef]
- Lumeij, J.T.; Hommers, C.J. Foraging ‘enrichment’ as treatment for pterotillomania. Appl. Anim. Behav. Sci. 2008, 111, 85–94. [Google Scholar] [CrossRef]
- Van Staaveren, N.; Ellis, J.; Baes, C.F.; Harlander-Matauschek, A. A meta analysis on the effect of environmental enrichment on feather pecking and feather damage in laying hens. Poult. Sci. 2021, 100, 397–411. [Google Scholar] [CrossRef]
- Martin, P.; Bateson, P. Measuring Behaviour: An Introductory Guide, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar] [CrossRef]
- Schielzeth, H.; Dingemanse, N.J.; Nakagawa, S.; Westneat, D.F.; Allegue, H.; Teplitsky, C.; Réale, D.; Dochtermann, N.A.; Garamszegi, L.Z.; Araya-Ajoy, Y.G. Robustness of linear mixed-effects models to violations of distributional assumptions. Methods Ecol. Evol. 2020, 11, 1141–1152. [Google Scholar] [CrossRef]
- Arnau, J.; Bendayan, R.; Blanca, M.J.; Bono, R. The effect of skewness and kurtosis on the robustness of linear mixed models. Behav. Res. 2013, 45, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Rudkin, C. Feather pecking and foraging uncorrelated—The redirection hypothesis revisited. Br. Poult. Sci. 2022, 63, 265–273. [Google Scholar] [CrossRef]
- Shi, H.; Li, B.; Tong, Q.; Zheng, W.; Zeng, D.; Feng, G. Effects of LED Light Color and Intensity on Feather Pecking and Fear Responses of Layer Breeders in Natural Mating Colony Cages. Animals 2019, 9, 814. [Google Scholar] [CrossRef]
- Schwarzer, A.; Plattner, C.; Bergmann, S.; Rauch, E.; Erhard, M.; Reese, S.; Louton, H. Feather pecking in non-beak-trimmed and beak-trimmed laying hens on commercial farms with aviaries. Animals 2021, 11, 3085. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.F.F.; de Azevedo, C.S.; Young, R.J.; Viau, P. Impacts of food-based enrichment on behaviour and physiology of male greater rheas (Rhea Americana, Rheidae, Aves). Papéis Avulsos Zool. 2019, 59, e20195911. [Google Scholar] [CrossRef]
- de Almeida, A.C.; Palme, R.; Moreira, N. How environmental enrichment affects behavioral and glucocorticoid responses in captive blue-and-yellow macaws (Ara ararauna). Appl. Anim. Behav. Sci. 2018, 201, 125–135. [Google Scholar] [CrossRef]
- Miglioli, A.; da Silva Vasconcellos, A. Can behavioural management improve behaviour and reproduction in captive blue-and-yellow macaws (Ara ararauna)? Appl. Anim. Behav. Sci. 2021, 241, 105386. [Google Scholar] [CrossRef]
- Congdon, S. Starlings and mynas. AFA Watchb. 1999, 26, 17–19. [Google Scholar]
- Hernowo, J.B.; Haquesta, S. Evaluation on Bali Mynah (Leucopsar rothschildi Stresemann, 1912) population, result of release process 1998–2015 in Bali Barat National Park, Indonesia. Biodiversitas 2021, 22, 2699–2710. [Google Scholar] [CrossRef]
- Miller, R.; Lambert, M.L.; Frohnwieser, A.; Brecht, K.F.; Bugnyar, T.; Crampton, I.; Garcia-Pelegrin, E.; Gould, K.; Greggor, A.L.; Izawa, E.; et al. Socio-ecological correlates of neophobia in corvids. Curr. Biol. 2022, 32, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Garcia-Pelegrin, E.; Danby, E. Neophobia and innovation in critically endangered Bali myna, Leucopsar rothschildi. R. Soc. Open Sci. 2022, 9, 211781. [Google Scholar] [CrossRef] [PubMed]
- Ghavamian, Y.; Minier, D.E.; Jaffe, K.E. Effects of complex feeding enrichment on the behavior of captive Malayan sun bears (Helarctos malayanus). J. Appl. Anim. Welf. Sci. 2022, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).