Comparing Predictors and Outcomes of Higher Allostatic Load across Zoo-Housed African Great Apes
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Biomarker Analyses
2.3. Allostatic Load Index
2.4. Predictors and Outcomes of Higher Allostatic Load
2.5. Quantitative Analyses
3. Results
3.1. Predictors of Higher Allostatic Load
3.2. Outcomes of Higher Allostatic Load
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seaward, B.L. The Physiology of Stress. In Managing Stress: Principles and Strategies for Health and Well Being; Seaward, B.L., Ed.; Jones and Bartlett Publishers: Boston, MA, USA, 2006; pp. 31–48. [Google Scholar]
- Everly, G.S.; Lating, J.M. The Anatomy and Physiology of the Human Stress Response. In A Clinical Guide to the Treatment of the Human Stress Response; Everly, G.S., Lating, J.M., Eds.; Springer: New York, NY, USA, 2013; pp. 53–65. ISBN 9781461455370. [Google Scholar]
- Cockrem, J.F. Individual Variation in Glucocorticoid Stress Responses in Animals. Gen. Comp. Endocrinol. 2013, 181, 45–58. [Google Scholar] [CrossRef] [PubMed]
- MacDougall-Shackleton, S.A.; Bonier, F.; Romero, L.M.; Moore, I.T. Glucocorticoids and “Stress” Are Not Synonymous. Integr. Org. Biol. 2019, 1, obz017. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. What Is the Confusion with Cortisol? Chronic Stress 2019, 3, 2470547019833647. [Google Scholar] [CrossRef] [PubMed]
- Glaser, R.; Kiecolt-Glaser, J.K. Stress-Induced Immune Dysfunction: Implications for Health. Nat. Rev. Immunol. 2005, 5, 243–261. [Google Scholar] [CrossRef]
- Hawkley, L.C.; Bosch, J.A.; Engeland, C.G.; Marucha, P.T.; Cacioppo, J.T. Loneliness, Dysphoria, Stress, and Immunity: A Role for Cytokines. In Cytokines: Stress and Immunity; Plotnikoff, N.P., Faith, R.E., Murgo, A.J., Good, R.A., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 67–85. [Google Scholar]
- McEwen, B.S.; Stellar, E. Stress and the Individual: Mechanisms Leading to Disease. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef]
- Seeman, T.E.; Singer, B.H.; Rowe, J.W.; Horwitz, R.I.; McEwen, B.S. Price of Adaptation—Allostatic Load and Its Health Consequences. Arch. Intern. Med. 1997, 157, 2259–2268. [Google Scholar] [CrossRef]
- Juster, R.-P.; McEwen, B.S.; Lupien, S.J. Allostatic Load Biomarkers of Chronic Stress and Impact on Health and Cognition. Neurosci. Biobehav. Rev. 2010, 35, 2–16. [Google Scholar] [CrossRef]
- Edes, A.N.; Crews, D.E. Allostatic Load and Biological Anthropology. Am. J. Phys. Anthropol. 2017, 162 (Suppl. 63), 44–70. [Google Scholar] [CrossRef]
- Johnson, S.C.; Cavallaro, F.L.; Leon, D.A. A Systematic Review of Allostatic Load in Relation to Socioeconomic Position: Poor Fidelity and Major Inconsistencies in Biomarkers Employed. Soc. Sci. Med. 2017, 192, 66–73. [Google Scholar] [CrossRef]
- Guidi, J.; Lucente, M.; Sonino, N.; Fava, G.A. Allostatic Load and Its Impact on Health: A Systematic Review. Psychother. Psychosom. 2020, 90, 11–27. [Google Scholar] [CrossRef]
- Christensen, D.S.; Flensborg-Madsen, T.; Garde, E.; Hansen, Å.M.; Masters Pedersen, J.; Mortensen, E.L. Early Life Predictors of Midlife Allostatic Load: A Prospective Cohort Study. PLoS ONE 2018, 13, e0202395. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, L.; Joshi, D.; Raina, P.; Griffith, L.E.; MacMillan, H.; Gonzalez, A. Social Engagement and Allostatic Load Mediate between Adverse Childhood Experiences and Multimorbidity in Mid to Late Adulthood: The Canadian Longitudinal Study on Aging. Psychol. Med. 2021, 1–11. [Google Scholar] [CrossRef]
- Glei, D.A.; Goldman, N.; Chuang, Y.-L.; Weinstein, M. Do Chronic Stressors Lead to Physiological Dysregulation? Testing the Theory of Allostatic Load. Psychosom. Med. 2007, 69, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Cave, L.; Cooper, M.N.; Zubrick, S.R.; Shepherd, C.C.J. Racial Discrimination and Allostatic Load among First Nations Australians: A Nationally Representative Cross-Sectional Study. BMC Public Health 2020, 20, 1881. [Google Scholar] [CrossRef]
- Shen, J.; Fuemmeler, B.F.; Guan, Y.; Zhao, H. Association of Allostatic Load and All Cancer Risk in the SWAN Cohort. Cancers 2022, 14, 3044. [Google Scholar] [CrossRef]
- Akinyemiju, T.; Wilson, L.E.; Deveaux, A.; Aslibekyan, S.; Cushman, M.; Gilchrist, S.; Safford, M.; Judd, S.; Howard, V. Association of Allostatic Load with All-Cause and Cancer Mortality by Race and Body Mass Index in the REGARDS Cohort. Cancers 2020, 12, 1695. [Google Scholar] [CrossRef]
- Karlamangla, A.S.; Singer, B.H.; McEwen, B.S.; Rowe, J.W.; Seeman, T.E. Allostatic Load as a Predictor of Functional Decline: MacArthur Studies of Successful Aging. J. Clin. Epidemiol. 2002, 55, 696–710. [Google Scholar] [CrossRef]
- Mattei, J.; Demissie, S.; Falcon, L.M.; Ordovas, J.M.; Tucker, K. Allostatic Load Is Associated with Chronic Conditions in the Boston Puerto Rican Health Study. Soc. Sci. Med. 2010, 70, 1988–1996. [Google Scholar] [CrossRef]
- Bruun-Rasmussen, N.E.; Napolitano, G.; Christiansen, C.; Bojesen, S.E.; Ellervik, C.; Jepsen, R.; Rasmussen, K.; Lynge, E. Allostatic Load as Predictor of Mortality: A Cohort Study from Lolland-Falster, Denmark. BMJ Open 2022, 12, e057136. [Google Scholar] [CrossRef]
- Parker, H.W.; Abreu, A.M.; Sullivan, M.C.; Vadiveloo, M.K. Allostatic Load and Mortality: A Systematic Review and Meta-Analysis. Am. J. Prev. Med. 2022, 63, 131–140. [Google Scholar] [CrossRef]
- Seeley, K.E.; Proudfoot, K.L.; Edes, A.N. The Application of Allostasis and Allostatic Load in Animal Species: A Scoping Review. PLoS ONE 2022, 17, e0273838. [Google Scholar] [CrossRef] [PubMed]
- Edes, A.N.; Wolfe, B.A.; Crews, D.E. Assessing Stress in Zoo-Housed Western Lowland Gorillas (Gorilla Gorilla Gorilla) Using Allostatic Load. Int. J. Primatol. 2016, 37, 241–259. [Google Scholar] [CrossRef]
- Edes, A.N.; Wolfe, B.A.; Crews, D.E. Rearing History and Allostatic Load in Adult Western Lowland Gorillas (Gorilla Gorilla Gorilla) in Human Care. Zoo Biol. 2016, 35, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Edes, A.N.; Wolfe, B.A.; Crews, D.E. The First Multi-Zoo Application of an Allostatic Load Index to Western Lowland Gorillas (Gorilla Gorilla Gorilla). Gen. Comp. Endocrinol. 2018, 266, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Edes, A.N.; Edwards, K.L.; Wolfe, B.A.; Brown, J.L.; Crews, D.E. Allostatic Load Indices With Cholesterol and Triglycerides Predict Disease and Mortality Risk in Zoo-Housed Western Lowland Gorillas (Gorilla Gorilla Gorilla). Biomark. Insights 2020, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Edes, A.N.; Wolfe, B.A.; Crews, D.E. Testing a Method to Improve Predictions of Disease and Mortality Risk in Western Lowland Gorillas (Gorilla Gorilla Gorilla) Using Allostatic Load. Stress 2021, 24, 76–86. [Google Scholar] [CrossRef]
- Gallo, L.C.; Fortmann, A.L.; Mattei, J. Allostatic Load and the Assessment of Cumulative Biological Risk in Biobehavioral Medicine: Challenges and Opportunities. Psychosom. Med. 2014, 76, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.T.; Bingham, B.A.; Aldana, P.C.; Chung, S.T.; Sumner, A.E. Variation in the Calculation of Allostatic Load Scores: 21 Examples from NHANES. J. Racial Ethn. Health Disparities 2017, 4, 455–461. [Google Scholar] [CrossRef]
- Harder, J.D. Reproduction and Hormones. In The Wildlife Techniques Manual; Silvy, N.J., Ed.; John Hopkins University Press: Baltimore, MD, USA, 2012; Volume 1, pp. 502–525. [Google Scholar]
- Orentreich, N.; Brind, J.L.; Rizer, R.L.; Vogelman, J.H. Age Changes and Sex Differences in Serum Dehydroepiandrosterone Sulfate Concentrations throughout Adulthood. J. Clin. Endocrinol. Metab. 1984, 59, 551–555. [Google Scholar] [CrossRef]
- Tworoger, S.S.; Hankinson, S.E. Collection, Processing, and Storage of Biological Samples in Epidemiological Studies: Sex Hormones, Carotenoids, Inflammatory Markers, and Proteomics as Examples. CEBP Focus Biorepos. Biospecim. Sci. 2006, 15, 1578–1581. [Google Scholar]
- Arts, E.E.A.; Popa, C.D.; Smith, J.P.; Arntz, O.J.; van de Loo, F.A.; Donders, R.; Semb, A.G.P.; Kitas, G.D.; van Riel, P.L.C.M.; Fransen, J. Serum Samples That Have Been Stored Long-Term (>10 Years) Can Be Used as a Suitable Data Source for Developing Cardiovascular Risk Prediction Models in Large Observational Rheumatoid Arthritis Cohorts. Biomed. Res. Int. 2014, 2014, 930925. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.L.; Bansiddhi, P.; Paris, S.; Galloway, M.; Brown, J.L. The Development of an Immunoassay to Measure Immunoglobulin A in Asian Elephant Feces, Saliva, Urine and Serum as a Potential Biomarker of Well-Being. Conserv. Physiol. 2019, 7, coy077. [Google Scholar] [PubMed]
- Edes, A.N.; Brown, J.L.; Edwards, K.L. Evaluating Individual Biomarkers for Predicting Health Risks in Zoo-Housed Chimpanzees (Pan troglodytes) and Bonobos (Pan paniscus). Am. J. Primatol. 2023, 85, e23457. [Google Scholar] [CrossRef] [PubMed]
- Badanes, L.S.; Watamura, S.E.; Hankin, B.L. Hypocortisolism as a Potential Marker of Allostatic Load in Children: Associations with Family Risk and Internalizing Disorders. Dev. Psychopathol. 2011, 23, 881–896. [Google Scholar] [CrossRef]
- Heim, C.; Ehlert, U.; Hellhammer, D.H. The Potential Role of Hypocortisolism in the Pathophysiology of Stress-Related Bodily Disorders. Psychoneuroendocrinology 2000, 25, 1–35. [Google Scholar] [CrossRef]
- Raison, C.L.; Miller, A.H. When Not Enough Is Too Much: The Role of Insufficient Glucocorticoid Signaling in the Pathophysiology of Stress-Related Disorders. Am. J. Psychiatry 2003, 160, 1554–1565. [Google Scholar] [CrossRef]
- Schatzkin, A.; Hoover, R.N.; Taylor, P.R.; Ziegler, R.G.; Carter, C.L.; Larson, D.B.; Ligitra, L.M. Serum Cholesterol and Cancer in the NHANES I Epidemiological Followup Study. Lancet 1987, 330, 298–301. [Google Scholar] [CrossRef]
- Isles, C.G.; Hole, D.J.; Gillis, C.R.; Hawthorne, V.M.; Lever, A.F. Plasma Cholesterol, Coronary Heart Disease, and Cancer in the Renfrew and Paisley Survey. BMJ Br. Med. J. 1989, 298, 920–924. [Google Scholar] [CrossRef]
- Harris, T.; Feldman, J.J.; Kleinman, J.C.; Ettinger, W.H., Jr.; Makuc, D.M.; Schatzkin, A.G. The Low Cholesterol-Mortality Association in a National Cohort. J. Clin. Epidemiol. 1992, 45, 595–601. [Google Scholar]
- Kronmal, R.A.; Cain, K.C.; Ye, Z.; Omenn, G.S. Total Serum Cholesterol Levels and Mortality Risk as a Function of Age: A Report Based on the Framingham Data. Arch. Intern. Med. 1993, 153, 1065–1073. [Google Scholar] [CrossRef]
- Species360 Zoological Information Management System (ZIMS). Available online: https://Zims.Species360.org (accessed on 15 October 2022).
- Lowenstine, L.J.; McManamon, R.; Terio, K.A. Comparative Pathology of Aging Great Apes: Bonobos, Chimpanzees, Gorillas, and Orangutans. Vet. Pathol. 2016, 53, 250–276. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.W.; Danforth, M.D.; Clyde, V.L. The Great Ape Heart Project. Int. Zoo Yearb. 2018, 52, 103–112. [Google Scholar] [CrossRef]
- Strong, V.J.; Martin, M.; Redrobe, S.; White, K.; Baiker, K. A Retrospective Review of Great Ape Cardiovascular Disease Epidemiology and Pathology. Int. Zoo Yearb. 2018, 52, 113–125. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Scheipl, F.; Grothendieck, G.; Green, P.; et al. Lme4: Linear Mixed-Effects Models Using “Eigen” and S4, R Package version 1.42.1; The R Project for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Therneau, T.M.; Lumley, T.; Atkinson, E.; Crowson, C. Survival: Survival Analysis, R Package version 1.42.1; The R Project for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Bartoń, K. MuMIn: Multi-Model Inference, R Package version 1.42.1; The R Project for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Crimmins, E.M.; Johnston, M.; Hayward, M.; Seeman, T.E. Age Differences in Allostatic Load: An Index of Physiological Dysregulation. Exp. Gerontol. 2003, 38, 731–734. [Google Scholar] [CrossRef]
- Seeman, T.E.; Merkin, S.S.; Crimmins, E.M.; Koretz, B.K.; Charette, S.; Karlamangla, A.S. Education, Income and Ethnic Differences in Cumulative Biological Risk Profiles in a National Sample of US Adults: NHANES III (1988–1994). Soc. Sci. Med. 2008, 66, 72–87. [Google Scholar] [CrossRef]
- Piazza, J.R.; Stawski, R.S.; Sheffler, J.L. Age, Daily Stress Processes, and Allostatic Load: A Longitudinal Study. J. Aging Health 2018, 31, 1671–1691. [Google Scholar] [CrossRef]
- Robertson, T.; Watts, E. The Importance of Age, Sex and Place in Understanding Socioeconomic Inequalities in Allostatic Load: Evidence from the Scottish Health Survey (2008–2011). BMC Public Health 2016, 16, 126. [Google Scholar] [CrossRef]
- van Deurzen, I.; Vanhoutte, B. A Longitudinal Study of Allostatic Load in Later Life: The Role of Sex, Birth Cohorts, and Risk Accumulation. Res. Aging 2019, 41, 419–442. [Google Scholar] [CrossRef]
- Crews, D.E. Assessing Composite Estimates of Stress in American Samoans. Am. J. Phys. Anthropol. 2007, 133, 1028–1034. [Google Scholar] [CrossRef]
- Crews, D.E.; Harada, H.; Aoyagi, K.; Maeda, T.; Alfarano, A.; Sone, Y.; Kusano, Y. Allostatic Load among Elderly Japanese Living on Hizen-Oshima Island. Int. J. Phys. Anthropol. 2012, 31, 18–29. [Google Scholar] [CrossRef]
- Lateef, S.S.; Al Najafi, M.; Dey, A.K.; Batool, M.; Abdelrahman, K.M.; Uceda, D.E.; Reddy, A.S.; Svirydava, M.D.; Nanda, N.; Ortiz, J.E.; et al. Relationship between Chronic Stress-Related Neural Activity, Physiological Dysregulation and Coronary Artery Disease in Psoriasis: Findings from a Longitudinal Observational Cohort Study. Atherosclerosis 2020, 310, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.P.; Gruenewald, T.; Karlamangla, A.; Hu, P.; Koretz, B.; Seeman, T.E. Social Relationships and Allostatic Load in the MIDUS Study. Health Psychol. 2014, 33, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Sedanur Macit, M.; Acar-Tek, N. Evaluation of Nutritional Status and Allostatic Load in Adult Patients with Type 2 Diabetes. Can. J. Diabetes 2019, 44, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Goldman, N.; Glei, D.A.; Seplaki, C.; Liu, I.W.; Weinstein, M. Perceived Stress and Physiological Dysregulation in Older Adults. Stress 2005, 8, 95–105. [Google Scholar] [CrossRef]
- Augustine, L.F.; Nair, K.M.; Rao, S.F.; Rao, M.V.V.; Ravinder, P.; Laxmaiah, A. Exploring the Bio-Behavioural Link between Stress, Allostatic Load & Micronutrient Status: A Cross-Sectional Study among Adolescent Boys. Indian J. Med. Res. 2016, 144, 378–384. [Google Scholar]
- Weinstein, M.; Goldman, D.; Hedley, A.; Yu-Hsuan, L.; Seeman, T.E. Social Linkages to Biological Markers of Health Among the Elderly. J. Biosoc. Sci. 2003, 35, 433–453. [Google Scholar] [CrossRef]
- Hellhammer, J.; Schlotz, W.; Stone, A.A.; Pirke, K.-M.; Hellhammer, D.H. Allostatic Load, Perceived Stress, and Health: A Prospective Study in Two Age Groups. In Biobehavioral Stress Response: Protective and Damaging Effects; Yehuda, R., McEwen, B.S., Eds.; New York Academy of Sciences: New York, NY, USA, 2004; Volume 1032, pp. 8–13. [Google Scholar]
- Arévalo, S.P.; Tucker, K.L.; Falcón, L.M. Life Events Trajectories, Allostatic Load, and the Moderating Role of Age at Arrival from Puerto Rico to the US Mainland. Soc. Sci. Med. 2014, 120, 301–310. [Google Scholar] [CrossRef]
- Berg, M.T.; Simons, R.L.; Barr, A.; Beach, S.R.H.; Philibert, R.A. Childhood/Adolescent Stressors and Allostatic Load in Adulthood: Support for a Calibration Model. Soc. Sci. Med. 2017, 193, 130–139. [Google Scholar] [CrossRef]
- Nugent, K.L.; Chiappelli, J.; Rowland, L.M.; Hong, L.E. Cumulative Stress Pathophysiology in Schizophrenia as Indexed by Allostatic Load. Psychoneuroendocrinology 2015, 60, 120–129. [Google Scholar]
- Danese, A.; McEwen, B.S. Adverse Childhood Experiences, Allostasis, Allostatic Load, and Age-Related Disease. Physiol. Behav. 2012, 106, 29–39. [Google Scholar]
- Horan, J.M.; Widom, C.S. From Childhood Maltreatment to Allostatic Load in Adulthood: The Role of Social Support. Child Maltreat. 2015, 20, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Seeman, T.E.; Singer, B.H.; Ryff, C.D.; Love, G.D.; Levy-Storms, L. Social Relationships, Gender, and Allostatic Load Across Two Age Cohorts. Psychosom. Med. 2002, 64, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Reiber, G.; Kohler, T.; Boyko, E.J. Peripheral Arterial Disease in a Multiethnic National Sample: The Role of Conventional Risk Factors and Allostatic Load. Ethn. Dis. 2007, 17, 669–675. [Google Scholar] [PubMed]
- Sabbah, W.; Watt, R.G.; Sheiham, A.; Tsakos, G. Effects of Allostatic Load on the Social Gradient in Ischaemic Heart Disease and Periodontal Disease: Evidence from the Third National Health and Nutrition Examination Survey. J. Epidemiol. Community Health 2008, 62, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Borrell, L.N.; Crawford, N.D. Social Disparities in Periodontitis among US Adults: The Effect of Allostatic Load. J. Epidemiol. Community Health 2011, 65, 144–149. [Google Scholar] [CrossRef]
- Sabry, S.M.; Hend, G.; Nadia, B.; Sanaa, R.; Ola, A. Prediction of Health Risk and Estimation of Associated Variables with Work Stress Using Allostatic Load Index. Biomed. Pharmacol. J. 2020, 13, 979–987. [Google Scholar] [CrossRef]
- Hwang, A.-C.; Peng, L.-N.; Wen, Y.-W.; Tsai, Y.-W.; Chang, L.-C.; Chiou, S.-T.; Chen, L.-K. Predicting All-Cause and Cause-Specific Mortality by Static and Dynamic Measurements of Allostatic Load: A 10-Year Population-Based Cohort Study in Taiwan. J. Post-Acute Long-Term Care Med. 2014, 15, 490–496. [Google Scholar] [CrossRef]
- Levine, M.E.; Crimmins, E.M. A Comparison of Methods for Assessing Mortality Risk. Am. J. Hum. Biol. 2014, 26, 768–776. [Google Scholar] [CrossRef]
- Howard, J.T.; Sparks, P.J. The Effects of Allostatic Load on Racial/Ethnic Mortality Differences in the United States. Popul. Res. Policy Rev. 2016, 35, 421–443. [Google Scholar] [CrossRef]
- Gruenewald, T.L.; Seeman, T.E.; Ryff, C.D.; Karlamangla, A.S.; Singer, B.H. Combinations of Biomarkers Predictive of Later Life Mortality. Proc. Natl. Acad. Sci. USA 2006, 103, 14158–14163. [Google Scholar] [CrossRef]
- Glei, D.A.; Goldman, N.; Rodríguez, G.; Weinstein, M. Beyond Self-Reports: Changes in Biomarkers as Predictors of Mortality. Popul. Dev. Rev. 2014, 40, 331–360. [Google Scholar] [CrossRef] [PubMed]
- Robertson, T.; Beveridge, G.; Bromley, C. Allostatic Load as a Predictor of All-Cause and Cause-Specific Mortality in the General Population: Evidence from the Scottish Health Survey. PLoS ONE 2017, 12, e0183297. [Google Scholar] [CrossRef] [PubMed]
- Castagné, R.; Garès, V.; Karimi, M.; Chadeau-Hyam, M.; Vineis, P.; Delpierre, C.; Kelly-Irving, M.; Lifepath Consortium. Allostatic Load and Subsequent All-Cause Mortality: Which Biological Markers Drive the Relationship? Findings from a UK Birth Cohort. Eur. J. Epidemiol. 2018, 33, 441–458. [Google Scholar] [PubMed]
- Tampubolon, G.; Maharani, A. Trajectories of Allostatic Load among Older Americans and Britons: Longitudinal Cohort Studies. BMC Geriatr. 2018, 18, 255. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.; Coley, R.L. Variations in Links between Educational Success and Health: Implications for Enduring Health Disparities. Cult. Divers. Ethn. Minor. Psychol. 2019, 25, 32–43. [Google Scholar] [CrossRef]
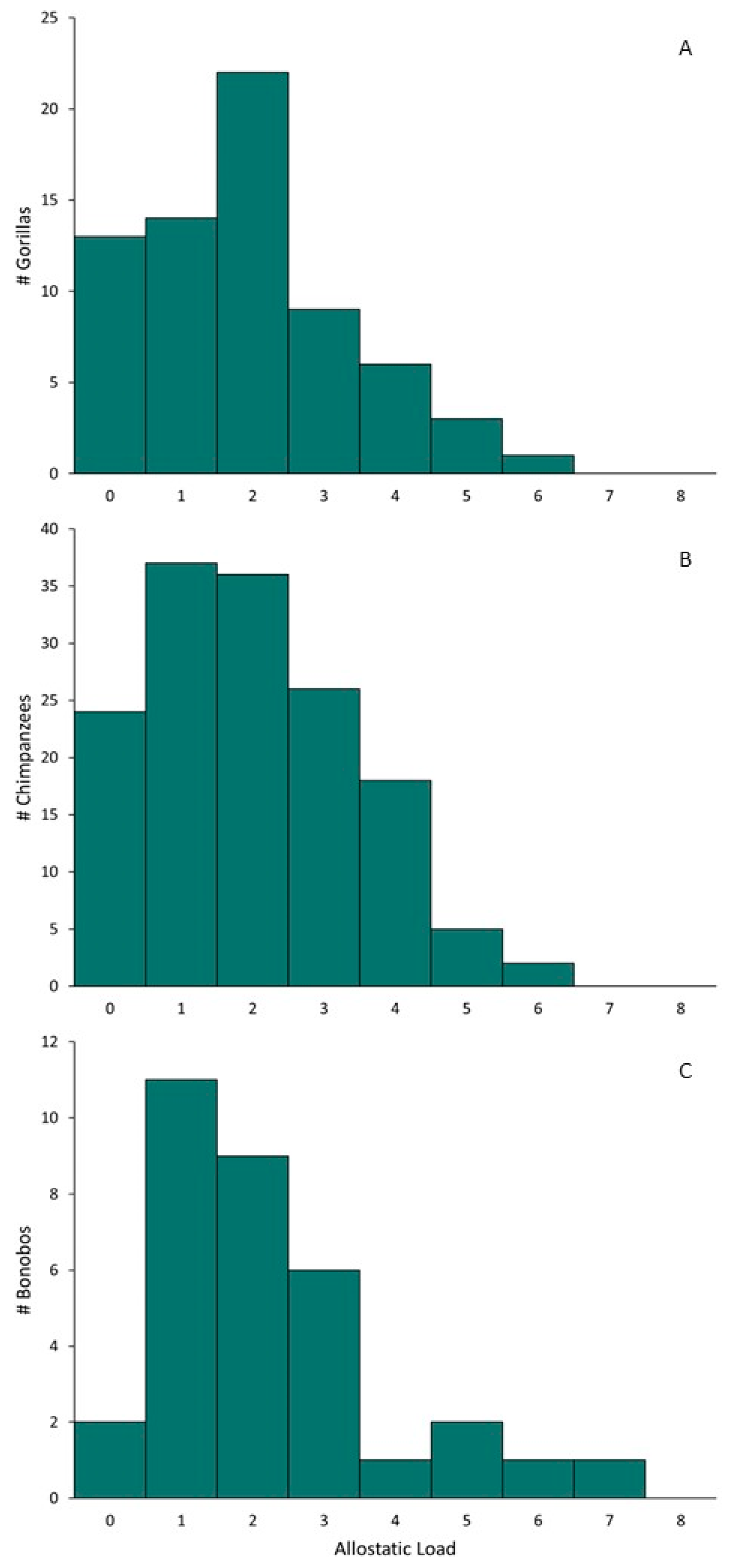
| Gorillas | Chimpanzees | Bonobos | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variation by Sex | Cut- Point (s) | Variation by Sex | Cut- Point (s) | Variation by Sex | Cut- Point (s) | ||||||||||
| n | β | SE | p | n | β | SE | p | n | β | SE | p | ||||
| Albumin | 63 | 3.897 | 1.087 | <0.001 | M ≤ 35.04; F ≤ 30.42 | 148 | 2.871 | 0.719 | <0.001 | M ≤ 30.94; F ≤ 28.50 | 33 | 0.763 | 1.101 | 0.494 | ≤31.57 |
| TC | 61 | 0.035 | 0.057 | 0.537 | ≤5.01, ≥7.90 | 148 | −0.030 | 0.180 | 0.867 | ≤2.90, ≥5.40 | 33 | −0.122 | 0.071 | 0.085 | ≤4.55, ≥6.28 |
| Cortisol | 68 | −0.662 | 0.114 | <0.001 | M ≤ 7.21, ≥14.38; F ≤ 8.67, ≥34.55 | 142 | −0.555 | 0.083 | <0.001 | M ≤ 9.70, ≥19.52; F ≤ 11.01, ≥42.99 | 32 | −0.369 | 0.117 | 0.002 | M ≤ 9.30, ≥15.14; F ≤ 10.64, ≥28.21 |
| DHEA-S | 68 | −0.012 | 0.265 | 0.964 | ≤16.85 | 142 | 0.457 | 0.118 | <0.001 | M ≤ 411.0; F ≤ 250.0 | 33 | −0.037 | 0.186 | 0.844 | ≤310.12 |
| Glucose | 62 | 0.031 | 0.073 | 0.670 | ≥4.77 | 148 | −0.033 | 0.060 | 0.582 | ≥5.97 | 33 | −0.174 | 0.094 | 0.065 | ≥4.81 |
| IL-6 | 68 | 0.222 | 0.184 | 0.229 | ≥6.38 | 134 | −0.181 | 0.217 | 0.404 | ≥11.43 | 33 | −1.457 | 0.405 | <0.001 | M ≥ 5.74; F ≥ 5.95 |
| TG | 61 | −0.314 | 0.137 | 0.022 | M ≥ 1.36; F ≥ 1.95 | 148 | −0.217 | 0.087 | 0.013 | M ≥ 1.19; F ≥ 1.49 | 33 | −0.110 | 0.153 | 0.472 | ≥1.14 |
| TNF-α | 68 | −0.804 | 0.260 | 0.002 | M ≥ 0.54; F ≥ 0.94 | 141 | 0.022 | 0.094 | 0.814 | ≥1.31 | 33 | −0.471 | 0.182 | 0.010 | M ≥ 0.75; F ≥ 0.99 |
| Gorillas | Chimpanzees | Bonobos | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | Range | SD | n | Range | SD | n | Range | SD | ||||
| Total stressful events | 3–151 | 35.78 | 26.60 | 1–156 | 33.97 | 32.60 | 1–87 | 16.25 | 19.43 | ||||
| Transfers | 0–6 | 1.38 | 1.21 | 0–5 | 1.16 | 1.12 | 0–3 | 1.30 | 0.98 | ||||
| Wounding | 0–133 | 23.99 | 23.47 | 0–147 | 26.32 | 30.86 | 1–45 | 11.50 | 12.15 | ||||
| Immobilizations | 1–26 | 9.68 | 6.10 | 1–42 | 8.90 | 8.71 | 1–44 | 8.5 | 10.20 | ||||
| Parity (females only) | 34 | 88 | 15 | ||||||||||
| Nulliparous | 12 | 10 | 0 | ||||||||||
| Parous | 20 | 1–16 | 32 | 1–9 | 7 | 2–6 | |||||||
| Unknown | 2 | 46 | 8 | ||||||||||
| Birthplace | |||||||||||||
| Wild | 10 | 30 | 8 | ||||||||||
| Zoo | 58 | 103 | 22 | ||||||||||
| Unknown | 15 | 3 | |||||||||||
| Rearing history (zoo-born) | 58 | 103 | |||||||||||
| Mother-reared | 24 | 70 | 19 | ||||||||||
| Nursery-reared | 33 | 27 | 3 | ||||||||||
| Surrogate-reared | 1 | ||||||||||||
| Peer-reared | 6 | ||||||||||||
| Chronic condition(s) | 31 | 81 | 6 | ||||||||||
| Cardiac disease | 23 | 49 | 5 | ||||||||||
| Dead | 29 | 51 | 6 | ||||||||||
| Gorillas | Chimpanzees | Bonobos | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | β | SE | p | ||
| Age | 0.678 | 0.289 | 0.010 | 0.014 | 0.005 | 0.003 | 0.006 | 0.010 | 0.535 | |
| Sex | −0.139 | 0.181 | 0.443 | −0.083 | 0.122 | 0.499 | −0.213 | 0.252 | 0.397 | |
| Stressful events | 0.000 | 0.003 | 0.946 | 0.005 | 0.002 | 0.022 | 0.001 | 0.007 | 0.778 | |
| Transfers | 0.077 | 0.072 | 0.289 | 0.002 | 0.059 | 0.977 | 0.093 | 0.109 | 0.393 | |
| Wounding | −0.001 | 0.004 | 0.754 | 0.003 | 0.002 | 0.142 | −0.005 | 0.013 | 0.673 | |
| Immobilizations | 0.016 | 0.014 | 0.238 | 0.014 | 0.008 | 0.077 | 0.008 | 0.013 | 0.547 | |
| Parity (females only) | 0.091 | 0.267 | 0.733 | 0.588 | 0.320 | 0.066 | ||||
| Zoo-born vs. wild-caught | −0.623 | 0.223 | 0.005 | −0.257 | 0.140 | 0.066 | −0.138 | 0.309 | 0.654 | |
| Mother- vs. nursery-reared | −0.321 | 0.228 | 0.159 | −0.033 | 0.171 | 0.847 | 0.693 | 0.606 | 0.252 | |
| Gorillas | Chimpanzees | Bonobos | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | SD | Range | SD | Range | SD | |||||
| Sex | Males | 0–4 | 1.68 | 1.12 | 0–6 | 2.33 | 1.65 | 0–7 | 2.17 | 1.54 |
| Females | 0–6 | 2.15 | 1.71 | 0–6 | 2.02 | 1.38 | 0–6 | 2.33 | 1.80 | |
| Parity | Nulliparous | 0–3 | 1.83 | 1.27 | 0–2 | 1.20 | 0.92 | |||
| Parous | 0–6 | 2.35 | 1.98 | 0–6 | 2.15 | 1.48 | ||||
| Birthplace | Wild-caught | 1–6 | 3.70 | 1.49 | 0–5 | 2.16 | 1.46 | 0–7 | 3.00 | 2.20 |
| Zoo-born | 0–5 | 1.60 | 1.21 | 0–6 | 1.93 | 1.44 | 0–6 | 2.09 | 1.44 | |
| Rearing (zoo-born) | Mother-reared | 0–4 | 1.42 | 1.21 | 0–6 | 1.90 | 1.44 | 0–6 | 2.26 | 1.48 |
| Nursery-reared | 0–5 | 1.73 | 1.23 | 0–5 | 1.93 | 1.57 | 1 | 1.00 | 0.00 | |
| Gorillas | Chimpanzees | Bonobos | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | SD | Range | SD | Range | SD | |||||
| All-cause morbidity | Unaffected (0) | 0–6 | 1.68 | 1.40 | 0–5 | 1.88 | 1.36 | 1–5 | 2.15 | 1.31 |
| Affected (1) | 0–5 | 2.19 | 1.49 | 0–6 | 2.11 | 1.52 | 1–3 | 2.00 | 0.89 | |
| Cardiac disease | Unaffected (0) | 0–6 | 1.91 | 1.55 | 0–6 | 1.98 | 1.43 | 1–5 | 2.15 | 1.28 |
| Affected (1) | 0–4 | 1.91 | 1.28 | 0–6 | 2.06 | 1.52 | 1–3 | 2.00 | 1.00 | |
| Mortality | Alive (0) | 0–4 | 1.49 | 0.97 | 0–5 | 1.87 | 1.30 | 0–6 | 2.15 | 1.46 |
| Dead (1) | 0–6 | 2.48 | 1.79 | 0–6 | 2.33 | 1.66 | 0–7 | 2.67 | 2.42 | |
| Allostatic Load | Age | Sex | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | AICc |
| Gorillas (n = 68) | 0.070 | 1.45 | 0.99–2.25 | 96.39 | ||||||
| 0.0003 | 1.12 | 1.06–1.21 | 79.18 | |||||||
| 0.033 | 2.98 | 1.11–8.40 | 95.25 | |||||||
| 0.0001 | 1.16 | 1.09–1.27 | 0.004 | 9.75 | 2.37–56.40 | 70.87 | ||||
| 0.452 | 1.23 | 0.72–2.18 | 0.0002 | 1.16 | 1.08–1.26 | 0.004 | 10.02 | 2.42–58.71 | 72.32 | |
| Chimpanzees (n = 148) | 0.536 | 1.08 | 0.85–1.37 | 201.70 | ||||||
| 0.0004 | 1.06 | 1.03–1.09 | 188.25 | |||||||
| 0.155 | 0.60 | 0.30–1.21 | 200.04 | |||||||
| 0.0008 | 1.05 | 1.02–1.09 | 0.473 | 0.76 | 0.36–1.60 | 189.88 | ||||
| 0.715 | 0.95 | 0.73–1.23 | 0.0005 | 1.06 | 1.03–1.09 | 190.26 | ||||
| Bonobos (n = 33) | 0.635 | 0.79 | 0.24–1.84 | 34.75 | ||||||
| 0.025 | 1.21 | 1.06–1.50 | 24.97 | |||||||
| 0.693 | 0.67 | 0.07–4.99 | 34.84 | |||||||
| 0.031 | 1.22 | 1.06–1.55 | 0.784 | 1.45 | 0.10–31.19 | 27.71 | ||||
| 0.201 | 0.37 | 0.05–1.33 | 0.037 | 1.33 | 1.09–1.86 | 25.57 | ||||
| Allostatic Load | Age | Sex | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | AICc |
| Gorillas (n = 68) | 0.593 | 1.12 | 0.75–1.70 | 92.03 | ||||||
| 0.024 | 1.05 | 1.01–1.11 | 86.76 | |||||||
| 0.002 | 6.25 | 2.05–22.08 | 81.50 | |||||||
| 0.005 | 1.09 | 1.03–1.17 | 0.001 | 13.08 | 3.29–76.73 | 73.63 | ||||
| 0.957 | 0.99 | 0.59–1.65 | 0.007 | 1.09 | 1.03–1.17 | 0.001 | 13.05 | 3.28–76.68 | 75.96 | |
| Chimpanzees (n = 148) | 0.894 | 1.02 | 0.80–1.30 | 191.70 | ||||||
| 0.029 | 1.03 | 1.00–1.06 | 186.83 | |||||||
| 0.901 | 1.05 | 0.51–2.13 | 191.70 | |||||||
| 0.024 | 1.03 | 1.01–1.07 | 0.521 | 1.28 | 0.60–2.72 | 188.56 | ||||
| 0.696 | 0.95 | 0.74–1.22 | 0.028 | 1.03 | 1.00–1.07 | 188.82 | ||||
| Bonobos (n = 33) | 0.594 | 0.74 | 0.18–1.87 | 31.84 | ||||||
| 0.080 | 1.10 | 1.00–1.26 | 28.35 | |||||||
| 0.924 | 1.11 | 0.11–10.91 | 32.15 | |||||||
| 0.081 | 1.11 | 1.00–1.29 | 0.610 | 1.92 | 0.16–33.67 | 30.90 | ||||
| 0.440 | 0.60 | 0.10–1.76 | 0.082 | 1.12 | 1.00–1.32 | 30.43 | ||||
| Gorillas | Chimpanzees | Bonobos | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | β | HR | SE | z | p | AICc | β | HR | SE | z | p | AICc | β | HR | SE | z | p | AICc | |
| AL | 0.41 | 1.51 | 0.16 | 2.60 | 0.009 | 204.7 | 0.16 | 1.18 | 0.10 | 1.67 | 0.096 | 426.1 | 0.22 | 1.24 | 0.26 | 0.82 | 0.412 | 28.8 | |
| Age | 0.07 | 1.07 | 0.01 | 4.83 | <0.001 | 189.3 | 0.04 | 1.04 | 0.01 | 3.79 | <0.001 | 415.0 | 0.07 | 1.07 | 0.04 | 1.60 | 0.109 | 26.9 | |
| Sex | 0.03 | 1.03 | 0.39 | 0.07 | 0.944 | 211.8 | 0.45 | 1.57 | 0.29 | 1.53 | 0.126 | 426.5 | 1.52 | 4.59 | 1.15 | 1.33 | 0.185 | 27.3 | |
| Age + Sex | 189.3 | 410.9 | 29.2 | ||||||||||||||||
| Age | 0.08 | 1.09 | 0.02 | 4.86 | <0.001 | 0.05 | 1.05 | 0.01 | 4.27 | <0.001 | 0.09 | 1.09 | 0.05 | 1.66 | 0.097 | ||||
| Sex | 0.69 | 2.00 | 0.45 | 1.56 | 0.120 | 0.78 | 2.18 | 0.31 | 2.53 | 0.011 | 1.75 | 5.76 | 1.19 | 1.47 | 0.142 | ||||
| Age and/or Sex + AL | 191.7 | 411.3 | 31.8 | ||||||||||||||||
| Age | 0.07 | 1.07 | 0.02 | 3.98 | <0.001 | 0.05 | 1.05 | 0.01 | 4.23 | <0.001 | 0.06 | 1.06 | 0.04 | 1.35 | 0.176 | ||||
| Sex | 0.73 | 2.09 | 0.31 | 2.38 | 0.017 | ||||||||||||||
| AL | 0.03 | 1.03 | 0.16 | 0.21 | 0.836 | 0.14 | 1.15 | 0.10 | 1.47 | 0.141 | 0.09 | 1.09 | 0.28 | 0.31 | 0.757 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edes, A.N.; Edwards, K.L.; Zimmerman, D.; Jourdan, B.; Crews, D.E.; Wolfe, B.A.; Neiffer, D.L.; Brown, J.L. Comparing Predictors and Outcomes of Higher Allostatic Load across Zoo-Housed African Great Apes. J. Zool. Bot. Gard. 2023, 4, 158-175. https://doi.org/10.3390/jzbg4010016
Edes AN, Edwards KL, Zimmerman D, Jourdan B, Crews DE, Wolfe BA, Neiffer DL, Brown JL. Comparing Predictors and Outcomes of Higher Allostatic Load across Zoo-Housed African Great Apes. Journal of Zoological and Botanical Gardens. 2023; 4(1):158-175. https://doi.org/10.3390/jzbg4010016
Chicago/Turabian StyleEdes, Ashley N., Katie L. Edwards, Dawn Zimmerman, Balbine Jourdan, Douglas E. Crews, Barbara A. Wolfe, Donald L. Neiffer, and Janine L. Brown. 2023. "Comparing Predictors and Outcomes of Higher Allostatic Load across Zoo-Housed African Great Apes" Journal of Zoological and Botanical Gardens 4, no. 1: 158-175. https://doi.org/10.3390/jzbg4010016
APA StyleEdes, A. N., Edwards, K. L., Zimmerman, D., Jourdan, B., Crews, D. E., Wolfe, B. A., Neiffer, D. L., & Brown, J. L. (2023). Comparing Predictors and Outcomes of Higher Allostatic Load across Zoo-Housed African Great Apes. Journal of Zoological and Botanical Gardens, 4(1), 158-175. https://doi.org/10.3390/jzbg4010016








