Abstract
Nearly 10% of IVF patients experience repeated implantation failure (RIF). Although several meta-analyses report improved outcomes following peripheral blood mononuclear cell (PBMC) administration, the uterine mechanisms remain poorly understood. We first analyzed PBMC composition and cytokine secretion in a preliminary cohort (n = 18), followed by endometrial immune profiling in a larger cohort (n = 70) before and after PBMC treatment. Embryo transfer was performed in 41 women, enabling the assessment of associations between immune profiles and implantation success. Successful implantation occurred in 16 of 41 embryo transfers (39%). PBMCs were predominantly composed of lymphocytes (60.7%), with T helper cells as the predominant T cell subset (Th/cytT ratio 1.44). Cytokine assays confirmed secretion of TNF-α, IL-6, IL-4, and IL-10. C-reactive protein levels remained below the threshold for systemic inflammation and were unaffected by PBMC administration. In the full cohort, PBMC infusion significantly enriched stromal macrophages and T helper cells, reflected by higher Th/T, Th/MΦ, and Th/cytotoxic T cell ratios and a reduced cytotoxic T/T cell ratio (all p ≤ 0.001). Importantly, women with successful implantation exhibited a significantly higher macrophage/T cell ratio (1.15 vs. 0.74; p = 0.024). These findings suggest that PBMC administration reshapes the endometrial immune landscape and that the macrophage/T cell ratio may serve as a promising biomarker of treatment efficacy.
1. Introduction
Recurrent implantation failure (RIF) remains one of the major challenges in assisted reproductive medicine, affecting approximately 10% of the women undergoing in vitro fertilization (IVF). Despite the many advances in assisted reproductive techniques, patients still experience repeated unsuccessful transfer cycles of good-quality embryos, resulting in considerable emotional and clinical burden.
Numerous studies have emphasized on the endometrial immune environment as a main determinant in the process of embryo implantation [1]. The balance between pro- and anti-inflammatory behavior of the immune cells in terms of cytokine expression is crucial for successful embryo implantation [2]. In a previous study, the balanced presence of uterine natural killer cells, T cells, and macrophages was found to be dysregulated in patients experiencing RIF [3].
Intrauterine administration of autologous peripheral blood mononuclear cells (PBMCs) has emerged as a potential therapeutic strategy for patients with RIF. Although a series of reviews and meta-analyses show the positive effect of uterine PBMC administration on the success rate of patients with recurrent implantation failures, the reproductive societies are cautious about recommending it in routine practice [4,5,6,7]. The reason behind this is that there are safety concerns, as PBMC administration may induce systemic inflammation and the mechanism of its action in the uterus is poorly studied [8].
There are many hypotheses for the mechanism of action of PBMC on the endometrium. The local immunomodulatory effects could be due to the action of the cytokines secreted by PBMC placed in the cavity [9,10]. A 2021 study examined the peripheral and local balance of Treg and Th17 lymphocytes after PBMC administration in mice [11]. According to it, the expression of Treg and Th17-associated transcription factors and cytokines in the local endometrium changes after 2.5 days of PBMC administration. Since naive T cells require more time to proliferate and differentiate, it is possible that earlier changes are induced by the migration of systemic immune cells into the endometrium [11]. In other similar studies, Wang et al. and Qin et al. demonstrated that intrauterine exogenous PBMCs release cytokines (TGF-β and IL-2) and chemokines (CCL2, CCL17, CCL21, and CCL22), thereby regulating the intrauterine immune microenvironment and recruiting Treg cells to the endometrium [9,10].
The aim of this study was to assess the effect of intrauterine administration of autologous PBMC on the endometrial immune environment and IVF outcome in patients with repeated implantation failure.
2. Materials and Methods
2.1. Patients and Experimental Design
This prospective pre/post-study includes 18 patients in an initial PBMC characterization study and 52 women in the main study cohort. All 70 women (aged between 20 and 45 years old) meet the following inclusion criteria: undergoing IVF with history of RIF, defined as the failure to achieve clinical pregnancy after transfer of at least four good-quality embryos in a minimum of three fresh or frozen cycles; being scheduled for intrauterine administration of autologous PBMC by their attending physician; undergoing embryo transfer up to 3 months following PBMC administration. Women with genetic abnormalities, autoimmune, or endocrine disorders as well as endometrial pathology such as endometriosis, endometritis, atrophic endometrium, endometrial polyps, adenomyosis, hyperplasia, carcinoma, or bacterial infection were excluded from the study. The whole study cohort (n = 70) comprised Bulgarian women of the Caucasian race Figure 1.
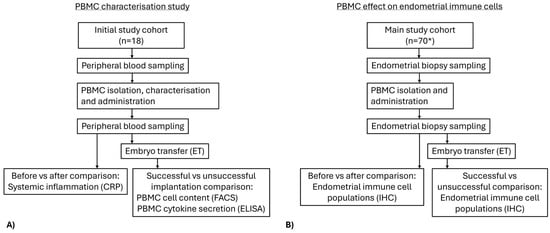
Figure 1.
Flowchart of the study design. (A) Initial study on characterization of PBMC content and cytokine secretion and effect of PBMC administration on systemic inflammation and embryo transfer outcome. (B) Main study on before/after PBMC administration comparison of endometrial immune cell populations and ratios and embryo transfer outcome following PBMC treatment. * Total number of participants including those from the initial PBMC characterization study.
In the preliminary study, the cellular composition and cytokine secretion profile of PBMCs isolated from 18 women were analyzed; their IVF outcome was also assessed. To evaluate the systemic effect of PBMC treatment, markers of systemic inflammation were analyzed before and after PBMC administration.
The experimental design included obtaining 2 endometrial biopsies from each patient before and after PBMC administration of all 70 women. The first endometrial biopsies were collected during the mid-luteal phase of the menstrual cycle, specifically seven days after the luteinizing hormone peak (LH + 7). In the subsequent cycle, 12 mL peripheral blood for PBMC isolation and cultivation were drawn. After 24 h of culture, intrauterine administration of PBMCs was performed on day LH + 5. After 2 days, a second endometrial biopsy was obtained on day LH + 7. The presence and abundance of immune cell populations in the paired endometrial biopsies, collected before and after intrauterine PBMC administration, were compared by immunohistochemistry. Subsequently, all patients underwent a standard in vitro fertilization (IVF) procedure and embryo transfer, all performed in accordance with established clinical practice.
The study was registered (Clin Trial ID: NCT05421364) and approved by the Institutional Review Board N:10/12.04.2022. All patients signed written informed consent. The study was conducted in accordance with the World Medical Association Declaration of Helsinki.
2.2. IVF Procedure
All 70 of the included patients underwent standard IVF procedure. Along the procedures 29 women dropped out of the study due to poor quality or aneuploid embryos or had their embryos frozen without further action. The remaining 41 women underwent standard IVF procedure without ovarian stimulation with single frozen embryo transfer of euploid embryo of high morphological grade to ensure embryo quality did not influence outcome variability. Embryo implantation outcome was assessed by peripheral blood hCG measurement 14 days after embryo transfer (Cobas e601; Roche Diagnostics, Basel, Switzerland).
2.3. PBMC Isolation and Administration
Peripheral blood mononuclear cells (PBMCs) were obtained on day 4 after LH peak (LH + 4). Briefly, 12 mL peripheral blood were diluted (1:1 with Dulbecco’s Phosphate-Buffered Saline (PBS) (VWR, VWRRMS01OQ, Radnor, PA, USA)) and subjected to 25 min of gradient centrifugation (Pancoll human, density 1.077 g/mL (Pan Biotech, P04-60100, Bavaria, Germany)) at 400 G on Eppendorf Centrifuge (5804 R). The buffy coat was collected, and after washing, was cultured at a concentration of 10 × 106/mL in RPMI 1640 (PAA, E15-842, Pasching, Austria) with added 1% human serum albumin (HSA) (Sage, ART-3001, Newcastle upon Tyne, England) and antibiotic (Pan Biotech, P06-07300, Aidenbach, Germany) in 36.6 °C 5% CO2 in incubator Galaxy 170R (Eppendorf, Hamburg, Germany). After 24 h of cultivation, PBMCs along with the culture media were administered into the uterine cavity using catheter (Gynétics Medical Products N.V, Lommel, Belgium) under ultrasonographic guidance.
2.4. PBMC Cytokine Secretion
The cytokines secreted from the cultivated PBMC in the culture media were quantified by enzyme-linked immunosorbent assay (ELISA). The amounts of IL-6, TNF-α, IL-4, and IL-10 were measured by, respectively, Human IL-6 (Interleukin 6) ELISA Kit (CSB-E04638h, Cusabio, Houston, TX, USA), Human Tumor necrosis factor α, TNF-α ELISA Kit (CSB-E04740h, Cusabio), Human IL-4 (Interleukin 4) ELISA Kit (CSB E04633h, Cusabio), and Human IL-10 (Interleukin 10) ELISA Kit (CSB E04593h, Cusabio), according to the manufacturer’s instructions.
2.5. PBMC Content
The isolated PBMC content (CD3+ total T lymphocytes, CD3+CD4+CD8- T helper cells, CD3+CD4-CD8+ cytotoxic T cells, CD3+CD4+CD8+ double-positive T cells, and CD3+CD4-CD8- double-negative T cells) was characterized by flowcytometrical analyses by antibodies against CD45 (PerCP-conjugated, BD Biosciences 345809, Franklin Lakes, NJ, USA), CD3 (PE-conjugated, Immuno Tools GmbH 21620034X2, Friesoythe, Germany), CD4 (FITC conjugated, Immuno Tools GmbH 21459043X2), and CD8 (APC-conjugated, Immuno Tools GmbH 21810086X2) using BD FACS Calibur flowcytometer and CellQuest software (v. 5.1). Lymphocytes, granulocytes, and monocytes were identified and gated by their forward and side scatter. Appropriate negative and positive controls were used. Gating strategy is shown in Figure A1.
2.6. Endometrial Biopsies
Two endometrial biopsies were obtained from each patient. Both were taken on day 7 after LH peak (LH + 7), the second was taken two days post-PBMC administration. Endometrial tissue was taken using a Pipelle for endometrial suction (CCD 1103000, Laboratoire CCD, Paris, France), under ultrasonographic guidance, and placed in 4% formaldehyde for subsequent immunohistochemical staining.
2.7. Endometrial Immune Cell Quantification
The endometrial immune cells, comprising total T lymphocytes, cytotoxic T cells, T helper cells, macrophages, and NK cells, were identified by immunohistochemistry using antibodies against CD3 (BRB063, Zytomed Systems, Berlin, Germany), CD8 (1-CD040-02, Quartett, Munich, Germany), CD4 (IS649, Dako, Santa Clara, CA, USA), CD68 (IS613, Dako), and CD56 (A00121-0007, ScyTek, Logan, UT, USA), respectively. The staining procedure was conducted as previously described [3], by using Novolink Polymer Detection System (Leica Biosystems, Nussloch, Germany) according to the manufacturer’s instructions on Leica Bond-Max clinical staining system (Leica Biosystems). Appropriate negative controls were also prepared.
2.8. Image Analysis
Endometrial immune cells were enumerated using HALO Image Analysis Software v3.4.2986.185 (Indica Labs, Albuquerque, NM, USA) with Cytonuclear Module v2.0.9. Five areas of each endometrial section were captured at 400× magnification with a high-resolution digital camera (IDS Imaging Development Systems GmbH, Obersulm, Germany).
A quantitative analysis of the number of stromal cells, the number stained with haematoxylin and eosin, and the number of cells stained with DAB was performed. The results are presented as the number of stromal cells and the number of positively stained cells. The analysis included the percentage of stained cells out of the total number of stromal cells for each preparation.
2.9. Statistical Analyses
Statistical analyses were conducted using Python (version 3.11) and SPSS v.21 (IBM, Chicago, IL, USA). Data distribution was analyzed for normality by the Kolmogorov–Smirnov Test. In cases of normal distribution, data was presented as mean and standard deviation (mean ± SD), otherwise data was presented as median and interquartile range (median [IQR]).
The distribution data between two patient groups were compared with the parametric Student’s t-test or the non-parametric Mann–Whitney U test. Pre–post comparison was performed using non-parametric Wilcoxon Signed Rank Test. p < 0.05 was considered significant. Figures were prepared using Python (version 3.11) and BioRender.com.
3. Results
3.1. PBMC Composition and Cytokine Secretion
PBMC subsets were quantified in the preliminary cohort. The mean percentages of each cell type and the standard deviation for the analyzed parameters are summarized in Table 1. In the isolated PBMCs, with slight variations, the highest percentage was lymphocytes (60.7%), which include T lymphocytes, B lymphocytes, and NK cells. Further investigation of the T lymphocyte fraction within the isolated PBMCs showed that T helper cells represented the predominant subset, with a Th/Tc ratio of 1.44.

Table 1.
PBMC characterization: cell composition and cytokine secretion.
Cytokine secretion analysis revealed that all examined cytokines (TNF-α, IL-6, IL-4, and IL-10) were present in the PBMC culture media with the highest amount of IL-6 and the lowest amount of IL-4 (Table 1). These findings established baseline PBMC immune activity prior to treatment.
3.2. PBMC Composition and Cytokine Secretion According to IVF Outcome in the Preliminary Cohort
Among the 18 women included in the preliminary cohort, IVF was successful in 36.4% (4/11) of the women and unsuccessful in 63.4% (7/11) of the women.
Women with successful embryo implantation showed similar proportions of lymphocytes, monocytes, granulocytes as well as T lymphocyte subsets when compared with unsuccessful patients. Cytokine secretion profiles also did not show variation by IVF outcome (Table 2).

Table 2.
PBMC composition and cytokine secretion according to IVF outcome in the preliminary analysis.
Together, these results suggest that both PBMC immune cell composition and cytokine secretion patterns cannot distinguish women who achieve pregnancy from those who do not in the preliminary cohort.
3.3. CRP Measurements in the Preliminary Cohort
CRP levels were measured before and after PBMC administration to assess systemic inflammation. No women had CRP above the threshold for peripheral inflammation (>0.6 mg/L) before or after PBMC administration. There was no significant difference in CRP levels between pre- and post-PBMC administration measurements (0.04 mg/L [0.14] vs. 0.05 mg/L [0.05], respectively, p = 0.497).
When comparing the change in CRP levels (δCRP) between successful and unsuccessful embryo implantation groups, no significant difference was observed. Median CRP levels before and after PBMC administration were 0.06 mg/L [0.05] vs. 0.05 [0.04] mg/L in the successful group and 0.07 mg/L [0.22] vs. 0.05 [0.07] mg/L in the unsuccessful group, respectively. The median δCRP was 0.01 mg/L [0.06] in the successful group vs. 0.00 mg/L [0.12] in the unsuccessful group, p = 0.412.
3.4. PBMC and Endometrial Immune Content Before and After Treatment
Endometrial immune cell analysis showed that PBMC administration leads to stromal enrichment of macrophages and T helper cells (Table 3, Figure 2). Furthermore, from the immune cell ratios examined, Th/T (0.13 [IQR 0.15] vs. 0.27 [IQR 0.28], p < 0.001), Th/MΦ (0.17 [IQR 0.23] vs. 0.33 [IQR 0.33], p < 0.001), and Th/cytT (0.16 [IQR 0.24] vs. 0.40 [IQR 0.52], p < 0.001) were significantly increased, while cytT/T (0.87 [IQR 0.23] vs. 0.72 [IQR 0.37], p = 0.001) was significantly decreased following PBMC administration (Table 3, Figure 3). Representative staining of successful and unsuccessful implantation cases can be observed in Figure A2 and Figure A3, respectively.

Table 3.
Endometrial immune cells and ratios before and after intrauterine PBMC administration.
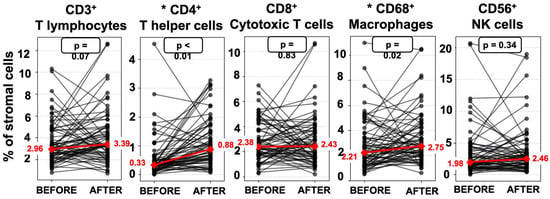
Figure 2.
Connected dot plots of endometrial immune cells before and after PBMC administration. Red line: median values change. * Statistically significant difference.
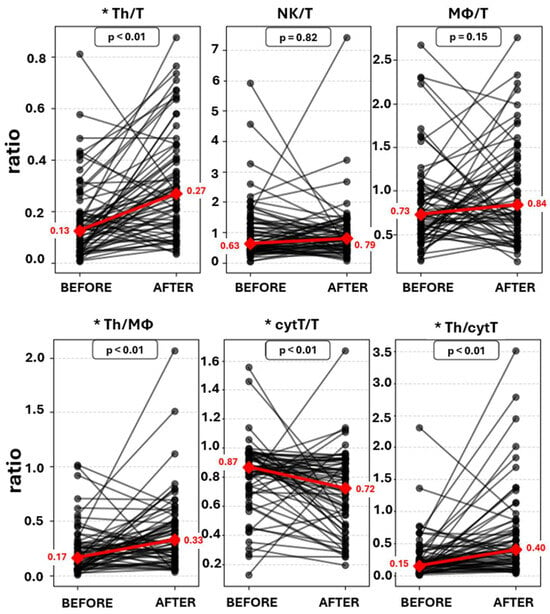
Figure 3.
Connected dot plots of endometrial immune cell ratios before and after PBMC administration. Red line: median values change. * Statistically significant change.
3.5. Association Between Endometrial Immune Cells and IVF Success
Among the 70 women included in the full cohort, 41 underwent embryo transfer, of which, 39% (16/41) achieved clinical pregnancy, while 61% (25/41) did not. The endometrial immune cell composition following PBMC administration, in terms of percentages of the stromal cells, did not differ significantly between the successful and unsuccessful groups. In contrast, the MΦ/T cell ratio was significantly higher in women with successful implantation outcome (1.15 [IQR 1.09]) compared to those with unsuccessful implantation (0.74 [IQR 0.60]; p = 0.024) (Table 4, Figure 4 and Figure 5).

Table 4.
Endometrial immune cells and ratios before and after intrauterine PBMC administration according to implantation outcome following embryo transfer.
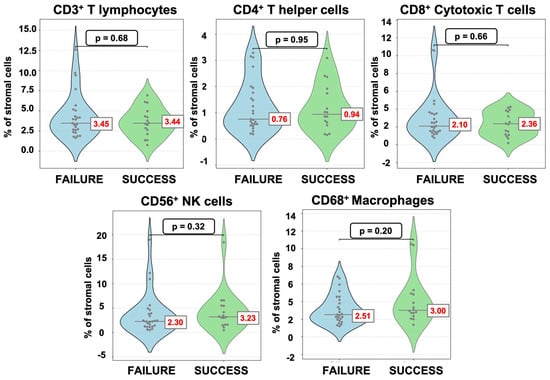
Figure 4.
Violin dot plots of endometrial immune cells after PBMC administration according to implantation outcome.
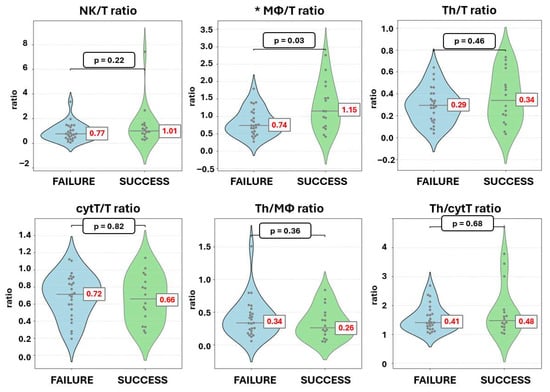
Figure 5.
Violin dot plots of endometrial immune cell ratios after PBMC administration according to implantation outcome. * Statistically significant change.
4. Discussion
This study aimed to evaluate the impact of intrauterine administration of autologous PBMC on the endometrial immune microenvironment and subsequent IVF outcomes in women with repeated implantation failure. In line with this objective, we observed that PBMC treatment was associated with favorable shifts in endometrial immune ratios, suggesting that modulation of the local immune landscape may have contributed to the implantation rates observed in this cohort.
As an approved systemic inflammation marker [12], peripheral blood C-reactive protein (CRP) in the peripheral blood of patients before and after PBMC administration was compared as a safety regulation. No increase or even differences in serum CRP levels was found following PBMC administration, showing no systemic inflammatory process after PBMC administration.
Looking into the mechanism of action of the administered PBMC, all four studied cytokines (IL-6, IL-10, IL-4, and TNF-α) were found in the medium of cultured PBMC. All of them have a role in the regulation of the endometrial immune environment. According to previous studies, PBMC-secreted cytokines modulate the endometrial immune microenvironment and facilitate the recruitment of Treg cells [9,10]. The anti-inflammatory cytokines IL-4 and IL-10 inhibit Th1 cells and macrophages and prevent embryo rejection, while the pro-inflammatory IL-6 and TNF-α, in balanced amounts, are involved in angiogenesis and trophoblast invasion [13] (Figure 6).
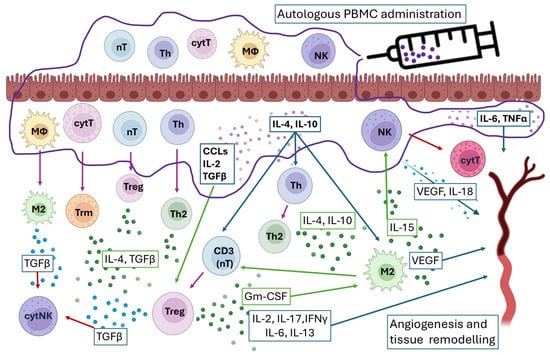
Figure 6.
Proposed mechanism of action of intrauterine administration of PBMC. Arrow colors: green—activation; purple—turn into; blue—cytokine interaction; red—blocking.
To examine the cellular mechanism of PBMC endometrial modulation, we compared the immune cell quantities and ratios in the endometrium before and after PBMC administration. Interestingly, the cellular composition of PBMCs, enriched in monocytes/macrophage precursors and T helper subsets, mirrors the immune cell types that were elevated in the endometrium following PBMC administration, suggesting a direct contribution of infused PBMCs to the observed immune shifts. This hypothesis needs further investigation in a separate experiment.
Previous studies on the PBMC mechanism of action discuss that possible migration of systemic immune cells rather than local T cell differentiation, due to changes in transcription factors and cytokine expression in fewer days than the naïve T cell differentiation, can occur [11]. This might be additional explanation for the results regarding the elevated Th in the post-PBMC endometrium (Figure 6).
It is known that CD4+ T helper cells play a fundamental role in establishing the immune response, performing diverse functions through the secretion of cytokines that induce pro- or anti-inflammatory responses [14]. According to the literature, the presence of increased numbers of CD4+ regulatory cells and Th2 type T helpers following uterine immune treatment is associated with higher implantation success in patients with recurrent failures [15,16]. There is limited consensus regarding the specific immune cell quantities associated with endometrial receptivity [8]. Therefore, it is reasonable to conclude that implantation depends not on absolute abundance but more on the immune equilibrium (Figure 6).
Here, we observed elevated endometrial Th/T, Th/MΦ, and Th/cytT ratios and lower cytT/T ratio following PBMC administration, indicating a shift toward a more receptive endometrial immune profile. According to our previous study, MΦ/T, Th/T, and Th/cytT ratios are indicative of the receptive state of the endometrium and were proposed as predictors and therapy guiders [3]. Building on this, T helper cells exert multifaceted control over CD8+ effector functions through immunosuppressive cytokines (IL-10, TGF-β), checkpoint molecule expression, and programming toward long-term memory differentiation [17]. In parallel, endometrial macrophages predominantly adopt an M2 phenotype under the influence of IL-10, TGF-β, glucocorticoids, IL-4, and IL-13, secreted by T helper cells [18]. In turn, macrophages amplify immune regulation by secreting IL-10, TGF-β, and IDO, suppressing cytotoxic T cell activity and inducing Treg differentiation, underscoring the layered complexity of macrophage–T cell interactions in tissue homeostasis and immune tolerance [19,20,21]. Such a crosstalk between T helper cells, macrophages, and cytotoxic subsets could create an immunological environment that supports embryo implantation (Figure 6 and Figure 7).
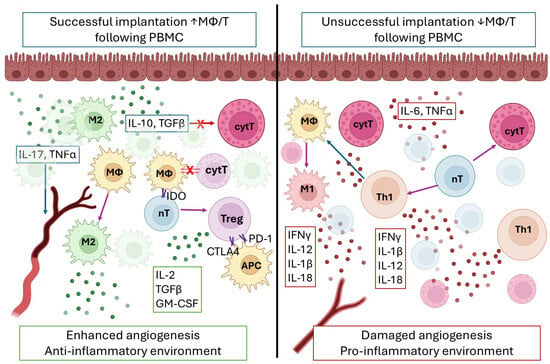
Figure 7.
Interaction between macrophages (MΦ) and T lymphocytes during the implantation window in successful (left) and unsuccessful (right) embryo implantation case. Note the higher MΦ/T on the left and the lower MΦ/T on the right. Arrow colors: green—activation; purple—turn into; blue—cytokine interaction; red—blocking. X—blocking; ↑—higher; ↓—lower.
In terms of clinical outcomes, although the sample size of 41 patients is relatively small, successful embryo implantation was observed in 39% of the women following intrauterine administration of PBMC. For comparison, according to CDC data from 2021, the average implantation success rate after a first in vitro fertilization cycle in women aged 35–40 years is 26% [22]. Thus, PBMC administration may account for more than a 10% increase in implantation success compared to the expected baseline. The positive effect of PBMC on implantation outcome was previously discussed in numerous clinical studies and meta-analyses [4,5,6,7]. Also, an ongoing registered clinical trial investigating the effects of intrauterine PBMC administration on implantation and pregnancy outcomes will provide further data to validate these observations [23].
The lack of efficacy observed in some cases does not appear to be attributable to differences in PBMC composition or cytokine secretion, as no significant variations were identified between successful and failed outcomes. These findings suggest that other mechanisms may be involved, potentially linking to the phenotypic characteristics of the resident immune cells within the pre-PBMC endometrial stroma. In particular, variations in the activation state of macrophages and T lymphocytes could influence the capacity of PBMCs to exert their intended immunomodulatory effects. This highlights the importance of assessing endometrial immune cell profiles in patients with recurrent implantation failure prior to initiating any form of immune-based treatment.
When comparing the post-PBMC endometrial immune profiles, a significant increase in the endometrial MΦ/T ratio was observed in the group with improved endometrial receptivity following PBMC administration. Our previous study identified this ratio as a sensitive indicator of receptivity [3]. The crosstalk between macrophages and T lymphocytes is central to endometrial regulation and embryo acceptance as mentioned. In cases of failed implantation, the shifted balance between macrophages to T lymphocytes may lead to a shift in the immune profile towards cytotoxicity. More specifically, CD68+ macrophages undergo phenotypic change into pro-inflammatory macrophages, in turn influencing naïve T cells to convert into cytotoxic T lymphocytes or T helper cells secreting pro-inflammatory cytokines. In this context, the endometrial environment, enriched in cytokines such as IFNγ, IL-12, IL-1β, IL-18, IL-6, and TNFα, turns pro-inflammatory, hindering embryo implantation and damaging angiogenesis (Figure 7).
In the other case, where successful embryo implantation is observed, the increase in MΦ/T ratio might lead to blocking through direct contact of the cytotoxic T subsets and naive T lymphocytes, leading to conversion into T helper cells secreting Th2-type cytokines. In turn the endometrial environment, enriched in IL-10, GM-CSF, and TGFβ, helps embryo invasion and enhances angiogenesis (Figure 6). Interestingly, MΦ/T ratio was previously associated with tumor progression, suggesting a similar mechanism in control of tissue invasion during embryo implantation [24].
This study has several limitations that should be acknowledged: firstly, the limited sample size; secondly, the absence of a control group for direct comparison of IVF outcomes with and without PBMC treatment; finally, the fate of the administered PBMCs in the endometrial stroma could not be followed, and in future studies, animal models should be used; future studies with larger cohorts, appropriate control groups, and longitudinal assessment of immune cell dynamics are warranted to validate and extend these findings.
5. Conclusions
In summary, intrauterine PBMC administration is a safe treatment for repeated implantation failure in patients as it does not induce systemic inflammation. Its action is expressed as shifting the endometrial immune microenvironment toward a more receptive profile, characterized by the enrichment of T helper cells and macrophages, increased Th/T, Th/MΦ, and Th/cytT ratios, and reduced cytT/T ratio. These immunological shifts may underlie the beneficial clinical effects of PBMC therapy observed in recurrent implantation failure. Beyond highlighting immune ratios as potential biomarkers, our findings provide mechanistic insight into how PBMC administration promotes receptivity, warranting further evaluation as a therapeutic option in IVF.
Author Contributions
Conceptualization, R.G.; methodology, R.G. and D.P.; software, D.P. and L.J.; validation, D.M. and T.T.; formal analysis, R.G.; investigation, M.H., J.S., L.J., and T.T.; resources, G.S., L.J., and T.T.; data curation, R.G. and D.P.; writing—original draft preparation, R.G.; writing—review and editing, M.R. and D.P.; visualization, R.G., M.R., and M.H.; supervision, G.S. and D.P.; project administration, G.S. and S.H.; funding acquisition, S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European UnionNextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No BG-RRP-2.004-0004-C01.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Nadezhda Women’s Health Hospital (N:10/12.04.2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original data presented in the study are openly available on zenodo.org at DOI: https://doi.org/10.5281/zenodo.17200353.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CCL | Chemokine C-C motif Ligand |
| CD | Cluster of Differentiation |
| CRP | C-reactive Protein |
| cytT | Cytotoxic T Cell |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| hCG | Human Chorion Gonadotropin |
| IL | Interleukin |
| IQR | Inter Quartile Range |
| IVF | In Vitro Fertilization |
| LH | Luteinizing Hormone |
| M2 | Macrophages Type 2 |
| MΦ | Macrophages |
| NK | Natural Killer |
| PBMCs | Peripheral Blood Mononuclear Cells |
| PBS | Phosphate-Buffered Saline |
| RIF | Repeated Implantation Failure |
| TGF-β | Transforming Growth Factor β |
| Th | T helper |
| TNF-α | Tumor Necrosis Factor α |
Appendix A
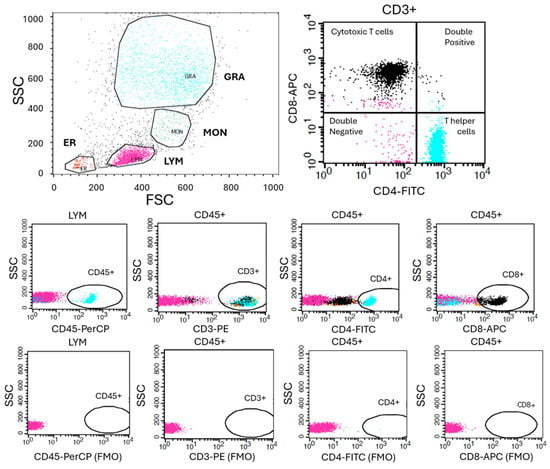
Figure A1.
Gating strategy for flow cytometric analysis. Representative gating strategy used for the identification of PBMC populations. Initial gating was performed on forward scatter (FSC) versus side scatter (SSC) to determine Lymphocyte, Monocyte and Granulocyte populations. Fluorescence-minus-one (FMO) controls were used to define positive gating boundaries for each fluorochrome. Within the live lymphocyte population, CD45+CD3+ T lymphocytes were identified and further subdivided into CD3+CD4+CD8− T helper cells, CD3+CD4−CD8+ cytotoxic T cells, CD3+CD4+CD8+ double-positive T cells, and CD3+CD4−CD8− double-negative T cells.
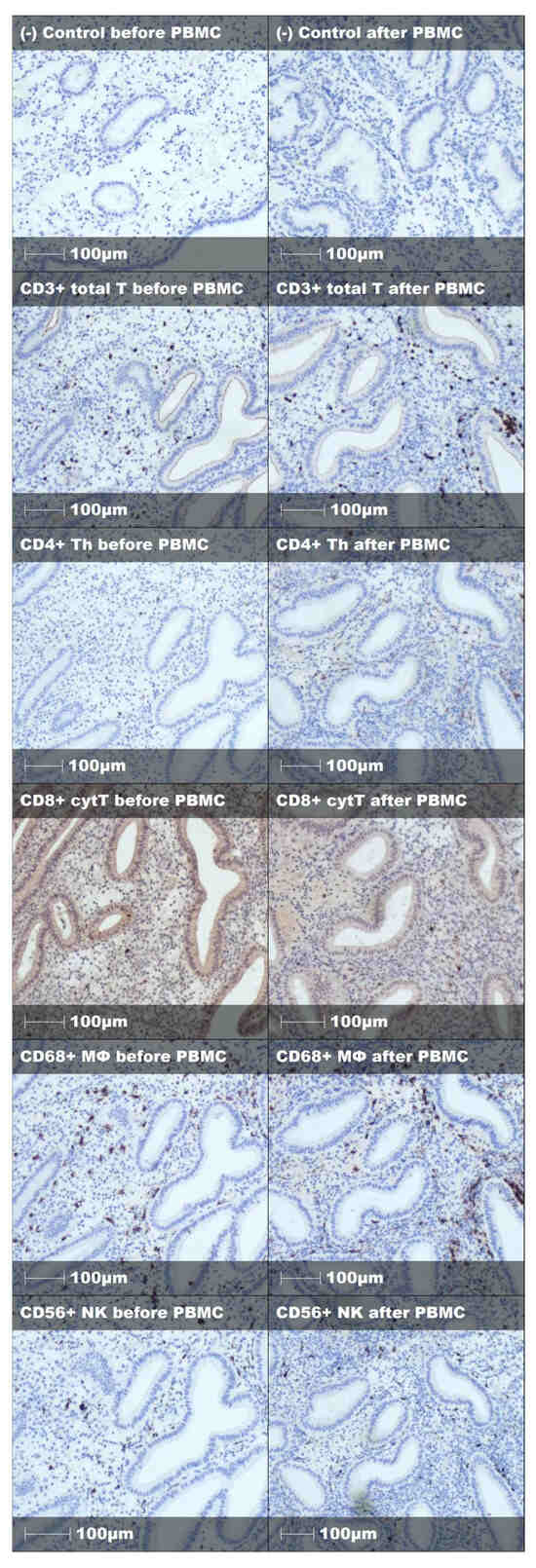
Figure A2.
Representative staining and negative control of endometrial immune cells before and after PBMC treatment in successful embryo implantation case.
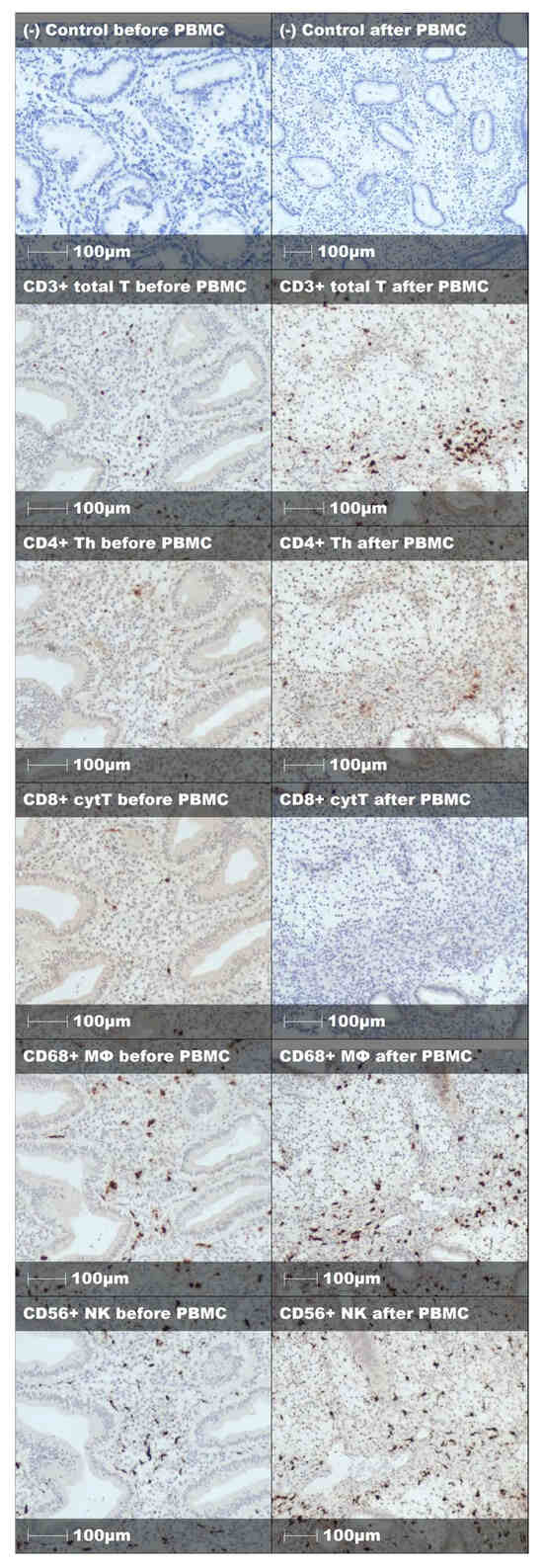
Figure A3.
Representative staining and negative control of endometrial immune cells before and after PBMC treatment in unsuccessful embryo implantation case.
References
- Saito, S. Role of immune cells in the establishment of implantation and maintenance of pregnancy and immunomodulatory therapies for patients with repeated implantation failure and recurrent pregnancy loss. Reprod. Med. Biol. 2024, 23, e12600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lédée, N.; Petitbarat, M.; Chevrier, L. The uterine immune profile may help women with repeated unexplained embryo implantation failure after in vitro fertilization. Am. J. Reprod. Immunol. 2016, 75, 388–401. [Google Scholar] [CrossRef]
- Ganeva, R.; Parvanov, D.; Vidolova, N.; Ruseva, M.; Handzhiyska, M.; Arsov, K.; Decheva, I.; Metodiev, D.; Moskova-Doumanova, V.; Stamenov, G. Endometrial immune cell ratios and implantation success in patients with recurrent implantation failure. J. Reprod. Immunol. 2023, 156, 103816. [Google Scholar] [CrossRef] [PubMed]
- Pourmoghadam, Z.; Abdolmohammadi-Vahid, S.; Pashazadeh, F.; Aghebati-Maleki, L.; Ansari, F.; Yousefi, M. Efficacy of intrauterine administration of autologous peripheral blood mononuclear cells on the pregnancy outcomes in patients with recurrent implantation failure: A systematic review and meta-analysis. J. Reprod. Immunol. 2020, 137, 103077. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, L.; Liu, L.; Yang, X.; Yan, P.; Yang, K.; Zhang, X. Autologous peripheral blood mononuclear cells intrauterine instillation to improve pregnancy outcomes after recurrent implantation failure: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2019, 300, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Yakin, K.; Oktem, O.; Urman, B. Intrauterine administration of peripheral mononuclear cells in recurrent implantation failure: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 3897. [Google Scholar] [CrossRef]
- Liu, M.; Yuan, Y.; Qiao, Y.; Tang, Y.; Sui, X.; Yin, P.; Yang, D. The effectiveness of immunomodulatory therapies for patients with repeated implantation failure: A systematic review and network meta-analysis. Sci. Rep. 2022, 12, 18434. [Google Scholar] [CrossRef]
- ESHREWorking Group on Recurrent Implantation Failure; Cimadomo, D.; de Los Santos, M.J.; Griesinger, G.; Lainas, G.; Le Clef, N.; McLernon, D.J.; Montjean, D.; Toth, B.; Vermeulen, N.; et al. ESHRE good practice recommendations on recurrent implantation failure. Hum. Reprod. Open 2023, 2023, hoad023. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhou, W.J.; Luo, X.Z.; Tao, Y.; Li, D.J. Synergistic effect of regulatory T cells and proinflammatory cytokines in angiogenesis in the endometriotic milieu. Hum. Reprod. 2017, 32, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Sui, Y.; Soloff, A.C.; Junecko, B.A.; Kirschner, D.E.; Murphey-Corb, M.A.; Watkins, S.C.; Tarwater, P.M.; Pease, J.E.; Barratt, S.M.; et al. Chemokine and cytokine mediated loss of regulatory T cells in lymph nodes during pathogenic simian immunodeficiency virus infection. J. Immunol. 2008, 180, 5530–5536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, L.; Sha, M.; Li, W.; Kang, Q.; Wu, J.; Chen, S.; Yu, N. Intrauterine administration of peripheral blood mononuclear cells (PBMCs) improves embryo implantation in mice by regulating local Treg/Th17 cell balance. J. Reprod. Dev. 2021, 67, 359–368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Menzel, A.; Samouda, H.; Dohet, F.; Loap, S.; Ellulu, M.S.; Bohn, T. Common and Novel Markers for Measuring Inflammation and Oxidative Stress Ex Vivo in Research and Clinical Practice-Which to Use Regarding Disease Outcomes? Antioxidants 2021, 10, 414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kitazawa, J.; Kimura, F.; Nakamura, A.; Morimune, A.; Takahashi, A.; Takashima, A.; Amano, T.; Tsuji, S.; Kaku, S.; Kasahara, K.; et al. Endometrial Immunity for Embryo Implantation and Pregnancy Establishment. Tohoku J. Exp. Med. 2020, 250, 49–60. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+T cells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmadi, M.; Abdolmohammadi-Vahid, S.; Ghaebi, M.; Aghebati-Maleki, L.; Dolati, S.; Farzadi, L.; Ghasemzadeh, A.; Hamdi, K.; Younesi, V.; Nouri, M.; et al. Regulatory T cells improve pregnancy rate in RIF patients after additional IVIG treatment. Syst. Biol. Reprod. Med. 2017, 63, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Ricaud, G.; Vaillancourt, C.; Blais, V.; Disdier, M.; Joao, F.; Johnson, B.; Benkhalifa, M.; Miron, P.; Bernier, J. Role of T cells in intrauterine administration of activated peripheral blood mononuclear cells in recurrent implantation failure. bioRxiv 2021. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Wang, X.H.; Zhou, W.J.; Jin, L.P.; Li, M.Q. Crosstalk between human endometrial stromal cells and decidual NK cells promotes decidualization in vitro by upregulating IL-25. Mol. Med. Rep. 2018, 17, 2869–2878. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heikkinen, J.; Möttönen, M.; Komi, J.; Alanen, A.; Lassila, O. Phenotypic characterization of human decidual macrophages. Clin. Exp. Immunol. 2003, 131, 498–505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, C.; Chang, M.Y.; Parker, K.H.; Beury, D.W.; DuHadaway, J.B.; Flick, H.E.; Boulden, J.; Sutanto-Ward, E.; Soler, A.P.; Laury-Kleintop, L.D.; et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012, 2, 722–735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Annacker, O.; Asseman, C.; Read, S.; Powrie, F. Interleukin-10 in the regulation of T cell-induced colitis. J. Autoimmun. 2003, 20, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Curtsinger, J.M.; Mescher, M.F. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 2010, 22, 333–340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- The Center for Disease Control and Prevention (CDC). Assisted Reproductive Technology (ART) Fertility Clinic and National Summary Report; The Center for Disease Control and Prevention (CDC): Atlanta, Georgia, 2021. [Google Scholar]
- Nadezhda Women’s Health Hospital. Effects of Intrauterine Administration of Autologous Pbmc on the Endometrial Cells Populations; National Library of Medicine (US): Bethesda, MD, USA, 2022.
- Xin, H.; Liang, D.; Zhang, M.; Ren, D.; Chen, H.; Zhang, H.; Li, S.; Ding, G.; Zhang, C.; Ding, Z.; et al. The CD68+ macrophages to CD8+ T-cell ratio is associated with clinical outcomes in hepatitis B virus (HBV)-related hepatocellular carcinoma. HPB 2021, 23, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).