Abstract
Recent advances in organoid technology have revolutionized cancer biology and therapeutic interventions, offering personalized immunotherapy treatment. Organoids, three-dimensional cell cultures derived from patient tumors, accurately replicate the tumor microenvironment, providing unprecedented insights into tumor-immune interactions and therapeutic responses. In this literature-based study, we discuss various culture methods for the diverse applications of organoids in cancer immunotherapy, including drug screening, personalized treatment strategies, and mechanistic studies. Additionally, we address the technological challenges associated with these methods and propose potential future solutions to accelerate the development of novel immunotherapeutic approaches. This review highlights the transformative potential of organoid models in advancing preclinical cancer immunotherapy modeling, screening, and evaluation, paving the way for more effective and personalized cancer treatments.
1. Introduction
Cancer immunotherapy represents a transformative approach in oncology, harnessing the body’s immune system to recognize and eradicate cancer cells. Unlike traditional treatments such as chemotherapy and radiation, which non-specifically target rapidly dividing cells, immunotherapy offers a more precise mechanism, aiming to enhance the immune system’s natural ability to combat cancer []. This specificity not only promises improved efficacy but also minimizes the adverse side effects commonly associated with conventional treatments. Clinically, immunotherapy has shown remarkable success in treating various cancers, including melanoma, non-small cell lung cancer, and certain types of lymphoma, marking a significant advancement in cancer care [].
In recent years, the development of organoid culture systems has emerged as a pivotal tool for advancing cancer immunotherapy. Organoids, three-dimensional structures derived from patient tissues, closely mimic real organs’ architecture and function, providing a realistic platform for studying tumor biology and the tumor-immune microenvironment []. These models bridge basic research and clinical application, significantly advancing our understanding of immune cell interactions within tumors. By co-culturing organoids with immune cells, researchers can observe cell migration and activation, test immune checkpoint inhibitors, and optimize immunotherapies before clinical trials [,].
Despite the promise of cancer immunotherapy, there remain substantial challenges that limit its efficacy for all patients. Tumor heterogeneity, immune evasion mechanisms, and variability in patient responses are significant obstacles [,]. Additionally, the current preclinical models often fail to accurately replicate the complexity of human tumors, impeding the translation of promising therapies from bench to bedside. These limitations underscore the urgent need for more predictive and representative models to facilitate the development of effective immunotherapies [].
This review aims to address the intersection of biotechnology and clinical application by exploring the role of organoid cultures in cancer immunotherapy. We will provide a comprehensive overview of the current landscape of immunotherapy, discuss the advancements and potential of organoid models in enhancing our understanding of the tumor-immune interface, and highlight how these models can be leveraged to overcome existing clinical challenges. Through this exploration, we aim to underscore the importance of integrating innovative biotechnological tools in the pursuit of more effective and personalized cancer treatments.
2. Utilization of Organoids in Preclinical Personalized Immunotherapy Screening and Evaluation
Immunotherapy is an advanced cancer treatment that leverages the patient’s immune system to combat malignancies. This strategy enhances or modifies immune functionality, improving its ability to detect and destroy cancer cells. The five main categories of immunotherapy are oncolytic virus therapies, cancer vaccines, cytokine therapies, immune checkpoint inhibitors, and adoptive cell transfer (Figure 1), []. Oncolytic virus therapies use genetically modified viruses to selectively infect and destroy cancer cells. The viruses replicate within the cancer cells, causing cell lysis and releasing new viral particles to infect neighboring cells, turning tumors into a more antigenic form and triggering a systemic immune response. Cancer vaccines stimulate the immune system to target tumor-associated antigens (TAAs) on cancer cells. Administered via intradermal injection, they activate dendritic cells, which present the antigens to T cells, leading to the proliferation of cytotoxic T lymphocytes (CTLs) that seek out and kill cancer cells, while helper T cells enhance the overall immune response []. Cytokine therapies use small proteins that play vital roles in immune signaling to enhance the anticancer immune response, boosting T cell activation, promoting antigen presentation, and modulating the tumor microenvironment. Immune checkpoint inhibitors block interactions between checkpoint proteins and their ligands, such as PD-1/PD-L1 and CTLA-4/CD80, preventing inhibitory signals and allowing T cells to maintain their cytotoxic activity, improving their ability to recognize and eliminate cancer cells []. Adoptive cell transfer (ACT) involves infusing patients with genetically modified T cells tailored to recognize cancer-specific antigens, engineered with T cell receptors (TCRs) or chimeric antigen receptors (CARs), which enhances their ability to target and destroy cancer cells [,].
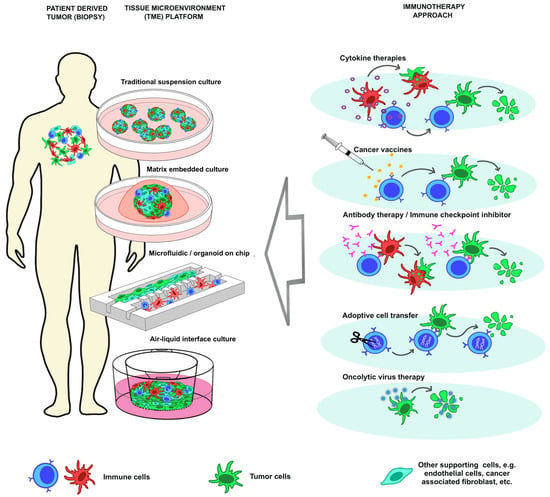
Figure 1.
Organoid-based tissue microenvironment construct for personalized immunotherapy model.
The use of patient-derived organoids in personalized cancer immunotherapy has shown great promise. These organoids retain the genetic and functional characteristics of the original tumors, allowing for the tailoring of immunotherapeutic strategies to each patient’s unique cancer profile [,]. By testing different immunotherapies on these personalized models, clinicians can predict the most effective treatments, improving patient outcomes []. Case studies, such as those involving colorectal and breast cancer, have demonstrated that organoid-based approaches can accurately guide personalized treatment plans, leading to significant tumor regression and enhanced clinical results [].
Votanopoulos et al. engineered a novel 3D mixed melanoma/lymph node organoid system to enable personalized immunotherapy screening by preserving tumor heterogeneity and the immune microenvironment []. Surgically obtained matched melanoma and lymph node biospecimens from the same patients were dissociated, incorporated into an extracellular matrix-based hydrogel, and biofabricated into immune-enhanced patient tumor organoids (iPTOs). This method retained the original tumor’s stroma and immune cells without sorting. The organoids were screened with various immunotherapies (nivolumab, pembrolizumab, ipilimumab, and dabrafenib/trametinib) over 72 h, and their responses were assessed using live/dead staining and quantitative metabolism assays. Histology and immunohistochemistry confirmed the resemblance between the original tumor and the organoid cells. In a pilot study, autologous peripheral T cells activated by iPTOs successfully killed tumor cells in naïve PTOs, indicating the generation of adaptive immunity. The study demonstrated a high establishment success rate (90%) and a strong correlation (85%) between iPTO response and clinical outcomes, showcasing its potential as a personalized immunotherapy testing platform [].
Forsythe et al. (2021) conducted a pioneering study to evaluate the efficacy of immunotherapy for appendiceal cancer using a personalized organoid model, addressing the scarcity of clinical trial data due to the cancer’s low incidence []. They created patient tumor organoids (PTOs) with and without enrichment from immune components and treated them with pembrolizumab, ipilimumab, or nivolumab. The study showed that immunotherapy responses were observed in some cases, particularly with pembrolizumab and nivolumab, but not with ipilimumab []. This research demonstrates the potential of immunotherapy for appendiceal cancer and the usefulness of immunocompetent organoids in selecting patients for clinical trials in rare cancers.
3. Primary Components for Constructing Immunotherapy Models
3.1. Cellular Components
The organoid model for immunotherapy incorporates key cellular components to faithfully replicate aspects of the tumor microenvironment [,,]. Derived from patient tumor biopsies, tumor cells form the foundational component, preserving genetic and phenotypic characteristics essential for modeling personalized cancer scenarios. Immune cells such as T cells, natural killer (NK) cells, and macrophages play a crucial role in studying immune-tumor interactions and can be autologous, ensuring the model’s relevance to individual patient responses. Cancer-associated fibroblasts (CAFs), which provide structural support and secrete signaling molecules, mimic the tumor’s supportive microenvironment and influence immune cell behavior. Endothelial cells within the organoid form vascular-like structures, enabling investigations into angiogenesis and the infiltration dynamics of immune cells within the tumor environment. Together, these components create a comprehensive model that enhances our understanding and testing of immunotherapeutic strategies against cancer [,].
There are multiple organoid culture strategies for modeling the tumor immune microenvironment, which can be broadly categorized into reconstitution approaches and holistic approaches []. The reconstitution approach involves reconstituting the tumor microenvironment with immune components, such as in submerged Matrigel culture. This method incorporates specific elements of the tumor and immune system into a controlled environment, allowing interactions between cancer cells and immune cells []. In contrast, holistic approaches aim to maintain the native tumor microenvironment (TME) with its immune components intact []. Examples include microfluidic 3D culture and air-liquid interface (ALI) culture. These methods preserve the complexity and heterogeneity of the TME, more closely mimicking patient conditions. By maintaining the original structure and composition of the tumor and its surrounding immune cells, holistic approaches provide a more accurate representation of how tumors interact with the immune system in the body, thereby enhancing the relevance of findings from organoid studies to clinical scenarios [,,,].
3.2. Non-Cellular Components
The extracellular matrix (ECM) forms the structural foundation for organoid formation, comprising proteins such as collagen, laminin, and fibronectin. Matrigel, a widely used hydrogel, mimics natural ECM properties by supporting cell attachment, growth, and differentiation within organoids [,,,]. Additionally, ECM components play a crucial role in cell signaling, influencing cellular behaviors and interactions crucial for organoid development and function [,,].
Synthetic or natural hydrogels like polyethylene glycol (PEG), alginate, or hyaluronic acid are utilized to encapsulate cells and create a 3D matrix environment for organoid cultures []. These hydrogels can be engineered to replicate the mechanical characteristics and biochemical cues of native tissues, providing an optimal milieu for organoid growth and physiological function [,,]. They serve as essential scaffolds for maintaining cellular integrity and fostering cellular interactions crucial for studying tumor biology and therapeutic responses [].
In organoid cultures, growth factors and cytokines are essential additives in the culture media, supporting cell proliferation, differentiation, and survival. Key formulations examples including DMEM/F12, HEPES, penicillin-streptomycin, Glutamax, N-acetyl-L-cysteine, and B-27 supplements for maintaining genetic and protein markers of lung-patient-derived organoids (PDOs) (e.g., EGFR, TTF-1, p63, cytokeratin 5) []. The culture medium was supplemented with small molecule activators or inhibitors such as A83-01, CHIR 99021, Noggin, Y-27632, and SAG, alongside growth factors like epidermal growth factor (EGF), fibroblast growth factor 4 (FGF4), transforming growth factor-beta (TGF-β), and FGF10, which supported successful long-term organoid maintenance [,,,]. These molecules are pivotal in recreating the complex signaling environment of the tumor microenvironment within organoid models, ensuring accurate representation of cellular responses and interactions crucial for studying cancer biology and therapeutic efficacy [,].
Advanced biomimetic scaffolds made from materials such as decellularized tissue [,,], synthetic polymers [], or nanofibers [,] offer enhanced tissue architecture and functionality in organoid cultures. These scaffolds provide robust mechanical support and can be tailored with specific ligands to optimize cell adhesion and interaction. By mimicking the structural and biochemical cues of native tissues, biomimetic scaffolds enhance the fidelity and relevance of organoid models in studying complex biological processes and therapeutic interventions in cancer research [].
4. Strategies for Constructing Organoid Based-Tissue Microenvironment (TME) Platforms for Personalized Immunotherapy
4.1. Traditional Suspension Culture
Suspension culture involves growing cells in a suspended state, allowing them to interact and form three-dimensional aggregates that mimic the in vivo tumor microenvironment without needing external scaffolds [,]. This direct cellular interaction enhances communication, signaling, and tissue organization, leading to improved tissue maturation and functionality []. Nevertheless, the absence of mechanical support poses a substantial limitation, as it plays a critical role in directing tissue organization and architecture. Additionally, nutrient and oxygen transfer limitations can cause necrotic cores, resulting in less predictable tissue formation and impeding the engineering of larger and more complex tissues [,].
The study by Koeck et al. investigates how tumor-associated fibroblasts and tumor microenvironment-derived cytokines influence the infiltration of CD3+CD8+ cytotoxic T lymphocytes in a multicellular co-culture system using A549 and Calu-6 cancer cell lines with SV80 fibroblasts []. Cultivated for 10 days using a hanging drop system, microtissues were analyzed after the addition of patient-derived PBMC with or without cytokine stimulation. Immunohistochemistry and multi-cytokine immunoassays revealed increased chemokine secretion in cancer cell-fibroblast microtissues, where PBMCs tended to localize at the microtissue periphery, and activated CD69+ and CD49d+ T lymphocytes showed enhanced infiltration []. These findings underscore the stromal influence on immune cell behavior within cancer microenvironments, particularly fibroblast-induced shifts towards activated T lymphocyte infiltration.
4.2. Matrix Embedded Culture
Matrix-embedded culture is particularly important for tumor or cancer organoids as it provides a 3D environment that closely replicates the natural tumor microenvironment []. This allows tumor biology study and drug responses in a more physiologically relevant context, facilitating the development of organoid-immunotherapy models [].
Matrigel is a commonly used extracellular matrix (ECM) for constructing a matrix embedded cancer organoid. Its advantages for organoid-immunotherapy models include its ability to support the formation of complex organoid structures and maintain long-term cultures, which are critical for studying prolonged immunotherapy responses and interactions within the tumor microenvironment [,]. Despite these benefits, Matrigel has several drawbacks, such as inherent variability in its composition leading to inconsistencies in experimental outcomes, high cost, and ethical concerns due to its animal origin [,].
Several types of matrices can be utilized as alternatives to Matrigel, available from both natural and synthetic sources. Natural sources, such as isolated ECM proteins like collagen [], fibronectin [] and laminin [], offer biocompatibility, cell adhesion support, and proliferation. Another matrix formed by synthetic polymer, such as hydrogels like polyethylene glycol (PEG) and alginate, also can be used as an alternative. These polymers provide a superior tunable mechanical properties and degradation rates. PEG hydrogels can be functionalized with cell-adhesive peptides to enhance cell attachment and growth [,]. while alginate offers a 3D environment conducive to cell encapsulation, proliferation, and differentiation [,]. These alternatives can mitigate some of the drawbacks associated with Matrigel, offering more consistent and ethically acceptable options for organoid culture [].
4.3. Microfluidic Culture and Organoid-Based Immunotherapy-on-Chip
Microfluidic and organoid-based immunotherapy-on-chip platforms are advanced technologies designed to replicate tumor-immune interactions in a controlled, miniature setting []. These systems integrate microfluidic channels with organoid cultures, allowing precise manipulation and observation of immune responses against tumor cells []. This platform enable the simulation and evaluation of diverse immunotherapy strategies, including assessing immune checkpoint inhibitors like PD-1/PD-L1, evaluating CAR-T cell therapy efficacy against patient-derived tumor organoids, studying cytokine therapy impacts on immune activation, and screening novel immunotherapeutic agents for personalized treatment strategies []. Strategies employed include advanced imaging for real-time cell interaction visualization, microsensor arrays for biochemical quantification, and high-throughput screening techniques for assessing drug responses at a single-cell level [,,].
Microfluidic organoid culture can represent ex vivo systems that mimic the tumor microenvironment and model dynamic responses to immune checkpoint blockade (ICB) can advance precision immuno-oncology and combination therapy development. The study by Jenkins et al. demonstrates the use of murine- and patient-derived organotypic tumor spheroids (MDOTS/PDOTS) to evaluate ex vivo responses to ICB. MDOTS/PDOTS, retaining autologous lymphoid and myeloid cell populations, respond to ICB in short-term 3D microfluidic culture []. Using MDOTS from immunocompetent mouse tumor models, the study recapitulated response and resistance to ICB. Profiling MDOTS showed that TBK1/IKKϵ inhibition enhanced the response to PD-1 blockade, predicting in vivo tumor responses. Systematic cytokine profiling in PDOTS captured features associated with PD-1 blockade response and resistance. Thus, MDOTS/PDOTS profiling offers a novel platform for evaluating ICB in murine models and clinically relevant patient specimens. This approach addresses the challenge of resistance to PD-1 blockade and the need for biomarkers to guide treatment, facilitating therapeutic combination development and precision immuno-oncology efforts [].
Al-samadi et al. developed a novel in vitro microfluidic chip to assess immunotherapy efficacy in head and neck squamous cell carcinoma (HNSCC) patients []. Initially validated using a tongue cancer cell line (HSC-3) and immune cells from healthy donors in a Myogel/fibrin matrix, the microfluidic chip was later tested with freshly isolated cancer cells, patient serum, and immune cells. They found that immune cell migration towards cancer cells was dependent on cancer cell density. The IDO1 inhibitor promoted immune cell migration towards HSC-3 cells and patient-derived samples. The efficacy of PD-L1 antibody and IDO1 inhibitor varied among patients, suggesting a patient-specific response. This humanized microfluidic chip represents a promising tool for predicting individual responses to immunotherapy in HNSCC patients [].
4.4. Air Liquid Interface Culture
The air-liquid interface (ALI) culture is a sophisticated method used in organoid-based immunotherapy models to cultivate complex tissue structures that mimic the in vivo environment. The basic principle involves exposing the apical side of the organoid to air while maintaining the basal side in contact with a nutrient-rich medium. This setup allows the development of differentiated cell layers and the formation of tissue-specific structures, closely resembling their natural counterparts []. The key advantages of this culture system are its ability to generate physiologically relevant models that can be used for studying disease mechanisms, drug testing, and personalized medicine. Additionally, ALI culture facilitates the interaction between immune cells and organoids, which is crucial for developing immunotherapy strategies [,] However, challenges remain, such as the complexity of replicating the exact in vivo conditions and ensuring the longevity and stability of the organoid cultures [].
In their 2018 study, Neal et al. developed an advanced air-liquid interface (ALI) method to culture patient-derived organoids (PDOs) from over 100 human biopsies and mouse tumors in syngeneic immunocompetent hosts, maintaining tumor epithelia along with their native immune cells (T, B, NK, and macrophages) []. This approach allowed the tumor microenvironment (TME) preservation without the need for artificial reconstitution. Using robust droplet-based, single-cell analysis, it was confirmed the co-cultured PDOs accurately retained the original tumor T cell receptor (TCR) spectrum. Moreover, these organoids effectively modeled immune checkpoint blockade (ICB) therapy with anti-PD-1 and/or anti-PD-L1, leading to the expansion and activation of tumor antigen-specific tumor-infiltrating lymphocytes (TILs) and inducing tumor cytotoxicity [].
5. Technological Challenges and Possible Innovative Solutions
A significant challenge in the application of 3D organoid models for cancer immunotherapy is achieving reproducibility and standardization across different laboratories []. Zhou et al. discuss the challenges of using tumor organoids to replicate tumor heterogeneity and biology, noting that variations in cell sources, culture conditions, and methodologies can lead to inconsistent results, hampering the reliability and comparability of studies []. To address these issues, the development and implementation of standardized protocols for organoid culture, including uniform procedures for cell isolation, extracellular matrix (ECM) composition, and co-culture conditions with immune cells, are essential [,] (Table 1). Additionally, implementing quality control measures, such as genetic and phenotypic characterization of organoids, can ensure consistency [].

Table 1.
Examples of patient-derived cancer organoid culture platforms for immunotherapy.
Mimicking the full complexity and heterogeneity of tumors remains a daunting task. Tumors exhibit vast diversity in their cellular composition, genetic mutations, and microenvironmental conditions. To tackle these difficulties, various strategies can be explored, such as using patient-derived organoids that inherently capture the genetic and phenotypic heterogeneity of individual tumors [,]. Another method involves integrating multiple cell types, such as stromal cells, endothelial cells, and various immune cells, to recreate the intricate tumor microenvironment. Despite these advancements, replicating the dynamic interactions and evolving nature of tumors continues to be a challenge, necessitating further technological innovations. Additionally, assessing the long-term viability and stability of organoid cultures, particularly when integrated with immune components, is crucial to ensuring consistent results in longitudinal studies [,].
Ongoing research and technological innovations offer promising solutions to these challenges. The use of gene editing allows precise manipulation of organoid genomes, enabling the study of specific genetic alterations and their impact on tumor-immune interactions. For instance, combining CRISPR-Cas9 with organoid technology can also facilitate the creation of more accurate disease models by introducing patient-specific mutations []. Furthermore, enhanced characterization techniques are being developed to identify specific markers or pathways involved in pharmacogenomic interactions reflecting therapy responses, which can help refine these models further [,].
Engineering approaches, such as 3D bioprinting and perfusion systems, can significantly enhance cancer organoid models. 3D bioprinting technology enables the construction of highly precise tissue architectures by depositing cells and biomaterials layer by layer [,]. This method allows for the creation of complex structures that better represent the native tumor architecture, including vascular networks and spatial distribution of different cell types [,]. Perfusion systems represent another innovative solution, providing continuous nutrient supply and waste removal, thus maintaining organoid viability and function over extended periods []. This dynamic culture environment more closely mimics in vivo conditions and supports the study of long-term treatment effects.
Advanced imaging techniques, such as live-cell imaging and intravital microscopy, are being incorporated to monitor organoid development and immune cell interactions in real-time. These technologies provide valuable insights into the dynamic processes within organoids, allowing researchers to observe immune cell infiltration, tumor growth, and response to therapies with high spatial and temporal resolution [,]. Schnalzger et al. developed a sensitive preclinical model using 3D patient-derived organoids (PDOs) to assay non autologous CAR-mediated cytotoxicity within a tumor-immune microenvironment []. They established a confocal live-cell imaging protocol to dynamically monitor cytotoxic activity at the single-organoid level, demonstrating stable effector-target cell interactions in co-cultures of NK cells with colorectal cancer (CRC) or normal organoids on an ECM layer. Additionally, CRC organoids were used to assess tumor antigen-specific cytotoxicity of EGFRvIII or FRIZZLED receptor-targeting CAR-engineered NK-92 cells, establishing a platform to evaluate CAR efficacy and tumor specificity. Furthermore, epithelial-only PDOs, although lacking stromal and immune components, can select and evaluate tumor-reactive T cells, enhancing the enrichment and stimulation of tumor-reactive lymphocytes [].
The integration of these innovative methodologies holds the potential to overcome current limitations in organoid technology, paving the way for more accurate and reliable cancer immunotherapy models. By combining standardized protocols, advanced gene editing, perfusion systems, 3D bioprinting, and real-time imaging, sophisticated organoid models that closely mimic the complexity and heterogeneity of human tumors can be developed, ultimately advancing personalized cancer treatment strategies.
6. Conclusions
Organoids derived from patient tissues faithfully recapitulate the complexity of tumors, including their genetic diversity and microenvironmental interactions. This fidelity allows for personalized testing of immunotherapies, enabling clinicians to predict treatment responses more accurately and tailor therapies to individual patients []. By incorporating immune cells and maintaining the tumor microenvironment, organoid models facilitate the study of immune-tumor interactions, drug screening, and the development of innovative treatment strategies.
Despite these advancements, a cautious approach is necessary when generalizing findings from organoid models, acknowledging their limitations and areas requiring further investigation. Although patient-derived organoids (PDOs) have shown great promise in predicting drug responses, they may not fully recapitulate the complex interactions between tumor cells and the surrounding stromal and immune cells, leading to an incomplete representation of the tumor microenvironment (TME) [,]. This limitation suggests that while PDOs can be valuable tools for personalized medicine, they may not always accurately predict therapeutic outcomes within the full immune TME.
Challenges such as achieving standardization across laboratories, replicating tumor heterogeneity, and ensuring the longevity of organoid cultures persist. Addressing these challenges requires ongoing innovation, including the development of standardized protocols, advanced gene-editing techniques, and the integration of perfusion systems and 3D bioprinting technology. Although organoids offer a promising platform, they are not without limitations. Issues related to standardization, reproducibility, and fully replicating the complexity of in vivo tumor biology are critical areas needing further investigation []. These approaches aim to enhance the reproducibility and reliability of organoid studies while better capturing the dynamic nature of tumors and their responses to therapies.
The continued integration of biotechnological advancements with organoid models holds promise for accelerating the translation of preclinical findings into clinical applications. By refining our understanding of the tumor-immune interface and overcoming current limitations, organoid-based approaches stand poised to contribute significantly to the future of personalized cancer treatment, offering hope for improved outcomes and enhanced therapeutic efficacy across a broad spectrum of malignancies.
Author Contributions
Conceptualization, F.G.T.; literature investigation, S.T.N., C.A. and F.G.T.; writing—original draft preparation, S.T.N. and C.A.; review and editing, F.G.T.; scientific illustration, F.G.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, C.; Yang, M.; Zhang, D.; Chen, M.; Zhu, D. Clinical cancer immunotherapy: Current progress and prospects. Front. Immunol. 2022, 13, 961805. [Google Scholar] [CrossRef]
- Kareff, S.A.; Samtani, S.; Burotto, M.; Prasad, V.; Kim, C. Current Landscape of Immunotherapy Trials Involving the Programmed Cell Death Protein 1/Programmed Death-Ligand 1 Axis in Intrathoracic Tumors. JTO Clin. Res. Rep. 2021, 2, 100149. [Google Scholar] [CrossRef]
- Wu, W.; Li, X.; Yu, S. Patient-derived Tumour Organoids: A Bridge between Cancer Biology and Personalised Therapy. Acta Biomater. 2022, 146, 23–36. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C.; Wang, L.; Xie, M.; Ge, X.; Wu, S.; He, Y.; Mou, X.; Ye, C.; Sun, Y. Patient-Derived Tumor Organoids: New Progress and Opportunities to Facilitate Precision Cancer Immunotherapy. Front. Oncol. 2022, 12, 872531. [Google Scholar] [CrossRef]
- Forsythe, S.D.; Erali, R.A.; Sasikumar, S.; Laney, P.; Shelkey, E.; D’AGostino, R.; Miller, L.D.; Shen, P.; Levine, E.A.; Soker, S.; et al. Organoid platform in preclinical investigation of personalized immunotherapy efficacy in appendiceal cancer: Feasibility study. Clin. Cancer Res. 2021, 27, 5141–5151. [Google Scholar] [CrossRef]
- Jiang, X.; Oyang, L.; Peng, Q.; Liu, Q.; Xu, X.; Wu, N.; Tan, S.; Yang, W.; Han, Y.; Lin, J.; et al. Organoids: Opportunities and challenges of cancer therapy. Front. Cell Dev. Biol. 2023, 11, 1232528. [Google Scholar] [CrossRef]
- Magré, L.; Verstegen, M.M.A.; Buschow, S.; Van Der Laan, L.J.W.; Peppelenbosch, M.; Desai, J. Emerging organoid-immune co-culture models for cancer research: From oncoimmunology to personalized immunotherapies. J. Immunother. Cancer 2023, 11, e006290. [Google Scholar] [CrossRef]
- Ngan Ngo, T.K.; Kuo, C.H.; Tu, T.Y. Recent advances in microfluidic-based cancer immunotherapy-on-a-chip strategies. Biomicrofluidics 2023, 17, 011501. [Google Scholar] [CrossRef]
- Qiu, Y.; Su, M.; Liu, L.; Tang, Y.; Pan, Y.; Sun, J. Clinical Application of Cytokines in Cancer Immunotherapy. Drug Des. Devel. Ther. 2021, 15, 2269–2287. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Restifo, N.P.; Yang, J.C.; Morgan, R.A.; Dudley, M.E. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 2008, 8, 299–308. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Parkhurst, M.R.; Robbins, P.F. Adoptive cell transfer immunotherapy for patients with solid epithelial cancers. Cancer Cell 2023, 41, 646–648. [Google Scholar] [CrossRef]
- Sun, C.P.; Lan, H.R.; Fang, X.L.; Yang, X.Y.; Jin, K.T. Organoid Models for Precision Cancer Immunotherapy. Front. Immunol. 2022, 13, 770465. [Google Scholar] [CrossRef]
- Xu, H.; Jiao, D.; Liu, A.; Wu, K. Tumor organoids: Applications in cancer modeling and potentials in precision medicine. J. Hematol. Oncol. 2022, 15, 58. [Google Scholar] [CrossRef]
- Grönholm, M.; Feodoroff, M.; Antignani, G.; Martins, B.; Hamdan, F.; Cerullo, V. Patient-derived organoids for precision cancer immunotherapy. Cancer Res. 2021, 81, 3149–3155. [Google Scholar] [CrossRef]
- Votanopoulos, K.I.; Forsythe, S.; Sivakumar, H.; Mazzocchi, A.; Aleman, J.; Miller, L.; Levine, E.; Triozzi, P.; Skardal, A. Model of Patient-Specific Immune-Enhanced Organoids for Immunotherapy Screening: Feasibility Study. Ann. Surg. Oncol. 2020, 27, 1956–1967. [Google Scholar] [CrossRef]
- Yuki, K.; Cheng, N.; Nakano, M.; Kuo, C.J. Organoid Models of Tumor Immunology. Trends Immunol. 2020, 41, 652–664. [Google Scholar] [CrossRef]
- Dominijanni, A.; Mazzocchi, A.; Shelkey, E.; Forsythe, S.; Devarsetty, M.; Soker, S. Bioengineered tumor organoids. Curr. Opin. Biomed. Eng. 2020, 13, 168–173. [Google Scholar] [CrossRef]
- Zhou, Z.; Van der Jeught, K.; Li, Y.; Sharma, S.; Yu, T.; Moulana, I.; Liu, S.; Wan, J.; Territo, P.R.; Opyrchal, M.; et al. A T Cell-Engaging Tumor Organoid Platform for Pancreatic Cancer Immunotherapy. Adv. Sci. 2023, 10, e2300548. [Google Scholar] [CrossRef]
- Gu, Z.; Wu, Q.; Shang, B.; Zhang, K.; Zhang, W. Organoid co-culture models of the tumor microenvironment promote precision medicine. Cancer Innov. 2024, 3, e101. [Google Scholar] [CrossRef]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Garreta, E.; Kamm, R.D.; Lopes, S.M.C.d.S.; Lancaster, M.A.; Weiss, R.; Trepat, X.; Hyun, I.; Montserrat, N. Rethinking organoid technology through bioengineering. Nat. Mater. 2021, 20, 145–155. [Google Scholar] [CrossRef]
- Mukhopadhyay, M. Recapitulating early cardiogenesis in vitro. Nat. Methods 2021, 18, 331. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Kim, H.Y.; Nelson, C.M. Extracellular matrix and cytoskeletal dynamics during branching morphogenesis. Organogenesis 2012, 8, 56–64. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Alam, K.; Roy, N.S.; Kaur, K.; Kaity, S.; Ravichandiran, V.; Roy, S. Exploring the interaction between extracellular matrix components in a 3D organoid disease model to replicate the pathophysiology of breast cancer. J. Exp. Clin. Cancer Res. 2023, 42, 343. [Google Scholar] [CrossRef]
- Spagnol, G.; Sensi, F.; De Tommasi, O.; Marchetti, M.; Bonaldo, G.; Xhindoli, L.; Noventa, M.; Agostini, M.; Tozzi, R.; Saccardi, C. Patient Derived Organoids (PDOs), Extracellular Matrix (ECM), Tumor Microenvironment (TME) and Drug Screening: State of the Art and Clinical Implications of Ovarian Cancer Organoids in the Era of Precision Medicine. Cancers 2023, 15, 2059. [Google Scholar] [CrossRef]
- Castellote-Borrell, M.; Merlina, F.; Rodríguez, A.R.; Guasch, J. Biohybrid Hydrogels for Tumoroid Culture. Adv. Biol. 2023, 7, e2300118. [Google Scholar] [CrossRef]
- Riewruja, K.; Aguglia, A.M.; Hines, S.; Makarcyzk, M.J.; Honsawek, S.; Lin, H. PEG Reinforced Scaffold Promotes Uniform Distribution of Human MSC-Created Cartilage Matrix. Gels 2022, 8, 794. [Google Scholar] [CrossRef]
- Scott, R.A.; Elbert, D.L.; Willits, R.K. Modular poly(ethylene glycol) scaffolds provide the ability to decouple the effects of stiffness and protein concentration on PC12 cells. Acta Biomater. 2011, 7, 3841–3849. [Google Scholar] [CrossRef][Green Version]
- Ashimova, A.; Yegorov, S.; Negmetzhanov, B.; Hortelano, G. Cell Encapsulation Within Alginate Microcapsules: Immunological Challenges and Outlook. Front. Bioeng. Biotechnol. 2019, 7, 380. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef]
- Huo, K.-G.; D’Arcangelo, E.; Tsao, M.-S. Patient-derived cell line, xenograft and organoid models in lung cancer therapy. Transl. Lung Cancer Res. 2020, 9, 2214–2232. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Kim, M.; Mun, H.; Sung, C.O.; Cho, E.J.; Jeon, H.-J.; Chun, S.-M.; Jung, D.J.; Shin, T.H.; Jeong, G.S.; Kim, D.K.; et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 2019, 10, 3991. [Google Scholar] [CrossRef]
- Witsch, E.; Sela, M.; Yarden, Y. Roles for Growth Factors in Cancer Progression. Physiology 2010, 25, 85–101. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, Y.; Zhou, R.; Yu, Y.; Xiao, Z.; Zhang, H. Lung cancer organoids, a promising model still with long way to go. Crit. Rev. Oncol. Hematol. 2022, 171, 103610. [Google Scholar] [CrossRef]
- Mimeault, M.; Batra, S.K. Interplay of distinct growth factors during epithelial–mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann. Oncol. 2007, 18, 1605–1619. [Google Scholar] [CrossRef]
- García-Gareta, E.; Pérez, M.Á.; García-Aznar, J.M. Decellularization of tumours: A new frontier in tissue engineering. J. Tissue Eng. 2022, 13, 20417314221091682. [Google Scholar] [CrossRef]
- Varinelli, L.; Guaglio, M.; Brich, S.; Zanutto, S.; Belfiore, A.; Zanardi, F.; Iannelli, F.; Oldani, A.; Costa, E.; Chighizola, M.; et al. Decellularized extracellular matrix as scaffold for cancer organoid cultures of colorectal peritoneal metastases. J. Mol. Cell Biol. 2022, 14, mjac064. [Google Scholar] [CrossRef]
- van Tienderen, G.S.; Rosmark, O.; Lieshout, R.; Willemse, J.; de Weijer, F.; Rendin, L.E.; Westergren-Thorsson, G.; Doukas, M.; Koerkamp, B.G.; van Royen, M.E.; et al. Extracellular matrix drives tumor organoids toward desmoplastic matrix deposition and mesenchymal transition. Acta Biomater. 2023, 158, 115–131. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, R.; Liang, Z.; Guo, J.; Chen, B.; Zhou, S.; Yu, D. Application of Electrospun Drug-Loaded Nanofibers in Cancer Therapy. Polymers 2024, 16, 504. [Google Scholar] [CrossRef]
- Shen, C.; Zhang, Z.-J.; Li, X.-X.; Huang, Y.-P.; Wang, Y.-X.; Zhou, H.; Xiong, L.; Wen, Y.; Zou, H.; Liu, Z.-T. Intersection of nanomaterials and organoids technology in biomedicine. Front. Immunol. 2023, 14, 1172262. [Google Scholar] [CrossRef]
- Górnicki, T.; Lambrinow, J.; Golkar-Narenji, A.; Data, K.; Domagała, D.; Niebora, J.; Farzaneh, M.; Mozdziak, P.; Zabel, M.; Antosik, P.; et al. Biomimetic Scaffolds—A Novel Approach to Three Dimensional Cell Culture Techniques for Potential Implementation in Tissue Engineering. Nanomaterials 2024, 14, 531. [Google Scholar] [CrossRef]
- Séraudie, I.; Pillet, C.; Cesana, B.; Bazelle, P.; Jeanneret, F.; Evrard, B.; Chalmel, F.; Bouzit, A.; Battail, C.; Long, J.-A.; et al. A new scaffold-free tumoroid model provides a robust preclinical tool to investigate invasion and drug response in Renal Cell Carcinoma. Cell Death Dis. 2023, 14, 622. [Google Scholar] [CrossRef]
- El Harane, S.; Zidi, B.; El Harane, N.; Krause, K.H.; Matthes, T.; Preynat-Seauve, O. Cancer Spheroids and Organoids as Novel Tools for Research and Therapy: State of the Art and Challenges to Guide Precision Medicine. Cells 2023, 12, 1001. [Google Scholar] [CrossRef]
- Zhu, Y.; Kang, E.; Wilson, M.; Basso, T.; Chen, E.; Yu, Y.; Li, Y.-R. 3D Tumor Spheroid and Organoid to Model Tumor Microenvironment for Cancer Immunotherapy. Organoids 2022, 1, 149–167. [Google Scholar] [CrossRef]
- Lv, J.; Du, X.; Wang, M.; Su, J.; Wei, Y.; Xu, C. Construction of tumor organoids and their application to cancer research and therapy. Theranostics 2024, 14, 1101–1125. [Google Scholar] [CrossRef]
- Hubert, C.G.; Rivera, M.; Spangler, L.C.; Wu, Q.; Mack, S.C.; Prager, B.C.; Couce, M.; McLendon, R.E.; Sloan, A.E.; Rich, J.N. A Three-Dimensional Organoid Culture System Derived from Human Glioblastomas Recapitulates the Hypoxic Gradients and Cancer Stem Cell Heterogeneity of Tumors Found In Vivo. Cancer Res. 2016, 76, 2465–2477. [Google Scholar] [CrossRef]
- Koeck, S.; Kern, J.; Zwierzina, M.; Gamerith, G.; Lorenz, E.; Sopper, S.; Zwierzina, H.; Amann, A. The influence of stromal cells and tumor-microenvironment-derived cytokines and chemokines on CD3 + CD8 + tumor infiltrating lymphocyte subpopulations. Oncoimmunology 2017, 6, e1323617. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Marinho, P.; Sartore, R.; Paulsen, B.; Mariante, R.; Castilho, L.; Rehen, S. Successful scale-up of human embryonic stem cell production in a stirred microcarrier culture system. Braz. J. Med. Biol. Res. 2009, 42, 515–522. [Google Scholar] [CrossRef]
- Jain, R.; Roy, S. Designing a bioactive scaffold from coassembled collagen–laminin short peptide hydrogels for controlling cell behaviour. RSC Adv. 2019, 9, 38745–38759. [Google Scholar] [CrossRef] [PubMed]
- Asadishekari, M.; Mpoyi, E.N.; Li, Y.; Eslami, J.; Walker, M.; Cantini, M.; Gourdon, D. Three-Dimensional Tunable Fibronectin-Collagen Platforms for Control of Cell Adhesion and Matrix Deposition. Front. Phys. 2022, 10, 806554. [Google Scholar] [CrossRef]
- Garg, K. Laminin Enriched Scaffolds for Tissue Engineering Applications. Adv. Tissue Eng. Regen. Med. Open Access 2017, 2, 194–200. [Google Scholar] [CrossRef][Green Version]
- Yu, T.; Yang, Q.; Peng, B.; Gu, Z.; Zhu, D. Vascularized organoid-on-a-chip: Design, imaging, and analysis. Angiogenesis 2024, 27, 147–172. [Google Scholar] [CrossRef]
- Zhu, J.; Ji, L.; Chen, Y.; Li, H.; Huang, M.; Dai, Z.; Wang, J.; Xiang, D.; Fu, G.; Lei, Z.; et al. Organoids and organs-on-chips: Insights into predicting the efficacy of systemic treatment in colorectal cancer. Cell Death Discov. 2023, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Papp, D.; Korcsmaros, T.; Hautefort, I. Revolutionizing immune research with organoid-based co-culture and chip systems. Clin. Exp. Immunol. 2024, 218, 40–54. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Aref, A.R.; Lizotte, P.H.; Ivanova, E.; Stinson, S.; Zhou, C.W.; Bowden, M.; Deng, J.; Liu, H.; Miao, D.; et al. Ex Vivo Profiling of PD-1 Blockade Using Organotypic Tumor Spheroids. Cancer Discov. 2018, 8, 196–215. [Google Scholar] [CrossRef]
- Al-Samadi, A.; Poor, B.; Tuomainen, K.; Liu, V.; Hyytiäinen, A.; Suleymanova, I.; Mesimaki, K.; Wilkman, T.; Mäkitie, A.; Saavalainen, P.; et al. In vitro humanized 3D microfluidic chip for testing personalized immunotherapeutics for head and neck cancer patients. Exp. Cell Res. 2019, 383, 111508. [Google Scholar] [CrossRef]
- Choi, K.Y.G.; Wu, B.C.; Lee, A.H.Y.; Baquir, B.; Hancock, R.E.W. Utilizing Organoid and Air-Liquid Interface Models as a Screening Method in the Development of New Host Defense Peptides. Front. Cell. Infect. Microbiol. 2020, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.-H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988. [Google Scholar] [CrossRef]
- Ringquist, R.; Ghoshal, D.; Jain, R.; Roy, K. Understanding and improving cellular immunotherapies against cancer: From cell-manufacturing to tumor-immune models. Adv. Drug Deliv. Rev. 2021, 179, 114003. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Y.; Wang, Z.; Liu, Y.; Yu, J.; Wang, W.; Chen, S.; Wu, W.; Wang, J.; Qian, G.; et al. Standardization of organoid culture in cancer research. Cancer Med. 2023, 12, 14375–14386. [Google Scholar] [CrossRef] [PubMed]
- Ning, R.X.; Liu, C.Y.; Wang, S.Q.; Li, W.K.; Kong, X.; He, Z.W. Application status and optimization suggestions of tumor organoids and CAR-T cell co-culture models. Cancer Cell Int. 2024, 24, 98. [Google Scholar] [CrossRef]
- Jacob, F.; Salinas, R.D.; Zhang, D.Y.; Nguyen, P.T.T.; Schnoll, J.G.; Wong, S.Z.H.; Thokala, R.; Sheikh, S.; Saxena, D.; Prokop, S.; et al. A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-tumoral Heterogeneity. Cell 2020, 180, 188–204. [Google Scholar] [CrossRef]
- Zhou, G.; Lieshout, R.; van Tienderen, G.S.; de Ruiter, V.; van Royen, M.E.; Boor, P.P.C.; Magré, L.; Desai, J.; Köten, K.; Kan, Y.Y.; et al. Modelling immune cytotoxicity for cholangiocarcinoma with tumour-derived organoids and effector T cells. Br. J. Cancer 2022, 127, 649–660. [Google Scholar] [CrossRef]
- Raimondi, G.; Mato-Berciano, A.; Pascual-Sabater, S.; Rovira-Rigau, M.; Cuatrecasas, M.; Fondevila, C.; Sánchez-Cabús, S.; Begthel, H.; Boj, S.F.; Clevers, H.; et al. Patient-derived pancreatic tumour organoids identify therapeutic responses to oncolytic adenoviruses. eBioMedicine 2020, 56, 102786. [Google Scholar] [CrossRef]
- Ou, L.; Liu, S.; Wang, H.; Guo, Y.; Guan, L.; Shen, L.; Luo, R.; Elder, D.E.; Huang, A.C.; Karakousis, G.; et al. Patient-derived melanoma organoid models facilitate the assessment of immunotherapies. eBioMedicine 2023, 92, 104614. [Google Scholar] [CrossRef]
- Zou, Z.; Lin, Z.; Wu, C.; Tan, J.; Zhang, J.; Peng, Y.; Zhang, K.; Li, J.; Wu, M.; Zhang, Y. Micro-Engineered Organoid-on-a-Chip Based on Mesenchymal Stromal Cells to Predict Immunotherapy Responses of HCC Patients. Adv. Sci. 2023, 10, e2302640. [Google Scholar] [CrossRef] [PubMed]
- Esser, L.K.; Branchi, V.; Leonardelli, S.; Pelusi, N.; Simon, A.G.; Klümper, N.; Ellinger, J.; Hauser, S.; Gonzalez-Carmona, M.A.; Ritter, M.; et al. Cultivation of Clear Cell Renal Cell Carcinoma Patient-Derived Organoids in an Air-Liquid Interface System as a Tool for Studying Individualized Therapy. Front. Oncol. 2020, 10, 1775. [Google Scholar] [CrossRef]
- D’Agosto, S.; Fiorini, E.; Pezzini, F.; Delfino, P.; Simbolo, M.; Vicentini, C.; Andreani, S.; Capelli, P.; Rusev, B.; Lawlor, R.T.; et al. Long-term organoid culture of a small intestinal neuroendocrine tumor. Front. Endocrinol. 2023, 14, 999792. [Google Scholar] [CrossRef]
- Geurts, M.H.; Clevers, H. CRISPR engineering in organoids for gene repair and disease modelling. Nat. Rev. Bioeng. 2023, 1, 32–45. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, S.; Yuan, Q.; Fu, J.; He, J.; Liu, Z.; Zhao, X.; Li, Y.; Zhao, Y.; Zhang, Y.; et al. Integrated characterization of hepatobiliary tumor organoids provides a potential landscape of pharmacogenomic interactions. Cell Rep. Med. 2024, 5, 101375. [Google Scholar] [CrossRef] [PubMed]
- Strobel, H.A.; Moss, S.M.; Hoying, J.B. Methods for vascularization and perfusion of tissue organoids. Mamm. Genome 2022, 33, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yang, X.; Ma, Z.; Sun, X.; Zhang, Y.; Li, W.; Yang, H.; Qiang, L.; Yang, Z.; Liu, Y.; et al. Developments and Opportunities for 3D Bioprinted Organoids. Int. J. Bioprint. 2024, 7, 364. [Google Scholar] [CrossRef] [PubMed]
- Beghin, A.; Grenci, G.; Sahni, G.; Guo, S.; Rajendiran, H.; Delaire, T.; Raffi, S.B.M.; Blanc, D.; de Mets, R.; Ong, H.T.; et al. Automated high-speed 3D imaging of organoid cultures with multi-scale phenotypic quantification. Nat. Methods 2022, 19, 881–892. [Google Scholar] [CrossRef]
- Schnalzger, T.E.; De Groot, M.H.; Zhang, C.; Mosa, M.H.; Michels, B.E.; Röder, J.; Darvishi, T.; Wels, W.S.; Farin, H.F. 3D model for CAR -mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J. 2019, 38, e100928. [Google Scholar] [CrossRef]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.A.; Francies, H.E.; Gavarró, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef]
- Werschler, N.; Quintard, C.; Nguyen, S.; Penninger, J. Engineering next generation vascularized organoid constructs. Atherosclerosis, 2024, in press. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).