Unlocking the Puzzle: Investigating the Role of Interleukin 17 Genetic Polymorphisms, Circulating Lymphocytes, and Serum Levels in Venezuelan Women with Recurrent Pregnancy Loss
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Samples
2.2. Antibodies and Reagents
2.3. Genetic Polymorphism Analysis
2.4. Analysis of Intracellular IL-17 in Different Cell Subpopulations
2.5. Determination of Il-17A from Serum Samples and Supernatants
2.6. Statistical Analysis
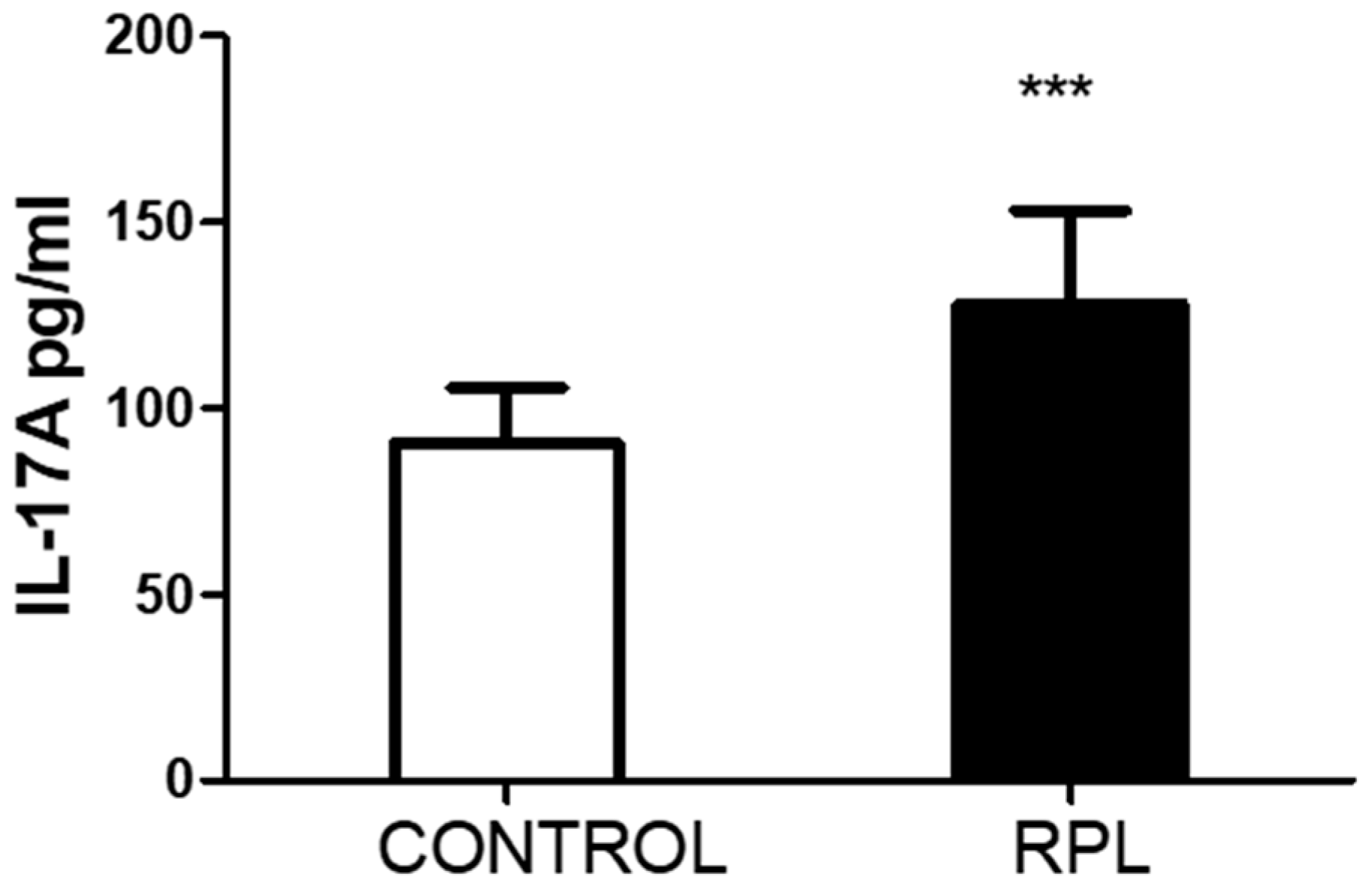
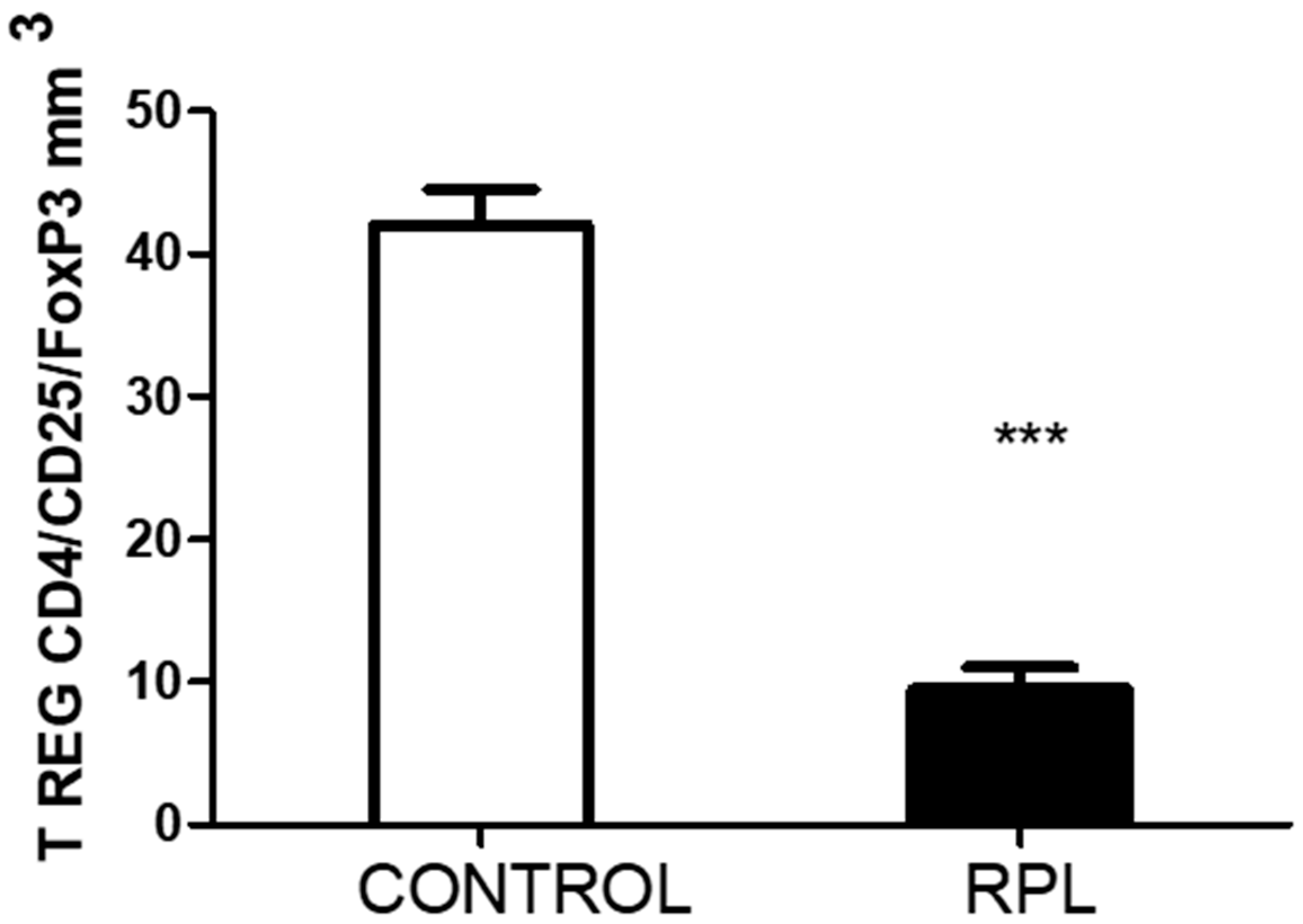
3. Results
4. Discussion
5. Conclusions
- There is no association between the two polymorphisms analyzed, IL-17A rs2275913 and IL-17F rs763780, with RPL. The values recorded from plasma of RPL patients were independent of the genetic polymorphisms.
- The peripheral lymphocytes of RPL patients were activated based on the percentage of HLA-DR expression. In addition, the number of T regulatory cells decreased.
- The number of IL-17-positive cells, CD8 and CD56, was significantly lower in RPL patients than in controls. However, IL-17 positiveness in the CD3CD4 subpopulation was higher in RPL patients than in controls.
- In stimulated cells, the response of all different cell populations and subpopulations was lower in RPL patients than in the controls. The effect of PMA/ionomycin stimulation on whole blood may be responsible for this effect.
- NK cells of RPL patients responded significantly more strongly upon PMA ionomycin stimulation than those of controls.
6. Limitations of the Study
- Other circulating cytokines were not measured in the present study. An analysis of inflammatory and anti-inflammatory cytokines may have helped us identify which Th17 cell subpopulation was circulating in these patients, as Navarron-Compán et al. [30] proposed in chronic diseases.
- Unfortunately, we could not quantify the number of CD4/CD25/FoxP3 cells in the stimulated samples, which could have been an essential point for comparing with IL-17. The significant differences recorded in the basal levels suggest that upon higher circulating levels of IL-17, the number of T regulatory cells decreases.
- Some of these RPL patients did not continue visits to the fertility clinic for other procedures due to the costs, so we could not perform a follow-up to ascertain if they had any successful pregnancies.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pillarisetty, L.S.; Mahdy, H. Recurrent Pregnancy Loss; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554460/ (accessed on 4 June 2024).
- Stephenson, M.D. Frequency of factors associated with habitual abortion in 197 couples. Fertil. Steril. 1996, 66, 24–29. [Google Scholar] [PubMed]
- Ford, H.B.; Schust, D.J. Recurrent pregnancy loss: Etiology, diagnosis, and therapy. Rev. Obstet. Gynecol. 2009, 2, 76–83. [Google Scholar] [PubMed]
- Cuadrado-Torroglosa, I.; García-Velasco, J.A.; Alecsandru, D. Maternal-Fetal Compatibility in Recurrent Pregnancy Loss. J. Clin. Med. 2024, 13, 2379. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Nakashima, A.; Ito, M.; Shima, T. Clinical implication of recent advances in our understanding of IL-17 and reproductive immunology. Expert. Rev. Clin. Immunol. 2011, 7, 649–657. [Google Scholar] [CrossRef]
- Fu, B.; Tian, Z.; Wei, H. TH17 cells in human recurrent pregnancy loss and pre-eclampsia. Cell. Mol. Immunol. 2014, 11, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Xu, Y.; Gong, G.; Zhang, Y. Roles of immune microenvironment in the female reproductive maintenance and regulation: Novel insights into the crosstalk of immune cells. Front. Immunol. 2023, 14, 1109122. [Google Scholar] [CrossRef]
- Cui, H.; Wang, N.; Li, H.; Bian, Y.; Wen, W.; Kong, X.; Wang, F. The dynamic shifts of IL-10-producing Th17 and IL-17-producing Treg in health and disease: Crosstalk between ancient "Yin-Yang" theory and modern immunology. Cell Commun. Signal. 2024, 22, 99. [Google Scholar] [CrossRef]
- Moura, G.A.; Rocha, Y.M.; Moura, F.L.D.; Freitas, J.O.; Rodrigues, J.P.V.; Gonçalves, V.P.; Nicolete, R. Immune system cells modulation in patients with reproductive issues: A systematic review approach. JBRA Assis. Reprod. 2024, 28, 78–89. [Google Scholar] [CrossRef]
- Najafi, S.; Hadinedoushan, H.; Eslami, G.; Aflatoonian, A. Association of IL-17A and IL-17 F gene polymorphisms with recurrent pregnancy loss in Iranian women. J. Assist. Reprod. Genet. 2014, 31, 1491–1496. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, J.; Bao, X.; Niu, W.; Wang, L.; Du, L.; Zhang, N.; Sun, Y. Association between Genetic Polymorphisms in Interleukin Genes and Recurrent Pregnancy Loss—A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169891. [Google Scholar] [CrossRef]
- Vahid, S.A.; Ghaebi, M.; Ahmadi, M.; Nouri, M.; Danaei, S.; Aghebati-Maleki, L.; Ardehaie, R.M.; Yousefi, B.; Hakimi, P.; Hojjat-Farsangi, M.; et al. Altered T-cell subpopulations in recurrent pregnancy loss patients with cellular immune abnormalities. J. Cell. Physiol. 2019, 234, 4924–4933. [Google Scholar] [CrossRef]
- Farshchi, M.; Abdollahi, E.; Saghafi, N.; Hosseini, A.; Fallahi, S.; Rostami, S.; Rostami, P.; Rafatpanah, H.; Habibagahi, M. Evaluation of Th17 and Treg cytokines in patients with unexplained recurrent pregnancy loss. J. Clin. Transl. Res. 2022, 8, 256–265. [Google Scholar]
- Ali, S.; Majid, S.; Ali, N.; Banday, M.Z.; Taing, S.; Wani, S.; Almuqbil, M.; Alshehri, S.; Shamim, K.; Rehman, M.U. Immunogenetic Role of IL17A Polymorphism in the Pathogenesis of Recurrent Miscarriage. J. Clin. Med. 2022, 11, 7448. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, M.; Ye, S.; Liu, Y.; Zhao, X.; Wang, Y. Association of IL-17 and IL-27 polymorphisms with susceptibility to recurrent pregnancy loss and pre-eclampsia: A systematic review and meta-analysis. Immun. Inflamm. Dis. 2023, 11, e1057. [Google Scholar] [CrossRef]
- Keshavarz Motamed, A.; Zarei, Z.H.; Mirfakhraee, H.; Shariatinia, F.; Akbari, M.; Ziagham, S.; Igder, S.; Zarei, N. Association of Interleukin-17A rs2275913 Polymorphism with Recurrent Miscarriage: A Systematic Review and Meta-Analysis Study. Int. J. Fertil. Steril. 2023, 18, 7–11. [Google Scholar]
- Li, D.; Uskenbayeva, N.; Fang, L.; Xu, Y.; Yan, H.; Zhang, K.; Wang, J. Genetic polymorphism of IL-17 influences susceptibility to recurrent pregnancy loss in a Chinese population. Medicine 2024, 103, e38333. [Google Scholar] [CrossRef]
- Gao, J.F.; Zhang, H.; Lv, J.; Wang, L.; Fan, Y.Y. Associations of the IL-17A rs2275913 and IL-17F rs763780 polymorphisms with the risk of digestive system neoplasms: A meta-analysis. Int. Immunopharmacol. 2019, 67, 248–259. [Google Scholar] [CrossRef]
- Li, J.; Tian, H.; Jiang, H.J.; Han, B. Interleukin-17 SNPs and serum levels increase ulcerative colitis risk: A meta-analysis. World J. Gastroenterol. 2014, 20, 15899–15909. [Google Scholar] [CrossRef]
- Stavros, S.; Panagopoulos, P.; Machairiotis, N.; Potiris, A.; Mavrogianni, D.; Sfakianakis, A.; Drakaki, E.; Christodoulaki, C.; Panagiotopoulos, D.; Sioutis, D.; et al. Association between cytokine polymorphisms and recurrent pregnancy loss: A review of current evidence. Int. J. Gynecol. Obstet. 2024, 167, 45–57. [Google Scholar] [CrossRef]
- Conesa, A.; Fernández-Mestre, M.; Padrón, D.; Toro, F.; Silva, N.; Tassinari, P.; Blanca, I.; Martin, M.P.; Carrington, M.; Layrisse, Z. Distribution of killer cell immunoglobulin-like receptor genes in the mestizo population from Venezuela. Tissue Antigens 2010, 75, 724–729. [Google Scholar] [CrossRef]
- del Fortes, P.M.; Gill, G.; Paredes, M.E.; Gamez, L.E.; Palacios, M.; Blanca, I.; Tassinari, P. Allele and haplotype frequencies at human leukocyte antigen class I and II genes in Venezuela’s population. Ann. Biol. Clin. 2012, 70, 175–181. [Google Scholar]
- Bryc, K.; Velez, C.; Karafet, T.; Moreno-Estrada, A.; Reynolds, A.; Auton, A.; Hammer, M.; Bustamante, C.D.; Ostrer, H. Colloquium paper: Genome-wide patterns of population structure and admixture among Hispanic/Latino populations. Proc. Natl. Acad. Sci. USA 2010, 107 (Suppl. S2), 8954–8961. [Google Scholar] [CrossRef]
- De Oliveira, T.C.; Secolin, R.; Lopes-Cendes, I. A review of ancestrality and admixture in Latin America and the Caribbean focusing on native American and African descendant populations. Front. Genet. 2023, 14, 1091269. [Google Scholar] [CrossRef]
- Niafar, M.; Samaie, V.; Soltani-Zangbar, M.S.; Motavalli, R.; Dolati, S.; Danaii, S.; Mehdizadeh, A.; Yousefi, M. The association of Treg and Th17 cells development factors and anti-TPO autoantibodies in patients with recurrent pregnancy loss. BMC Res. Notes 2023, 16, 302. [Google Scholar] [CrossRef]
- Peña, M.J.; De Sanctis, C.V.; De Sanctis, J.B.; Garmendia, J.V. Frequency of Gene Polymorphisms in Admixed Venezuelan Women with Recurrent Pregnancy Loss: Microsomal Epoxy Hydroxylase (rs1051740) and Enos (rs1799983). Curr. Issues Mol. Biol. 2024, 46, 3460–3469. [Google Scholar] [CrossRef]
- Xie, Z.; Ding, X.; Wang, Y.; Zhang, M. The rs2275913 polymorphism of the interleukin-17A gene is associated with the risk of ovarian endometriosis. J. Obstet. Gynaecol. 2023, 43, 2199852. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Z.; Tai, W.; Feng, W.; Zhang, D.; Gu, X.; Yang, R. Decreased Frequency of IL-17F rs763780 Site Allele G is Associated With Genetic Susceptibility to Immune Thrombocytopenia in a Chinese Population. Clin. Appl. Thromb. Hemost. 2017, 23, 466–471. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, J.Y.; Hur, S.E.; Kim, C.J.; Na, B.J.; Lee, M.; Gilman-Sachs, A.; Kwak-Kim, J. An imbalance in interleukin-17-producing T and Foxp3⁺ regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum. Reprod. 2011, 26, 2964–2971. [Google Scholar] [CrossRef]
- Navarro-Compán, V.; Puig, L.; Vidal, S.; Ramírez, J.; Llamas-Velasco, M.; Fernández-Carballido, C.; Almodóvar, R.; Pinto, J.A.; Galíndez-Aguirregoikoa, E.; Zarco, P.; et al. The paradigm of IL-23-independent production of IL-17F and IL-17A and their role in chronic inflammatory diseases. Frontier. Immunol. 2023, 14, 1191782. [Google Scholar] [CrossRef]
- Mills, K.H. Induction, function and regulation of IL-17-producing T cells. Eur. J. Immunol. 2008, 38, 2636–2649. [Google Scholar] [CrossRef]
- Purvis, H.A.; Stoop, J.N.; Mann, J.; Woods, S.; Kozijn, A.E.; Hambleton, S.; Robinson, J.H.; Isaacs, J.D.; Anderson, A.E.; Hilkens, C.M. Low-strength T-cell activation promotes Th17 responses. Blood 2010, 116, 4829–4837. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhou, Y.; Yu, J.; Mao, L.; Bosco, M.J.; Wang, J.; Lu, Y.; Mao, L.; Wu, X.; Wang, F.; et al. Establishment of the Reference Intervals of Lymphocyte Function in Healthy Adults Based on IFN-γ Secretion Assay upon Phorbol-12-Myristate-13-Acetate/Ionomycin Stimulation. Front. Immunol. 2018, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.D.; Al-Jaderi, Z.; Høglund, R.A.; Holmøy, T.; Harbo, H.F.; Norgauer, J.; Maghazachi, A.A. Identification of human NK17/NK1 cells. PLoS ONE 2011, 6, e26780. [Google Scholar] [CrossRef]

| Controls | RPL | |
|---|---|---|
| n | 50 | 50 |
| Age (years) | 34.3 ± 6.5 | 34.1 ± 4.5 |
| # Pregnancies (%) | 1 (10%) | 2 (50%) |
| 2 (60%) | 3 (35%) | |
| 3 (30%) | >3 (15%) | |
| # Miscarriages (%) | 0 | 2 (40%) |
| >2 (60%) | ||
| Duration of pregnancy (weeks) | 37.3 ± 2.2 | 8.1 ± 2.5 |
| Polymorphism | Control | RPL | p | OR |
|---|---|---|---|---|
| rs2275913 | ||||
| Genotype | ||||
| GG | 40 | 41 | 0.9 | 1.0 |
| GA | 8 | 8 | 0.9 | |
| AA | 2 | 1 | ||
| G | 48 | 50 | 0.8 | 1.0 |
| A | 10 | 9 | 0.8 | |
| rs763780 | ||||
| Genotype | ||||
| AA | 47 | 47 | 0.9 | 1.0 |
| GA | 1 | 2 | 0.9 | |
| GG | 2 | 1 | ||
| A | 48 | 50 | 0.9 | 1.0 |
| G | 3 | 3 | 0.9 |
| Control | RPL | p | |
|---|---|---|---|
| Total leukocytes | 6700 ± 814 | 6766 ± 727 | 0.7 |
| Total lymphocytes | 2105 ± 241 | 2359 ± 239 | >0.001 |
| CD3 T lymphocytes | 1530 ± 208 | 1614 ± 186 | 0.03 |
| NK cells (CD56/CD16) | 186 ± 37 | 261 ± 36 | >0.001 |
| NKT cells (CD3/CD56) | 34 ± 11 | 35 ± 14 | 0.7 |
| Index T cell/NK cell | 8.5 ± 1.6 | 6.2 ± 0.8 | >0.001 |
| CD3/CD45RA | 839 ± 134 | 1266 ± 165 | >0.001 |
| CD3/CD45RO | 928 ± 123 | 1076 ± 151 | >0.001 |
| HLA DR+ cells | 153 ± 43 | 247 ± 49 | >0.001 |
| CD3/HLA DR+ | 22 ± 8 | 82 ± 52 | >0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garmendia, J.V.; Blanca, I.; Peña, M.J.; De Sanctis, C.V.; De Sanctis, J.B. Unlocking the Puzzle: Investigating the Role of Interleukin 17 Genetic Polymorphisms, Circulating Lymphocytes, and Serum Levels in Venezuelan Women with Recurrent Pregnancy Loss. Immuno 2024, 4, 301-311. https://doi.org/10.3390/immuno4040019
Garmendia JV, Blanca I, Peña MJ, De Sanctis CV, De Sanctis JB. Unlocking the Puzzle: Investigating the Role of Interleukin 17 Genetic Polymorphisms, Circulating Lymphocytes, and Serum Levels in Venezuelan Women with Recurrent Pregnancy Loss. Immuno. 2024; 4(4):301-311. https://doi.org/10.3390/immuno4040019
Chicago/Turabian StyleGarmendia, Jenny Valentina, Isaac Blanca, María Johanna Peña, Claudia Valentina De Sanctis, and Juan Bautista De Sanctis. 2024. "Unlocking the Puzzle: Investigating the Role of Interleukin 17 Genetic Polymorphisms, Circulating Lymphocytes, and Serum Levels in Venezuelan Women with Recurrent Pregnancy Loss" Immuno 4, no. 4: 301-311. https://doi.org/10.3390/immuno4040019
APA StyleGarmendia, J. V., Blanca, I., Peña, M. J., De Sanctis, C. V., & De Sanctis, J. B. (2024). Unlocking the Puzzle: Investigating the Role of Interleukin 17 Genetic Polymorphisms, Circulating Lymphocytes, and Serum Levels in Venezuelan Women with Recurrent Pregnancy Loss. Immuno, 4(4), 301-311. https://doi.org/10.3390/immuno4040019







