Exploring Biological Risk Factors in Treatment-Resistant Depression
Abstract
1. Introduction
2. Literature Search and Selection
3. Neurochemical Imbalances
3.1. Neurotransmitters
3.1.1. Serotonin
Genetic and Epigenetic Influence on Serotonergic Function
3.1.2. Norepinephrine
3.1.3. Dopamine
Genetic Influence on Dopaminergic Function
3.1.4. Glutamate
3.1.5. Gamma-Aminobutyric Acid (GABA)
Genetic Influence on the GABAergic System
3.1.6. Additional Neurotransmitters in TRD
3.1.7. Clinical and Translational Implications of Neurotransmitters
3.2. Pharmacokinetics and Antidepressant Metabolism
Clinical and Translational Implications of Pharmacokinetics and Antidepressant Metabolism
3.3. Growth Factors
3.3.1. Brain-Derived Neurotrophic Factor (BDNF)
Genetic Influence on BDNF and Treatment Response
3.3.2. Insulin-like Growth Factor 1 (IGF-I)
3.3.3. Vascular Endothelial Growth Factor (VEGF)
Genetic Insights into Angiogenesis and TRD
3.3.4. Clinical and Translational Implications of Growth Factors
3.4. Hypothalamic–Pituitary–Adrenal (HPA) Axis
3.4.1. Genetic and Epigenetic Influence on HPA Axis Dysregulation
3.4.2. Clinical and Translational Implications of HPA Axis Dysregulation
3.5. Other Endocrinopathies Related to TRD
3.5.1. Genetic Links Between Endocrine Dysregulation and TRD
3.5.2. Clinical and Translational Implications of Other Endocrinopathies
4. Chronic Inflammation
4.1. Chronic Inflammation and TRD
4.1.1. Inflammation-Related Genetic Variants and TRD
4.1.2. Clinical and Translational Implications of Chronic Inflammation
5. Obesity and Metabolism
5.1. Obesity
5.1.1. Genetic Implications of Metabolism in TRD
5.1.2. Clinical and Translational Implications of Obesity and Metabolism
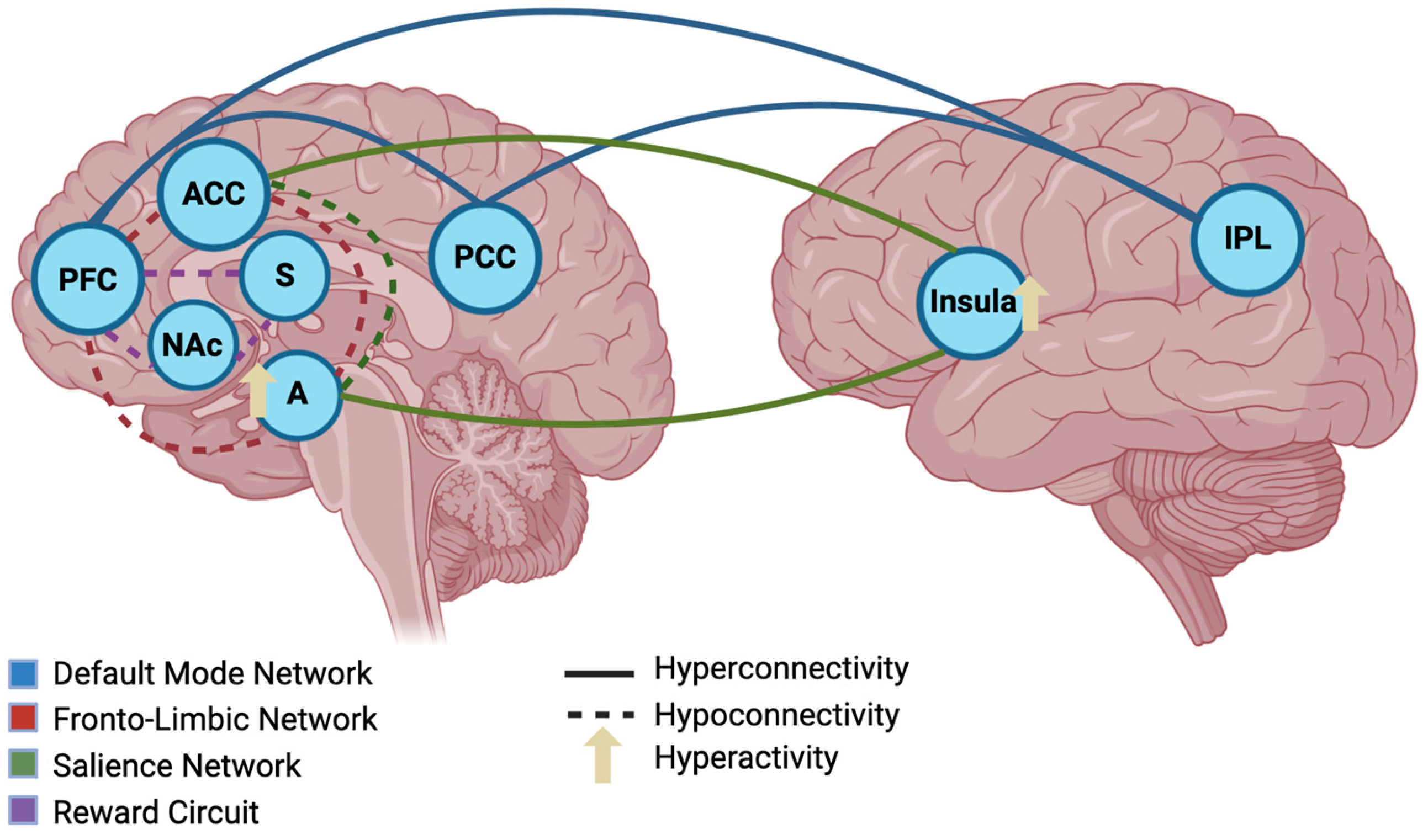
6. Alterations in Brain Connectivity
6.1. Default Mode Network (DMN)
6.2. Fronto-Limbic Network
6.3. Salience Network
6.4. Reward Circuit
6.5. Clinical and Translational Implications of Brain Connectivity Alterations
7. Gut Microbiota
Clinical and Translational Implications of Gut Microbiota
8. Oxidative Stress
Clinical and Translational Implications of Oxidative Stress
9. Psychosocial Factors
9.1. Chronic Stress
9.2. Childhood Adversity
9.3. Social Isolation
9.4. Clinical and Translational Implications of Psychosocial Factors
10. Animal Models and Non-Clinical Studies in TRD
11. Limitations and Future Directions
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| ACTH | Adrenocorticotropic hormone |
| AGDS | Australian Genetics of Depression Study |
| BDNF | Brain-Derived Neurotrophic Factor |
| BMI | Body Mass Index |
| CAT | Catalase |
| COMT | Catechol-O-methyltransferase |
| CPIC | Clinical Pharmacogenetics Implementation Consortium |
| CPNDS | Canadian Pharmacogenomics Network for Drug Safety |
| CRH | Corticotropin-releasing hormone |
| CRP | C-reactive protein |
| DBS | Deep brain stimulation |
| DPWG | Dutch Pharmacogenetics Working Group |
| ECT | Electroconvulsive therapy |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| GABA | Gamma-aminobutyric acid |
| GPX | Glutathione peroxidase |
| GR | Glucocorticoid receptor |
| GWAS | Genome-wide association studies |
| HPA | Hypothalamic–pituitary–adrenal |
| IDO | Indoleamine 2,3-dioxygenase |
| IGF-I | Insulin-like Growth Factor 1 |
| IL | Interleukin |
| LPS | Lipopolysaccharides |
| MDA | Malondialdehyde |
| MDD | Major depressive disorder |
| Met | Methionine |
| mPFC | Medial prefrontal cortex |
| MR | Mineralocorticoid receptor |
| MRAs | Mineralocorticoid receptor antagonists |
| NAc | Nucleus accumbens |
| PCC | Posterior cingulate cortex |
| ROS | Reactive oxygen species |
| rTMS | Repetitive transcranial magnetic stimulation |
| SCFAs | Short-chain fatty acids |
| SERT | Serotonin transporter |
| SNRI | Serotonin-norepinephrine reuptake inhibitors |
| SOD | Superoxide dismutase |
| SSRI | Serotonin reuptake inhibitors |
| T2DM | Type 2 diabetes mellitus |
| TCAs | Tricyclic antidepressants |
| TNF | Tumor Necrosis Factor |
| TRD | Treatment-resistant depression |
| TRIs | Triple reuptake inhibitors |
| Val | Valine |
| vALIC | Ventral anterior limb of the internal capsule |
| VEGF | Vascular Endothelial Growth Factor |
| vmPFC | Ventromedial prefrontal cortex |
References
- Goodwin, R.D.; Dierker, L.C.; Wu, M.; Galea, S.; Hoven, C.W.; Weinberger, A.H. Trends in U.S. Depression Prevalence From 2015 to 2020: The Widening Treatment Gap. Am. J. Prev. Med. 2022, 63, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Gaynes, B.N.; Lux, L.; Gartlehner, G.; Asher, G.; Forman-Hoffman, V.; Green, J.; Boland, E.; Weber, R.P.; Randolph, C.; Bann, C.; et al. Defining Treatment-Resistant Depression. Depress. Anxiety 2020, 37, 134–145. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Alsuwaidan, M.; Baune, B.T.; Berk, M.; Demyttenaere, K.; Goldberg, J.F.; Gorwood, P.; Ho, R.; Kasper, S.; Kennedy, S.H.; et al. Treatment-resistant Depression: Definition, Prevalence, Detection, Management, and Investigational Interventions. World Psychiatry 2023, 22, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Kverno, K.S.; Mangano, E. Treatment-Resistant Depression: Approaches to Treatment. J. Psychosoc. Nurs. Ment. Health Serv. 2021, 59, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.; Cars, T.; Lööv, S.Å.; Söderling, J.; Sundström, J.; Tiihonen, J.; Leval, A.; Gannedahl, A.; Björkholm, C.; Själin, M.; et al. Association of Treatment-Resistant Depression With Patient Outcomes and Health Care Resource Utilization in a Population-Wide Study. JAMA Psychiatry 2023, 80, 167–175. [Google Scholar] [CrossRef]
- Zhdanava, M.; Pilon, D.; Ghelerter, I.; Chow, W.; Joshi, K.; Lefebvre, P.; Sheehan, J.J. The Prevalence and National Burden of Treatment-Resistant Depression and Major Depressive Disorder in the United States. J. Clin. Psychiatry 2021, 82, 29169. [Google Scholar] [CrossRef]
- Kern, D.M.; Canuso, C.M.; Daly, E.; Johnson, J.C.; Fu, D.J.; Doherty, T.; Blauer-Peterson, C.; Cepeda, M.S. Suicide-Specific Mortality among Patients with Treatment-Resistant Major Depressive Disorder, Major Depressive Disorder with Prior Suicidal Ideation or Suicide Attempts, or Major Depressive Disorder Alone. Brain Behav. 2023, 13, e3171. [Google Scholar] [CrossRef]
- Halaris, A.; Sohl, E.; Whitham, E.A. Treatment-Resistant Depression Revisited: A Glimmer of Hope. J. Pers. Med. 2021, 11, 155. [Google Scholar] [CrossRef]
- Pourhamzeh, M.; Moravej, F.G.; Arabi, M.; Shahriari, E.; Mehrabi, S.; Ward, R.; Ahadi, R.; Joghataei, M.T. The Roles of Serotonin in Neuropsychiatric Disorders. Cell. Mol. Neurobiol. 2022, 42, 1671–1692. [Google Scholar] [CrossRef]
- Coplan, J.D.; Gopinath, S.; Abdallah, C.G.; Berry, B.R. A Neurobiological Hypothesis of Treatment-Resistant Depression–Mechanisms for Selective Serotonin Reuptake Inhibitor Non-Efficacy. Front. Behav. Neurosci. 2014, 8, 189. [Google Scholar] [CrossRef]
- Rink, L.; Adams, A.; Braun, C.; Bschor, T.; Kuhr, K.; Baethge, C. Dose-Response Relationship in Selective Serotonin and Norepinephrine Reuptake Inhibitors in the Treatment of Major Depressive Disorder: A Meta-Analysis and Network Meta-Analysis of Randomized Controlled Trials. Psychother. Psychosom. 2022, 91, 84–93. [Google Scholar] [CrossRef]
- Hieronymus, F.; Nilsson, S.; Eriksson, E. A Mega-Analysis of Fixed-Dose Trials Reveals Dose-Dependency and a Rapid Onset of Action for the Antidepressant Effect of Three Selective Serotonin Reuptake Inhibitors. Transl. Psychiatry 2016, 6, e834. [Google Scholar] [CrossRef] [PubMed]
- Murgaš, M.; Milz, C.; Stöhrmann, P.; Unterholzner, J.; Nics, L.; Kranz, G.S.; Hahn, A.; Hacker, M.; Kasper, S.; Lanzenberger, R.; et al. In Vivo Serotonin 1A Receptor Distribution in Treatment-Resistant Depression. Transl. Psychiatry 2025, 15, 186. [Google Scholar] [CrossRef]
- Caldiroli, A.; Capuzzi, E.; Tagliabue, I.; Capellazzi, M.; Marcatili, M.; Mucci, F.; Colmegna, F.; Clerici, M.; Buoli, M.; Dakanalis, A. Augmentative Pharmacological Strategies in Treatment-resistant Major Depression: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 13070. [Google Scholar] [CrossRef]
- López-Echeverri, Y.P.; Cardona-Londoño, K.J.; Garcia-Aguirre, J.F.; Orrego-Cardozo, M. Effects of Serotonin Transporter and Receptor Polymorphisms on Depression. Rev. Colomb. De Psiquiatr. 2023, 52, 130–138. [Google Scholar] [CrossRef]
- Jarčušková, D.; Tkáč, I.; Hlaváčová, N.; Yaluri, A.S.; Kozárová, M.; Habalová, V.; Klimčáková, L.; Židzik, J.; Javorský, M.; Bednářová, A. Serotonin Transporter 5-HTTLPR Polymorphism and Escitalopram Treatment Response in Patients with Major Depressive Disorder. BMC Psychiatry 2024, 24, 690. [Google Scholar] [CrossRef]
- Schiele, M.A.; Zwanzger, P.; Schwarte, K.; Arolt, V.; Baune, B.T.; Domschke, K. Serotonin Transporter Gene Promoter Hypomethylation as a Predictor of Antidepressant Treatment Response in Major Depression: A Replication Study. Int. J. Neuropsychopharmacol. 2021, 24, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhai, X.; Tan, H.; Ai, Y.; Zhao, L. Associations between the 1438A/G, 102T/C, and Rs7997012G/A Polymorphisms of HTR2A and the Safety and Efficacy of Antidepressants in Depression: A Meta-Analysis. Pharmacogenomics J 2021, 21, 200–215. [Google Scholar] [CrossRef]
- Dai, J.; Kim, H.; You, Z.; McCabe, M.F.; Zhang, S.; Wang, S.; Lim, G.; Chen, L.; Mao, J. Role of 5-HT1A-Mediated Upregulation of Brain Indoleamine 2,3 Dioxygenase 1 in the Reduced Antidepressant and Antihyperalgesic Effects of Fluoxetine during Maintenance Treatment. Front. Pharmacol. 2022, 13, 1084108. [Google Scholar] [CrossRef] [PubMed]
- Turcotte-Cardin, V.; Vahid-Ansari, F.; Luckhart, C.; Daigle, M.; Geddes, S.D.; Tanaka, K.F.; Hen, R.; James, J.; Merali, Z.; Béïque, J.C.; et al. Loss of Adult 5-HT1A Autoreceptors Results in a Paradoxical Anxiogenic Response to Antidepressant Treatment. J. Neurosci. 2019, 39, 1334–1346. [Google Scholar] [CrossRef]
- Sun, M.; Brivio, P.; Shan, L.; Docq, S.; Heltzel, L.C.M.W.; Smits, C.A.J.; Middelman, A.; Vrooman, R.; Spoelder, M.; Verheij, M.M.M.; et al. Offspring’s Own Serotonin Transporter Genotype, Independently from the Maternal One, Increases Anxiety- and Depression-like Behavior and Alters Neuroplasticity Markers in Rats. J. Affect. Disord. 2024, 350, 89–101. [Google Scholar] [CrossRef]
- Ren, F.; Ma, Y.; Zhu, X.; Guo, R.; Wang, J.; He, L. Pharmacogenetic Association of Bi- and Triallelic Polymorphisms of SLC6A4 with Antidepressant Response in Major Depressive Disorder. J. Affect. Disord. 2020, 273, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Cheng, Z.; Piao, J.; Cui, R.; Li, B. Dopamine Receptors: Is It Possible to Become a Therapeutic Target for Depression? Front. Pharmacol. 2022, 13, 947785. [Google Scholar] [CrossRef]
- Ventorp, F.; Lindahl, J.; Van Westen, D.; Jensen, J.; Björkstrand, J.; Lindqvist, D. Preliminary Evidence of Efficacy and Target Engagement of Pramipexole in Anhedonic Depression. Psych. Res. Clin. Pract. 2022, 4, 42–47. [Google Scholar] [CrossRef]
- Cook, J.; Halaris, A. Adjunctive Dopaminergic Enhancement of Esketamine in Treatment-Resistant Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 119, 110603. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, S.; Guo, D.; Wang, H. Association between COMT Gene Val108/158Met and Antidepressive Treatment Response: A Meta-Analysis. Gene 2020, 734, 144333. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Niu, L.; Ma, Y.; Qiu, Y.; Li, S.; Guobule, N.; Cao, H.; Li, J. Polymorphisms of COMT and CREB1 Are Associated with Treatment-Resistant Depression in a Chinese Han Population. J. Neural Transm. 2022, 129, 85–93. [Google Scholar] [CrossRef]
- Yang, J.H.; Presby, R.E.; Cayer, S.; Rotolo, R.A.; Perrino, P.A.; Fitch, R.H.; Correa, M.; Chesler, E.J.; Salamone, J.D. Effort-Related Decision Making in Humanized COMT Mice: Effects of Val158Met Polymorphisms and Possible Implications for Negative Symptoms in Humans. Pharmacol. Biochem. Behav. 2020, 196, 172975. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, T.E.; Lee, S.H.; Koo, J.W. The Role of Glutamate Underlying Treatment-Resistant Depression. Clin. Psychopharmacol. Neurosci. 2023, 21, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Vecera, C.M.; Courtes, A.C.; Jones, G.; Soares, J.C.; Machado-Vieira, R. Pharmacotherapies Targeting GABA-Glutamate Neurotransmission for Treatment-Resistant Depression. Pharmaceuticals 2023, 16, 1572. [Google Scholar] [CrossRef]
- Godfrey, K.E.M.; Muthukumaraswamy, S.D.; Stinear, C.M.; Hoeh, N. Effect of RTMS on GABA and Glutamate Levels in Treatment-Resistant Depression: An MR Spectroscopy Study. Psychiatry Res. Neuroimaging 2021, 317, 111377. [Google Scholar] [CrossRef]
- Spurny-Dworak, B.; Godbersen, G.M.; Reed, M.B.; Unterholzner, J.; Vanicek, T.; Baldinger-Melich, P.; Hahn, A.; Kranz, G.S.; Bogner, W.; Lanzenberger, R.; et al. The Impact of Theta-Burst Stimulation on Cortical GABA and Glutamate in Treatment-Resistant Depression: A Surface-Based MRSI Analysis Approach. Front. Mol. Neurosci. 2022, 15, 913274. [Google Scholar] [CrossRef]
- Chen, M.H.; Kao, C.F.; Tsai, S.J.; Li, C.T.; Lin, W.C.; Hong, C.J.; Bai, Y.M.; Tu, P.C.; Su, T.P. Treatment Response to Low-Dose Ketamine Infusion for Treatment-Resistant Depression: A Gene-Based Genome-Wide Association Study. Genomics 2021, 113, 507–514. [Google Scholar] [CrossRef]
- Gerhard, D.M.; Pothula, S.; Liu, R.J.; Wu, M.; Li, X.Y.; Girgenti, M.J.; Taylor, S.R.; Duman, C.H.; Delpire, E.; Picciotto, M.; et al. GABA Interneurons Are the Cellular Trigger for Ketamine’s Rapid Antidepressant Actions. J. Clin. Investig. 2020, 130, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Arias, H.R.; Targowska-Duda, K.M.; García-Colunga, J.; Ortells, M.O. Is the Antidepressant Activity of Selective Serotonin Reuptake Inhibitors Mediated by Nicotinic Acetylcholine Receptors? Molecules 2021, 26, 2149. [Google Scholar] [CrossRef] [PubMed]
- Fogaça, M.V.; Wu, M.; Li, C.; Li, X.Y.; Duman, R.S.; Picciotto, M.R. M1 Acetylcholine Receptors in Somatostatin Interneurons Contribute to GABAergic and Glutamatergic Plasticity in the MPFC and Antidepressant-like Responses. Neuropsychopharmacology 2023, 48, 1277–1287. [Google Scholar] [CrossRef]
- Rizzo, A.; Garçon-Poca, M.Z.; Essmann, A.; Souza, A.J.; Michaelides, M.; Ciruela, F.; Bonaventura, J. The Dopaminergic Effects of Esketamine Are Mediated by a Dual Mechanism Involving Glutamate and Opioid Receptors. Mol. Psychiatry 2025, 30, 3443–3454. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Jain, R. Glutamatergic Modulators for Major Depression from Theory to Clinical Use. CNS Drugs 2024, 38, 869–890. [Google Scholar] [CrossRef]
- Zimmer, L.; Newman-Tancredi, A. Serotonin 5-HT1A Receptor Biased Agonists: The Challenge of Translating an Innovative Neuropharmacological Concept into Therapeutics. Neuropharmacology 2025, 265, 110267. [Google Scholar] [CrossRef] [PubMed]
- Milic, J.; Jovic, S.; Sapic, R. Advancing Depression Management Through Biomarker Discovery with a Focus on Genetic and Epigenetic Aspects: A Comprehensive Study on Neurobiological, Neuroendocrine, Metabolic, and Inflammatory Pathways. Genes 2025, 16, 487. [Google Scholar] [CrossRef]
- Hicks, J.K.; Bishop, J.R.; Sangkuhl, K.; Muller, D.J.; Ji, Y.; Leckband, S.G.; Leeder, J.S.; Graham, R.L.; Chiulli, D.L.; LLerena, A.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin. Pharmacol. Ther. 2015, 98, 127–134. [Google Scholar] [CrossRef]
- Swen, J.J.; Nijenhuis, M.; De Boer, A.; Grandia, L.; Maitland-Van Der Zee, A.H.; Mulder, H.; Rongen, G.A.P.J.M.; Van Schaik, R.H.N.; Schalekamp, T.; Touw, D.J.; et al. Pharmacogenetics: From Bench to Byte an Update of Guidelines. Clin. Pharmacol. Ther. 2011, 89, 662–673. [Google Scholar] [CrossRef]
- ClinPGx: A Comprehensive Clinical Pharmacogenomics (PGx) Resource. Available online: https://www.clinpgx.org (accessed on 29 August 2025).
- Thorn, C.F.; Klein, T.E.; Altman, R.B. PharmGKB: The Pharmacogenomics Knowledge Base. Methods Mol. Biol. 2013, 1015, 311–320. [Google Scholar] [CrossRef]
- Thiele, L.S.; Ishtiak-Ahmed, K.; Thirstrup, J.P.; Agerbo, E.; Lunenburg, C.A.T.C.; Müller, D.J.; Gasse, C. Clinical Impact of Functional CYP2C19 and CYP2D6 Gene Variants on Treatment with Antidepressants in Young People with Depression: A Danish Cohort Study. Pharmaceuticals 2022, 15, 870. [Google Scholar] [CrossRef]
- Mitchell, B.; Martin, N.; Medland, S.E. Genetic and Environmental Predictors of Treatment Resistant Depression. Eur. Neuropsychopharmacol. 2024, 87, 25–26. [Google Scholar] [CrossRef]
- Zemanova, N.; Anzenbacher, P.; Anzenbacherova, E. The Role of Cytochromes P450 in the Metabolism of Selected Antidepressants and Anxiolytics under Psychological Stress. Biomed. Pap. 2022, 166, 140–149. [Google Scholar] [CrossRef]
- Dodd, S.; Bauer, M.; Carvalho, A.F.; Eyre, H.; Fava, M.; Kasper, S.; Kennedy, S.H.; Khoo, J.P.; Lopez Jaramillo, C.; Malhi, G.S.; et al. A Clinical Approach to Treatment Resistance in Depressed Patients: What to Do When the Usual Treatments Don’t Work Well Enough? World J. Biol. Psychiatry 2021, 22, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Persson, A.; Sim, S.C.; Virding, S.; Onishchenko, N.; Schulte, G.; Ingelman-Sundberg, M. Decreased Hippocampal Volume and Increased Anxiety in a Transgenic Mouse Model Expressing the Human CYP2C19 Gene. Mol. Psychiatry 2014, 19, 733–741. [Google Scholar] [CrossRef]
- Tolledo, C.; Stocco, M.R.; Miksys, S.; Gonzalez, F.J.; Tyndale, R.F. Human CYP2D6 Is Functional in Brain In Vivo: Evidence from Humanized CYP2D6 Transgenic Mice. Mol. Neurobiol. 2020, 57, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Deyama, S.; Fogaça, M.V. Role of BDNF in the Pathophysiology and Treatment of Depression: Activity-Dependent Effects Distinguish Rapid-Acting Antidepressants. Eur. J. Neurosci. 2021, 53, 126–139. [Google Scholar] [CrossRef]
- Zelada, M.I.; Garrido, V.; Liberona, A.; Jones, N.; Zúñiga, K.; Silva, H.; Nieto, R.R. Brain-Derived Neurotrophic Factor (BDNF) as a Predictor of Treatment Response in Major Depressive Disorder (MDD): A Systematic Review. Int. J. Mol. Sci. 2023, 24, 14810. [Google Scholar] [CrossRef]
- Mosiołek, A.; Mosiołek, J.; Jakima, S.; Pięta, A.; Szulc, A. Effects of Antidepressant Treatment on Neurotrophic Factors (BDNF and IGF-1) in Patients with Major Depressive Disorder (MDD). J. Clin. Med. 2021, 10, 3377. [Google Scholar] [CrossRef]
- Pathak, P.; Mehra, A.; Ram, S.; Pal, A.; Grover, S. Association of Serum BDNF Level and Val66Met Polymorphism with Response to Treatment in Patients of Major Depressive Disease: A Step towards Personalized Therapy. Behav. Brain Res. 2022, 430, 113931. [Google Scholar] [CrossRef]
- Pardossi, S.; Fagiolini, A.; Cuomo, A. Variations in BDNF and Their Role in the Neurotrophic Antidepressant Mechanisms of Ketamine and Esketamine: A Review. Int. J. Mol. Sci. 2024, 25, 13098. [Google Scholar] [CrossRef]
- Lin, P.Y.; Ma, Z.Z.; Mahgoub, M.; Kavalali, E.T.; Monteggia, L.M. A Synaptic Locus for TrkB Signaling Underlying Ketamine Rapid Antidepressant Action. Cell Rep. 2021, 36, 109513. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Gu, L.M.; Zhou, Y.L.; Wang, C.Y.; Lan, X.F.; Zhang, B.; Shi, H.S.; Wang, D.F.; Ning, Y.P. Association of VEGF With Antianhedonic Effects of Repeated-Dose Intravenous Ketamine in Treatment-Refractory Depression. Front. Psychiatry 2021, 12, 780975. [Google Scholar] [CrossRef]
- Ibarrola, J.; Jaffe, I.Z. The Mineralocorticoid Receptor in the Vasculature: Friend or Foe? Annu. Rev. Physiol. 2025, 86, 49–70. [Google Scholar] [CrossRef]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major Depressive Disorder: Hypothesis, Mechanism, Prevention and Treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Deyama, S.; Bang, E.; Wohleb, E.S.; Li, X.Y.; Kato, T.; Gerhard, D.M.; Dutheil, S.; Dwyer, J.M.; Taylor, S.R.; Picciotto, M.R.; et al. Role of Neuronal VEGF Signaling in the Prefrontal Cortex in the Rapid Antidepressant Effects of Ketamine. Am. J. Psychiatry 2019, 176, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Valiuliene, G.; Valiulis, V.; Dapsys, K.; Vitkeviciene, A.; Gerulskis, G.; Navakauskiene, R.; Germanavicius, A. Brain Stimulation Effects on Serum BDNF, VEGF, and TNFα in Treatment-Resistant Psychiatric Disorders. Eur. J. Neurosci. 2021, 53, 3791–3802. [Google Scholar] [CrossRef]
- Fukuda, A.M.; Hindley, L.E.; Kang, J.W.D.; Tirrell, E.; Tyrka, A.R.; Ayala, A.; Carpenter, L.L. Peripheral Vascular Endothelial Growth Factor Changes after Transcranial Magnetic Stimulation in Treatment-Resistant Depression. Neuroreport 2020, 31, 1121–1127. [Google Scholar] [CrossRef]
- Biwer, L.A.; Wallingford, M.C.; Jaffe, I.Z. Vascular Mineralocorticoid Receptor: Evolutionary Mediator of Wound Healing Turned Harmful by Our Modern Lifestyle. Am. J. Hypertens. 2019, 32, 123–134. [Google Scholar] [CrossRef]
- Nieckarz, A.; Graff, B.; Burnier, M.; Marcinkowska, A.B.; Narkiewicz, K. Aldosterone in the Brain and Cognition: Knowns and Unknowns. Front. Endocrinol. 2024, 15, 1456211. [Google Scholar] [CrossRef]
- Pastena, P.; Campagnoli, G.; Rahmani, A.R.; Kalogeropoulos, A.P. Mineralocorticoid Receptor Antagonists and Cognitive Outcomes in Cardiovascular Disease and Beyond: A Systematic Review. J. Pers. Med. 2025, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Shibata, H. Evolution of Mineralocorticoid Receptor Antagonists, Aldosterone Synthase Inhibitors, and Alternative Treatments for Managing Primary Aldosteronism. Hypertens. Res. 2025, 48, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-J.; Kao, C.-F.; Su, T.-P.; Li, C.-T.; Lin, W.-C.; Hong, C.-J.; Bai, Y.-M.; Tu, P.-C.; Chen, M.-H. Cytokine- and Vascular Endothelial Growth Factor-Related Gene-Based Genome-Wide Association Study of Low-Dose Ketamine Infusion in Patients with Treatment-Resistant Depression. CNS Drugs 2023, 37, 243–253. [Google Scholar] [CrossRef]
- Wang, J.; Meng, F.; Wang, L.; Li, Z. Vascular Endothelial Growth Factor: A Key Factor in the Onset and Treatment of Depression. Front. Cell. Neurosci. 2025, 19, 1645437. [Google Scholar] [CrossRef] [PubMed]
- Dziurkowska, E.; Wesolowski, M. Cortisol as a Biomarker of Mental Disorder Severity. J. Clin. Med. 2021, 10, 5204. [Google Scholar] [CrossRef]
- Lebedeva, A.; Sundström, A.; Lindgren, L.; Stomby, A.; Aarsland, D.; Westman, E.; Winblad, B.; Olsson, T.; Nyberg, L. Longitudinal Relationships among Depressive Symptoms, Cortisol, and Brain Atrophy in the Neocortex and the Hippocampus. Acta Psychiatr. Scand. 2018, 137, 491–502. [Google Scholar] [CrossRef]
- Vaseghi, S.; Mostafavijabbari, A.; Alizadeh, M.-S.; Ghaffarzadegan, R.; Kholghi, G.; Zarrindast, M. Intricate Role of Sleep Deprivation in Modulating Depression: Focusing on BDNF, VEGF, Serotonin, Cortisol, and TNF-α. Metab. Brain Dis. 2023, 38, 195–219. [Google Scholar] [CrossRef]
- James, K.A.; Stromin, J.I.; Steenkamp, N.; Combrinck, M.I. Understanding the Relationships between Physiological and Psychosocial Stress, Cortisol and Cognition. Front. Endocrinol. 2023, 14, 1085950. [Google Scholar] [CrossRef]
- Chan, I.I.; Wu, A.M.S. Assessing the Role of Cortisol in Anxiety, Major Depression, and Neuroticism: A Mendelian Randomization Study Using SERPINA6/SERPINA1 Variants. Biol. Psychiatry Glob. Open Sci. 2024, 4, 100294. [Google Scholar] [CrossRef]
- Hassamal, S. Chronic Stress, Neuroinflammation, and Depression: An Overview of Pathophysiological Mechanisms and Emerging Anti-Inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef]
- Puglisi, S.; Perini, A.M.E.; Botto, C.; Oliva, F.; Terzolo, M. Long-Term Consequences of Cushing Syndrome: A Systematic Literature Review. J. Clin. Endocrinol. Metab. 2024, 109, e901–e919. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.A.; Hickle, S.D.; Penna, S.; Ioachimescu, A.G.; Tone, E.B. Comparative Analysis of Cognitive and Psychiatric Functioning in People With Cushing’s Disease in Biochemical Remission and People With Nonfunctioning Adenomas. Behav. Neurol. 2024, 2024, 4393169. [Google Scholar] [CrossRef]
- Lee, M.R.; Rio, D.; Kwako, L.; George, D.T.; Heilig, M.; Momenan, R. Corticotropin-Releasing Factor Receptor 1 (CRF1) Antagonism in Patients with Alcohol Use Disorder and High Anxiety Levels: Effect on Neural Response during Trier Social Stress Test Video Feedback. Neuropsychopharmacology 2023, 48, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Milo, T.; Nir Halber, S.; Raz, M.; Danan, D.; Mayo, A.; Alon, U. Hormone Circuit Explains Why Most HPA Drugs Fail for Mood Disorders and Predicts the Few That Work. Mol. Syst. Biol. 2025, 21, 254–273. [Google Scholar] [CrossRef]
- Fan, B.; Ma, J.; Zhang, H.; Liao, Y.; Wang, W.; Zhang, S.; Lu, C.; Guo, L. Association of FKBP5 Gene Variants with Depression Susceptibility: A Comprehensive Meta-Analysis. Asia-Pac. Psychiatry 2021, 13, e12464. [Google Scholar] [CrossRef] [PubMed]
- Wietfeldt, N.; Boileau, A.J. FKBP5 Gene Variants as Predictors for Antidepressant Response in Individuals with Major Depressive Disorder Who Have Experienced Childhood Trauma. A Systematic Review. Int. J. Med. Stud. 2020, 8, 126–134. [Google Scholar] [CrossRef]
- Zhang, Y.; Yue, W.; Li, J. The Association of FKBP5 Gene Polymorphism with Genetic Susceptibility to Depression and Response to Antidepressant Treatment- a Systematic Review. BMC Psychiatry 2024, 24, 274. [Google Scholar] [CrossRef]
- Maier, H.B.; Moschny, N.; Eberle, F.; Jahn, K.; Folsche, T.; Schülke, R.; Bleich, S.; Frieling, H.; Neyazi, A. DNA Methylation of POMC and NR3C1-1F and Its Implication in Major Depressive Disorder and Electroconvulsive Therapy. Pharmacopsychiatry 2023, 56, 64–72. [Google Scholar] [CrossRef]
- Silva, R.C.; Dattilo, V.; Perusi, G.; Mazzelli, M.; Maffioletti, E.; Bazzanella, R.; Bortolomasi, M.; Cattaneo, A.; Gennarelli, M.; Minelli, A. Transcriptional Modulation of Stress-Related Genes in Association with Early Life Stress Exposure and Trauma-Focused Psychotherapy in Treatment-Resistant Depression Patients. J. EMDR Pract. Res. 2023, 17, 119–138. [Google Scholar] [CrossRef]
- Häusl, A.S.; Brix, L.M.; Hartmann, J.; Pöhlmann, M.L.; Lopez, J.P.; Menegaz, D.; Brivio, E.; Engelhardt, C.; Roeh, S.; Bajaj, T.; et al. The Co-Chaperone Fkbp5 Shapes the Acute Stress Response in the Paraventricular Nucleus of the Hypothalamus of Male Mice. Mol. Psychiatry 2021, 26, 3060–3076. [Google Scholar] [CrossRef] [PubMed]
- Codagnone, M.G.; Kara, N.; Ratsika, A.; Levone, B.R.; van de Wouw, M.; Tan, L.A.; Cunningham, J.I.; Sanchez, C.; Cryan, J.F.; O’Leary, O.F. Inhibition of FKBP51 Induces Stress Resilience and Alters Hippocampal Neurogenesis. Mol. Psychiatry 2022, 27, 4928–4938. [Google Scholar] [CrossRef] [PubMed]
- Nuguru, S.P.; Rachakonda, S.; Sripathi, S.; Khan, M.I.; Patel, N.; Meda, R.T. Hypothyroidism and Depression: A Narrative Review. Cureus 2022, 14, e28201. [Google Scholar] [CrossRef]
- Trifu, S.; Popescu, A.; Dragoi, A.M.; Trifu, A.I. Thyroid Hormones as a Third Line of Augmentation Medication in Treatment-Resistant Depression. Acta Endocrinol. 2020, 16, 256–261. [Google Scholar] [CrossRef]
- Lorentzen, R.; Kjær, J.N.; Østergaard, S.D.; Madsen, M.M. Thyroid Hormone Treatment in the Management of Treatment-resistant Unipolar Depression: A Systematic Review and Meta-analysis. Acta Psychiatr. Scand. 2020, 141, 316–326. [Google Scholar] [CrossRef]
- Wang, Z.; Robbins, B.; Zhuang, R.; van Bruggen, R.; Sandini, T.; Li, X.M.; Zhang, Y. Psilocybin Mitigates Behavioral Despair and Cognitive Impairment in Treatment-Resistant Depression Model Using Wistar Kyoto Rats. Sci. Rep. 2025, 15, 18432. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Ayani, N.; Abe, Y.; Nakayama, C.; Tsujikawa, T.; Oda, M.; Narumoto, J. Efficacy of Electroconvulsive Therapy for Treatment-Resistant Depression Caused by Hyperparathyroidism. J. ECT 2020, 36, e12–e13. [Google Scholar] [CrossRef]
- Lipsitz, O.; McIntyre, R.S.; Rodrigues, N.B.; Lee, Y.; Cha, D.S.; Gill, H.; Subramaniapillai, M.; Kratiuk, K.; Lin, K.; Ho, R.; et al. Intravenous Ketamine for Postmenopausal Women with Treatment-Resistant Depression: Results from the Canadian Rapid Treatment Center of Excellence. J. Psychiatr. Res. 2021, 136, 444–451. [Google Scholar] [CrossRef]
- Dwyer, J.B.; Aftab, A.; Radhakrishnan, R.; Widge, A.; Rodriguez, C.I.; Carpenter, L.L.; Nemeroff, C.B.; McDonald, W.M.; Kalin, N.H.; APA Council of Research Task Force on Novel Biomarkers and Treatments; et al. Hormonal Treatments for Major Depressive Disorder: State of the Art. Am. J. Psychiatry 2020, 177, 686–705. [Google Scholar] [CrossRef]
- Anderson, D.J.; Vazirnia, P.; Loehr, C.; Sternfels, W.; Hasoon, J.; Viswanath, O.; Kaye, A.D.; Urits, I. Testosterone Replacement Therapy in the Treatment of Depression. Health Psychol. Res. 2022, 10, 38956. [Google Scholar] [CrossRef]
- Dichtel, L.E.; Carpenter, L.L.; Nyer, M.; Mischoulon, D.; Kimball, A.; Deckersbach, T.; Dougherty, D.D.; Schoenfeld, D.A.; Fisher, L.; Cusin, C.; et al. Low-Dose Testosterone Augmentation for Antidepressant-Resistant Major Depressive Disorder in Women: An 8-Week Randomized Placebo-Controlled Study. Am. J. Psychiatry 2020, 177, 965–973. [Google Scholar] [CrossRef]
- Gagne, C.; Piot, A.; Brake, W.G. Depression, Estrogens, and Neuroinflammation: A Preclinical Review of Ketamine Treatment for Mood Disorders in Women. Front. Psychiatry 2022, 12, 797577. [Google Scholar] [CrossRef]
- Herson, M.; Kulkarni, J. Hormonal Agents for the Treatment of Depression Associated with the Menopause. Drugs Aging 2022, 39, 607–618. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Feng, L.; Xiao, L.; Yang, R.; Zhu, X.; Shi, H.; Hu, Y.; Chen, R.; Boyce, P.; et al. Venlafaxine vs. Fluoxetine in Postmenopausal Women with Major Depressive Disorder: An 8-Week, Randomized, Single-Blind, Active-Controlled Study. BMC Psychiatry 2021, 21, 260. [Google Scholar] [CrossRef]
- Shah, S.B.; Peddada, T.N.; Song, C.; Mensah, M.; Sung, H.; Yavi, M.; Yuan, P.; Zarate, C.A.; Mickey, B.J.; Burmeister, M.; et al. Exome-Wide Association Study of Treatment-Resistant Depression Suggests Novel Treatment Targets. Sci. Rep. 2023, 13, 12467. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Whybrow, P.C. Role of Thyroid Hormone Therapy in Depressive Disorders. J. Endocrinol. Investig. 2021, 44, 2341–2347. [Google Scholar] [CrossRef] [PubMed]
- Salvador, A.F.; de Lima, K.A.; Kipnis, J. Neuromodulation by the Immune System: A Focus on Cytokines. Nat. Rev. Immunol. 2021, 21, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Cheng, Z.-Y.; Shan, F.; Cao, Y.; Xia, Q.-R. Serum Indoleamine 2, 3-Dioxygenase and Tryptophan-2, 3-Dioxygenase: Potential Biomarkers for the Diagnosis of Major Depressive Disorder. Psychopharmacology 2024, 241, 1093–1099. [Google Scholar] [CrossRef]
- Fellendorf, F.T.; Bonkat, N.; Dalkner, N.; Schönthaler, E.M.D.; Manchia, M.; Fuchs, D.; Reininghaus, E.Z. Indoleamine 2,3-Dioxygenase (IDO)-Activity in Severe Psychiatric Disorders: A Systemic Review. Curr. Top. Med. Chem. 2022, 22, 2107–2118. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Sun, Z.; Ren, S.; Liu, M.; Wang, G.; Yang, J. Microglia in Depression: An Overview of Microglia in the Pathogenesis and Treatment of Depression. J. Neuroinflamm. 2022, 19, 132. [Google Scholar] [CrossRef]
- Afridi, R.; Suk, K. Microglial Responses to Stress-Induced Depression: Causes and Consequences. Cells 2023, 12, 1521. [Google Scholar] [CrossRef]
- Li, B.; Yang, W.; Ge, T.; Wang, Y.; Cui, R. Stress Induced Microglial Activation Contributes to Depression. Pharmacol. Res. 2022, 179, 106145. [Google Scholar] [CrossRef]
- Beckett, C.W.; Niklison-Chirou, M.V. The Role of Immunomodulators in Treatment-Resistant Depression: Case Studies. Cell Death Discov. 2022, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Nady, E.; El-Derany, M.; Michel, H.; El-Demerdash, E. Pathophysiology of Depression: Inflammation and Its Relation with Oxidative Stress and the Hypothalamic-Pituitary-Adrenal Axis. Arch. Pharm. Sci. Ain Shams Univ. 2024, 8, 13–28. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, C.; Lan, X.; Li, H.; Chao, Z.; Ning, Y. Plasma Inflammatory Cytokines and Treatment-Resistant Depression with Comorbid Pain: Improvement by Ketamine. J. Neuroinflammation 2021, 18, 200. [Google Scholar] [CrossRef]
- Gong, H.; Su, W.J.; Deng, S.L.; Luo, J.; Du, Z.L.; Luo, Y.; Lv, K.Y.; Zhu, D.M.; Fan, X.T. Anti-Inflammatory Interventions for the Treatment and Prevention of Depression among Older Adults: A Systematic Review and Meta-Analysis. Transl. Psychiatry 2025, 15, 114. [Google Scholar] [CrossRef]
- Duong, A.; Jeong, H.; El Soufi El Sabbagh, D.; Andreazza, A.C. Systemic Inflammatory Biomarkers in DSM-5–Defined Disorders and COVID-19: Evidence From Published Meta-Analyses. Biol. Psychiatry Glob. Open Sci. 2023, 3, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Castro, V.M.; Ripperger, M.; Venkatesh, S.; Burstein, D.; Linnér, R.K.; Rocha, D.B.; Hu, Y.; Wilimitis, D.; Morley, T.; et al. Genome-Wide Association Study of Treatment-Resistant Depression: Shared Biology With Metabolic Traits. Am. J. Psychiatry 2024, 181, 608–619. [Google Scholar] [CrossRef]
- Fulton, S.; Décarie-Spain, L.; Fioramonti, X.; Guiard, B.; Nakajima, S. The Menace of Obesity to Depression and Anxiety Prevalence. Trends Endocrinol. Metab. 2022, 33, 18–35. [Google Scholar] [CrossRef]
- McLaughlin, A.P.; Lambert, E.; Milton, R.; Mariani, N.; Kose, M.; Nikkheslat, N.; Patsalos, O.; Ferraro, L.; Chamseddine, G.; Panagiotopoulos, S.; et al. Peripheral Inflammation Associated with Depression and Reduced Weight Loss: A Longitudinal Study of Bariatric Patients. Psychol. Med. 2024, 54, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Munshi, S.; Burrows, K.; Kuplicki, R.; Figueroa-Hall, L.K.; Aupperle, R.L.; Khalsa, S.S.; Teague, T.K.; Taki, Y.; Paulus, M.P.; et al. Leptin’s Inverse Association With Brain Morphology and Depressive Symptoms: A Discovery and Confirmatory Study Across 2 Independent Samples. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2024, 9, 714–725. [Google Scholar] [CrossRef]
- Krupa, A.J.; Chrobak, A.A.; Sołtys, Z.; Dudek, D.; Szewczyk, B.; Siwek, M. Insulin Resistance, Clinical Presentation and Resistance to Selective Serotonin and Noradrenaline Reuptake Inhibitors in Major Depressive Disorder. Pharmacol. Rep. 2024, 76, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.Y.; Yau, S.Y. From Obesity to Hippocampal Neurodegeneration: Pathogenesis and Non-Pharmacological Interventions. Int. J. Mol. Sci. 2020, 22, 201. [Google Scholar] [CrossRef]
- Guo, H.; Han, J.; Xiao, M.; Chen, H. Functional Alterations in Overweight/Obesity: Focusing on the Reward and Executive Control Network. Rev. Neurosci. 2024, 35, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Dębski, J.; Przybyłowski, J.; Skibiak, K.; Czerwińska, M.; Walędziak, M.; Różańska-Walędziak, A. Depression and Obesity—Do We Know Everything about It? A Narrative Review. Nutrients 2024, 16, 3383. [Google Scholar] [CrossRef]
- Jakubowska, P.; Balcerczyk-Lis, M.; Fortuna, M.; Janiak, A.; Kopaczyńska, A.; Skwira, S.; Młynarska, E.; Rysz, J.; Franczyk, B. Influence of Metabolic Dysregulation in the Management of Depressive Disorder-Narrative Review. Nutrients 2024, 16, 1665. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Casanova, F.; O’Loughlin, J.; Green, H.; McKinley, T.J.; Bowden, J.; Tyrrell, J. Body Mass Index and Inflammation in Depression and Treatment-Resistant Depression: A Mendelian Randomisation Study. BMC Med. 2023, 21, 355. [Google Scholar] [CrossRef]
- Chang, R.; Huang, Z.; Zhao, S.; Zou, J.; Li, Y.; Tan, S. Emerging Roles of FTO in Neuropsychiatric Disorders. BioMed Res. Int. 2022, 2022, 2677312. [Google Scholar] [CrossRef]
- Liu, S.; Xiu, J.; Zhu, C.; Meng, K.; Li, C.; Han, R.; Du, T.; Li, L.; Xu, L.; Liu, R.; et al. Fat Mass and Obesity-Associated Protein Regulates RNA Methylation Associated with Depression-like Behavior in Mice. Nat. Commun. 2021, 12, 6937. [Google Scholar] [CrossRef]
- Sun, L.; Ma, L.; Zhang, H.; Cao, Y.; Wang, C.; Hou, N.; Huang, N.; von Deneen, K.M.; Zhao, C.; Shi, Y.; et al. FTO Deficiency Reduces Anxiety- and Depression-like Behaviors in Mice via Alterations in Gut Microbiota. Theranostics 2019, 9, 721–733. [Google Scholar] [CrossRef]
- Kurban, N.; Qin, Y.; Zhao, H.L.; Hu, X.; Chen, X.; Zhao, Y.Y.; Peng, Y.S.; Wang, H.B.; Cui, S.Y.; Zhang, Y.H. Chronic Stress-Induced Elevation of Melanin-Concentrating Hormone in the Locus Coeruleus Inhibits Norepinephrine Production and Associated With Depression-Like Behaviors in Rats. Int. J. Neuropsychopharmacol. 2024, 27, pyad069. [Google Scholar] [CrossRef]
- Shi, L.; He, Y.; Lian, Y.; Luo, J.; Zhu, X.; Zhao, H. Melanin-Concentrating Hormone: A Promising Target for Antidepressant Treatment. Pharmacol. Biochem. Behav. 2025, 250, 173999. [Google Scholar] [CrossRef]
- Fabbri, C.; Pain, O.; Hagenaars, S.P.; Lewis, C.M.; Serretti, A. Transcriptome-Wide Association Study of Treatment-Resistant Depression and Depression Subtypes for Drug Repurposing. Neuropsychopharmacology 2021, 46, 1821–1829. [Google Scholar] [CrossRef]
- Watson, K.; Akil, H.; Rasgon, N. Toward a Precision Treatment Approach for Metabolic Depression: Integrating Epidemiology, Neuroscience, and Psychiatry. Biol. Psychiatry Glob. Open Sci. 2023, 3, 623–631. [Google Scholar] [CrossRef]
- Heinen, D.; Heissel, A.; Heinzel, S.; Fydrich, T.; Ströhle, A.; Rapp, M.A.; Vogel, H. Effect of Acute and Long-Term Exercise on Leptin Levels in Depressed Outpatients. BMC Public Health 2023, 23, 2509. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; You, K.Y.; Lee, J.H.; Jeon, M.K.; Lee, B.H.; Ryu, J.Y.; Oh, K.S. Identification and New Indication of Melanin-Concentrating Hormone Receptor 1 (MCHR1) Antagonist Derived from Machine Learning and Transcriptome-Based Drug Repositioning Approaches. Int. J. Mol. Sci. 2022, 23, 3807. [Google Scholar] [CrossRef] [PubMed]
- Menon, V. 20 Years of the Default Mode Network: A Review and Synthesis. Neuron 2023, 111, 2469–2487. [Google Scholar] [CrossRef] [PubMed]
- Ilomäki, M.; Lindblom, J.; Salmela, V.; Flykt, M.; Vänskä, M.; Salmi, J.; Tolonen, T.; Alho, K.; Punamäki, R.-L.; Wikman, P. Early Life Stress Is Associated with the Default Mode and Fronto-Limbic Network Connectivity among Young Adults. Front. Behav. Neurosci. 2022, 16, 958580. [Google Scholar] [CrossRef]
- Runia, N.; Yücel, D.E.; Lok, A.; de Jong, K.; Denys, D.A.J.P.; van Wingen, G.A.; Bergfeld, I.O. The Neurobiology of Treatment-Resistant Depression: A Systematic Review of Neuroimaging Studies. Neurosci. Biobehav. Rev. 2022, 132, 433–448. [Google Scholar] [CrossRef]
- Sun, J.; Ma, Y.; Guo, C.; Du, Z.; Chen, L.; Wang, Z.; Li, X.; Xu, K.; Luo, Y.; Hong, Y.; et al. Distinct Patterns of Functional Brain Network Integration between Treatment-Resistant Depression and Non Treatment-Resistant Depression: A Resting-State Functional Magnetic Resonance Imaging Study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 120, 110621. [Google Scholar] [CrossRef]
- Spreen, A.; Alkhoury, D.; Walter, H.; Müller, S. Optogenetic Behavioral Studies in Depression Research: A Systematic Review. iScience 2024, 27, 109776. [Google Scholar] [CrossRef]
- Papp, M.; Gruca, P.; Litwa, E.; Lason, M.; Willner, P. Optogenetic Stimulation of Transmission from Prelimbic Cortex to Nucleus Accumbens Core Overcomes Resistance to Venlafaxine in an Animal Model of Treatment-Resistant Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 123, 110715. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.M.; Alexander, L.; Alsiö, J.; Santangelo, A.M.; McIver, L.; Cockcroft, G.J.; Roberts, A.C. Chemogenetics Identifies Separate Area 25 Brain Circuits Involved in Anhedonia and Anxiety in Marmosets. Sci. Transl. Med. 2023, 15, eade1779. [Google Scholar] [CrossRef] [PubMed]
- Mesbah, R.; Koenders, M.A.; van der Wee, N.J.A.; Giltay, E.J.; van Hemert, A.M.; de Leeuw, M. Association Between the Fronto-Limbic Network and Cognitive and Emotional Functioning in Individuals With Bipolar Disorder. JAMA Psychiatry 2023, 80, 432–440. [Google Scholar] [CrossRef]
- Lai, C.-H. Fronto-Limbic Neuroimaging Biomarkers for Diagnosis and Prediction of Treatment Responses in Major Depressive Disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 107, 110234. [Google Scholar] [CrossRef]
- Dam, S.; Batail, J.-M.; Robert, G.H.; Drapier, D.; Maurel, P.; Coloigner, J. Structural Brain Connectivity and Treatment Improvement in Mood Disorder. Brain Connect. 2024, 14, 239–251. [Google Scholar] [CrossRef]
- Becker, L.J.; Fillinger, C.; Waegaert, R.; Journée, S.H.; Hener, P.; Ayazgok, B.; Humo, M.; Karatas, M.; Thouaye, M.; Gaikwad, M.; et al. The Basolateral Amygdala-Anterior Cingulate Pathway Contributes to Depression-like Behaviors and Comorbidity with Chronic Pain Behaviors in Male Mice. Nat. Commun. 2023, 14, 2198. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, Z.H.; Bi, N.; Gu, X.; Huang, C.; Zhou, R.; Liu, H.; Wang, H.L. Dysfunction of the Medial Prefrontal Cortex Contributes to BPA-Induced Depression- and Anxiety-like Behavior in Mice. Ecotoxicol. Environ. Saf. 2023, 259, 115034. [Google Scholar] [CrossRef]
- Amemori, S.; Graybiel, A.M.; Amemori, K.I. Cingulate Microstimulation Induces Negative Decision-Making via Reduced Top-down Influence on Primate Fronto-Cingulo-Striatal Network. Nat. Commun. 2024, 15, 4201. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Li, J.; Hu, Y.; Zhang, M.; Wang, H. Optogenetics and Chemogenetics: Key Tools for Modulating Neural Circuits in Rodent Models of Depression. Front. Neural Circuits 2025, 19, 1516839. [Google Scholar] [CrossRef] [PubMed]
- Schimmelpfennig, J.; Topczewski, J.; Zajkowski, W.; Jankowiak-Siuda, K. The Role of the Salience Network in Cognitive and Affective Deficits. Front. Hum. Neurosci. 2023, 17, 1133367. [Google Scholar] [CrossRef]
- Pinto, A.M.; Geenen, R.; Wager, T.D.; Lumley, M.A.; Häuser, W.; Kosek, E.; Ablin, J.N.; Amris, K.; Branco, J.; Buskila, D.; et al. Emotion Regulation and the Salience Network: A Hypothetical Integrative Model of Fibromyalgia. Nat. Rev. Rheumatol. 2023, 19, 44–60. [Google Scholar] [CrossRef]
- Godfrey, K.E.M.; Muthukumaraswamy, S.D.; Stinear, C.M.; Hoeh, N. Decreased Salience Network FMRI Functional Connectivity Following a Course of RTMS for Treatment-Resistant Depression. J. Affect. Disord. 2022, 300, 235–242. [Google Scholar] [CrossRef]
- Idlett-Ali, S.L.; Salazar, C.A.; Bell, M.S.; Short, E.B.; Rowland, N.C. Neuromodulation for Treatment-Resistant Depression: Functional Network Targets Contributing to Antidepressive Outcomes. Front. Hum. Neurosci. 2023, 17, 1125074. [Google Scholar] [CrossRef]
- Zeisler, Z.R.; London, L.; Janssen, W.G.; Fredericks, J.M.; Elorette, C.; Fujimoto, A.; Zhan, H.; Russ, B.E.; Clem, R.L.; Hof, P.R.; et al. Single Basolateral Amygdala Neurons in Macaques Exhibit Distinct Connectional Motifs with Frontal Cortex. Neuron 2023, 111, 3307–3320.e5. [Google Scholar] [CrossRef]
- Rengasamy, M.; Brundin, L.; Griffo, A.; Panny, B.; Capan, C.; Forton, C.; Price, R.B. Cytokine and Reward Circuitry Relationships in Treatment-Resistant Depression. Biol. Psychiatry Glob. Open Sci. 2022, 2, 45–53. [Google Scholar] [CrossRef]
- Fenoy, A.J.; Quevedo, J.; Soares, J.C. Deep Brain Stimulation of the “Medial Forebrain Bundle”: A Strategy to Modulate the Reward System and Manage Treatment-Resistant Depression. Mol. Psychiatry 2022, 27, 574–592. [Google Scholar] [CrossRef]
- Pallikaras, V.; Shizgal, P. The Convergence Model of Brain Reward Circuitry: Implications for Relief of Treatment-Resistant Depression by Deep-Brain Stimulation of the Medial Forebrain Bundle. Front. Behav. Neurosci. 2022, 16, 851067. [Google Scholar] [CrossRef] [PubMed]
- Sobstyl, M.; Prokopienko, M.; Pietras, T. The Ventral Capsule and Ventral Striatum—Stereotactic Targets for the Management of Treatment-Resistant Depression. A Systematic Literature Review. Front. Psychiatry 2023, 14, 1100609. [Google Scholar] [CrossRef]
- Hur, K.-H.; Meisler, S.L.; Yassin, W.; Frederick, B.B.; Kohut, S.J. Prefrontal-Limbic Circuitry Is Associated With Reward Sensitivity in Nonhuman Primates. Biol. Psychiatry 2024, 96, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, J.; Kurokawa, S.; Iwamoto, C.; Miyaho, K.; Takamiya, A.; Ishii, C.; Hirayama, A.; Sanada, K.; Fukuda, S.; Mimura, M.; et al. Intestinal Metabolites Predict Treatment Resistance of Patients with Depression and Anxiety. Gut Pathog. 2024, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.-C.; Buffington, S.A.; Salas, R. Microbiota-Gut-Brain Axis in Psychiatry: Focus on Depressive Disorders. Curr. Epidemiol. Rep. 2024, 11, 222–232. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, H.; Ju, Y.; Liu, J.; Wang, M.; Liu, B.; Zhang, Y. Gut Microbiota-Derived Short-Chain Fatty Acids and Depression: Deep Insight into Biological Mechanisms and Potential Applications. Gen. Psychiatry 2024, 37, e101374. [Google Scholar] [CrossRef]
- Akram, N.; Faisal, Z.; Irfan, R.; Shah, Y.A.; Batool, S.A.; Zahid, T.; Zulfiqar, A.; Fatima, A.; Jahan, Q.; Tariq, H.; et al. Exploring the Serotonin-Probiotics-Gut Health Axis: A Review of Current Evidence and Potential Mechanisms. Food Sci. Nutr. 2024, 12, 694–706. [Google Scholar] [CrossRef]
- Kearns, R. Gut–Brain Axis and Neuroinflammation: The Role of Gut Permeability and the Kynurenine Pathway in Neurological Disorders. Cell. Mol. Neurobiol. 2024, 44, 64. [Google Scholar] [CrossRef]
- Wasiak, J.; Gawlik-Kotelnicka, O. Intestinal Permeability and Its Significance in Psychiatric Disorders—A Narrative Review and Future Perspectives. Behav. Brain Res. 2023, 448, 114459. [Google Scholar] [CrossRef]
- Madison, A.A.; Bailey, M.T. Stressed to the Core: Inflammation and Intestinal Permeability Link Stress-Related Gut Microbiota Shifts to Mental Health Outcomes. Biol. Psychiatry 2024, 95, 339–347. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, H.; Yuan, Y.; Guan, X.; Xie, Q.; Dong, Z. Baseline Gut Microbiota Profiles Affect Treatment Response in Patients with Depression. Front. Microbiol. 2024, 15, 1429116. [Google Scholar] [CrossRef] [PubMed]
- Siopi, E.; Galerne, M.; Rivagorda, M.; Saha, S.; Moigneu, C.; Moriceau, S.; Bigot, M.; Oury, F.; Lledo, P.M. Gut Microbiota Changes Require Vagus Nerve Integrity to Promote Depressive-like Behaviors in Mice. Mol. Psychiatry 2023, 28, 3002–3012. [Google Scholar] [CrossRef]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J. Inflamm. Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef] [PubMed]
- Lespérance, P.; Desbeaumes Jodoin, V.; Drouin, D.; Racicot, F.; Miron, J.P.; Longpré-Poirier, C.; Fournier-Gosselin, M.P.; Thebault, P.; Lapointe, R.; Arbour, N.; et al. Vagus Nerve Stimulation Modulates Inflammation in Treatment-Resistant Depression Patients: A Pilot Study. Int. J. Mol. Sci. 2024, 25, 2679. [Google Scholar] [CrossRef]
- Conway, C.R.; Aaronson, S.T.; Sackeim, H.A.; George, M.S.; Zajecka, J.; Bunker, M.T.; Duffy, W.; Stedman, M.; Riva-Posse, P.; Allen, R.M.; et al. Vagus Nerve Stimulation in Treatment-Resistant Depression: A One-Year, Randomized, Sham-Controlled Trial. Brain Stimul. 2025, 18, 676–689. [Google Scholar] [CrossRef]
- Fontana, A.; Manchia, M.; Panebianco, C.; Paribello, P.; Arzedi, C.; Cossu, E.; Garzilli, M.; Montis, M.A.; Mura, A.; Pisanu, C.; et al. Exploring the Role of Gut Microbiota in Major Depressive Disorder and in Treatment Resistance to Antidepressants. Biomedicines 2020, 8, 311. [Google Scholar] [CrossRef]
- Jiang, Y.; Qu, Y.; Shi, L.; Ou, M.; Du, Z.; Zhou, Z.; Zhou, H.; Zhu, H. The Role of Gut Microbiota and Metabolomic Pathways in Modulating the Efficacy of SSRIs for Major Depressive Disorder. Transl. Psychiatry 2024, 14, 493. [Google Scholar] [CrossRef] [PubMed]
- Ammer-Herrmenau, C.; Hamm, J.; Neesse, A.; Günther, K.; Besse, M.; Zilles-Wegner, D. Response to Electroconvulsive Therapy Is Associated with a More Diverse Oral Microbiome—A Prospective Longitudinal Cohort Pilot Study. Eur. Arch. Psychiatry Clin. Neurosci. 2025, 275, 1851–1858. [Google Scholar] [CrossRef]
- Zhang, W.; Qu, W.; Wang, H.; Yan, H. Antidepressants Fluoxetine and Amitriptyline Induce Alterations in Intestinal Microbiota and Gut Microbiome Function in Rats Exposed to Chronic Unpredictable Mild Stress. Transl. Psychiatry 2021, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Borgiani, G.; Possidente, C.; Fabbri, C.; Oliva, V.; Bloemendaal, M.; Arias Vasquez, A.; Dinan, T.G.; Vieta, E.; Menchetti, M.; De Ronchi, D.; et al. The Bidirectional Interaction between Antidepressants and the Gut Microbiota: Are There Implications for Treatment Response? Int. Clin. Psychopharmacol. 2025, 40, 3–26. [Google Scholar] [CrossRef]
- Wang, H.Y.; Liu, L.X.; Chen, X.Y.; Zhang, Y.D.; Li, W.X.; Li, W.W.; Wang, L.; Mo, X.L.; Wei, H.; Ji, P.; et al. Comprehensive Analysis of the Gut Microbiome and Post-Translational Modifications Elucidates the Route Involved in Microbiota-Host Interactions. Zool. Res. 2024, 45, 95–107. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–Gut–Brain Axis and Its Therapeutic Applications in Neurodegenerative Diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Magzal, F.; Turroni, S.; Fabbrini, M.; Barone, M.; Vitman Schorr, A.; Ofran, A.; Tamir, S. A Personalized Diet Intervention Improves Depression Symptoms and Changes Microbiota and Metabolite Profiles among Community-Dwelling Older Adults. Front. Nutr. 2023, 10, 1234549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bi, Y.; Zhang, B.; Jiang, Q.; Mou, C.K.; Lei, L.; Deng, Y.; Li, Y.; Yu, J.; Liu, W.; et al. Current Landscape of Fecal Microbiota Transplantation in Treating Depression. Front. Immunol. 2024, 15, 1416961. [Google Scholar] [CrossRef]
- Schaub, A.C.; Schneider, E.; Vazquez-Castellanos, J.F.; Schweinfurth, N.; Kettelhack, C.; Doll, J.P.K.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Clinical, Gut Microbial and Neural Effects of a Probiotic Add-on Therapy in Depressed Patients: A Randomized Controlled Trial. Transl. Psychiatry 2022, 12, 227. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Patergnani, S.; Bouhamida, E.; Leo, S.; Pinton, P.; Rimessi, A. Mitochondrial Oxidative Stress and “Mito-Inflammation”: Actors in the Diseases. Biomedicines 2021, 9, 216. [Google Scholar] [CrossRef]
- Riveros, M.E.; Ávila, A.; Schruers, K.; Ezquer, F. Antioxidant Biomolecules and Their Potential for the Treatment of Difficult-to-Treat Depression and Conventional Treatment-Resistant Depression. Antioxidants 2022, 11, 540. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef]
- Bhatt, S.; Pandey, D.K.; Patil, C.R.; Nagappa, A.N. Oxidative Stress in Depression and Other Comorbid Disorders. In Role of Oxidative Stress in Pathophysiology of Diseases; Springer: Singapore, 2020; pp. 149–162. [Google Scholar]
- Buoli, M.; Capuzzi, E.; Caldiroli, A.; Ceresa, A.; Esposito, C.M.; Posio, C.; Auxilia, A.M.; Capellazzi, M.; Tagliabue, I.; Surace, T.; et al. Clinical and Biological Factors Are Associated with Treatment-Resistant Depression. Behav. Sci. 2022, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Ait Tayeb, A.E.K.; Poinsignon, V.; Chappell, K.; Bouligand, J.; Becquemont, L.; Verstuyft, C. Major Depressive Disorder and Oxidative Stress: A Review of Peripheral and Genetic Biomarkers According to Clinical Characteristics and Disease Stages. Antioxidants 2023, 12, 942. [Google Scholar] [CrossRef]
- Lievanos-Ruiz, F.J.; Fenton-Navarro, B. Enzymatic Biomarkers of Oxidative Stress in Patients with Depressive Disorders. A Systematic Review. Clin. Biochem. 2024, 130, 110788. [Google Scholar] [CrossRef]
- Bradlow, R.C.J.; Berk, M.; Kalivas, P.W.; Back, S.E.; Kanaan, R.A. The Potential of N-Acetyl-L-Cysteine (NAC) in the Treatment of Psychiatric Disorders. CNS Drugs 2022, 36, 451–482. [Google Scholar] [CrossRef]
- Peng, T.-R.; Lin, H.-H.; Tseng, T.-L.; Huang, Y.-H.; Tsai, P.-Y.; Lin, C.-Y.; Lee, M.-C.; Chen, S.-M. Efficacy of N-Acetylcysteine for Patients with Depression: An Updated Systematic Review and Meta-Analysis. Gen. Hosp. Psychiatry 2024, 91, 151–159. [Google Scholar] [CrossRef]
- Larrea, A.; Sánchez-Sánchez, L.; Diez-Martin, E.; Elexpe, A.; Torrecilla, M.; Astigarraga, E.; Barreda-Gómez, G. Mitochondrial Metabolism in Major Depressive Disorder: From Early Diagnosis to Emerging Treatment Options. J. Clin. Med. 2024, 13, 1727. [Google Scholar] [CrossRef]
- Kolasa, M.; Faron-Górecka, A. Preclinical Models of Treatment-Resistant Depression: Challenges and Perspectives. Pharmacol. Rep. 2023, 75, 1326–1340. [Google Scholar] [CrossRef] [PubMed]
- Rocks, D.; Liston, C. Synaptic Plasticity in Fronto-Insular Circuits Underlying Stress Susceptibility and Resilience. Neurosci. Res. 2025, 211, 24–36. [Google Scholar] [CrossRef]
- Tang, H.; Xia, Y.; Gao, C.; Cai, Y.; Shao, Y.; Chen, W.; Yuan, Y.; Liu, C.; Zhang, Z.; Xu, Z. Association of Psychosocial Factors and Biological Pathways Identified from Rare-Variant Analysis with Longitudinal Trajectories of Treatment Response in Major Depressive Disorder. BMC Psychiatry 2025, 25, 505. [Google Scholar] [CrossRef]
- Giampetruzzi, E.; Tan, A.C.; LoPilato, A.; Kitay, B.; Riva Posse, P.; McDonald, W.M.; Hermida, A.P.; Crowell, A.; Hershenberg, R. The Impact of Adverse Childhood Experiences on Adult Depression Severity and Treatment Outcomes. J. Affect. Disord. 2023, 333, 233–239. [Google Scholar] [CrossRef]
- Yrondi, A.; Vaiva, G.; Walter, M.; D Amato, T.; Bellivier, F.; Bennabi, D.; Bougerol, T.; Camus, V.; Doumy, O.; Genty, J.B.; et al. Childhood Trauma Increases Suicidal Behaviour in a Treatment-Resistant Depression Population: A FACE-DR Report. J. Psychiatr. Res. 2021, 135, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, G.; Cavallo, C.; Palmieri, S.; Ruggiero, G.M.; Sassaroli, S.; Caselli, G. Adverse Childhood Experiences and Repetitive Negative Thinking in Adulthood: A Systematic Review. Clin. Psychol. Psychother. 2021, 28, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Sokołowski, A.; Kowalski, J.; Dragan, M. Neural Functional Connectivity during Rumination in Individuals with Adverse Childhood Experiences. Eur. J. Psychotraumatol. 2022, 13, 2057700. [Google Scholar] [CrossRef]
- King, A.R. Childhood Adversity Links to Self-Reported Mood, Anxiety, and Stress-Related Disorders. J. Affect. Disord. 2021, 292, 623–632. [Google Scholar] [CrossRef]
- Stein, C.R.; Sheridan, M.A.; Copeland, W.E.; Machlin, L.S.; Carpenter, K.L.H.; Egger, H.L. Association of Adversity with Psychopathology in Early Childhood: Dimensional and Cumulative Approaches. Depress. Anxiety 2022, 39, 524–535. [Google Scholar] [CrossRef]
- Fung, H.W.; Chien, W.T.; Ling, H.W.-H.; Ross, C.A.; Lam, S.K.K. The Mediating Role of Post-Traumatic Stress Disorder Symptoms in the Relationship between Childhood Adversities and Depressive Symptoms in Two Samples. Child Abus. Negl. 2022, 131, 105707. [Google Scholar] [CrossRef]
- Kleindienst, N.; Vonderlin, R.; Bohus, M.; Lis, S. Childhood Adversity and Borderline Personality Disorder. Analyses Complementing the Meta-Analysis by Porter et al. (2020). Acta Psychiatr. Scand. 2021, 143, 183–184. [Google Scholar] [CrossRef]
- Porter, C.; Palmier-Claus, J.; Branitsky, A.; Mansell, W.; Warwick, H.; Varese, F. Childhood Adversity and Borderline Personality Disorder: A Meta-Analysis. Acta Psychiatr. Scand. 2020, 141, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Lui, C.K.; Witbrodt, J.; Li, L.; Tam, C.C.; Williams, E.; Guo, Z.; Mulia, N. Associations between Early Childhood Adversity and Behavioral, Substance Use, and Academic Outcomes in Childhood through Adolescence in a U.S. Longitudinal Cohort. Drug Alcohol. Depend. 2023, 244, 109795. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Kong, X.; Han, F.; Tian, H.; Sun, S.; Sun, Y.; Feng, W.; Wu, Y. Association between Social Isolation and Depression: Evidence from Longitudinal and Mendelian Randomization Analyses. J. Affect. Disord. 2024, 350, 182–187. [Google Scholar] [CrossRef]
- Yoshii, T.; Oishi, N.; Sotozono, Y.; Watanabe, A.; Sakai, Y.; Yamada, S.; Matsuda, K.I.; Kido, M.; Ikoma, K.; Tanaka, M.; et al. Validation of Wistar-Kyoto Rats Kept in Solitary Housing as an Animal Model for Depression Using Voxel-Based Morphometry. Sci. Rep. 2024, 14, 3601. [Google Scholar] [CrossRef]
- Rodrigues, M.F.; Quagliato, L.; Appolinario, J.C.; Nardi, A.E. Online Mindfulness-Based Cognitive Therapy for Treatment-Resistant Depression: A Parallel-Arm Randomized Controlled Feasibility Trial. Front. Psychol. 2024, 15, 1412483. [Google Scholar] [CrossRef] [PubMed]
- Stefanidou, T.; Ambler, G.; Bartl, G.; Barber, N.; Billings, J.; Bogatsu, T.; Carroll, R.; Chipp, B.; Conneely, M.; Downey, A.M.; et al. Randomised Controlled Trial of the Community Navigator Programme to Reduce Loneliness and Depression for Adults with Treatment-Resistant Depression in Secondary Community Mental Health Services: Trial Protocol. Trials 2023, 24, 652. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lievanos-Ruiz, F.J.; Fenton-Navarro, B. Exploring Biological Risk Factors in Treatment-Resistant Depression. Psychiatry Int. 2025, 6, 134. https://doi.org/10.3390/psychiatryint6040134
Lievanos-Ruiz FJ, Fenton-Navarro B. Exploring Biological Risk Factors in Treatment-Resistant Depression. Psychiatry International. 2025; 6(4):134. https://doi.org/10.3390/psychiatryint6040134
Chicago/Turabian StyleLievanos-Ruiz, Francisco Javier, and Bertha Fenton-Navarro. 2025. "Exploring Biological Risk Factors in Treatment-Resistant Depression" Psychiatry International 6, no. 4: 134. https://doi.org/10.3390/psychiatryint6040134
APA StyleLievanos-Ruiz, F. J., & Fenton-Navarro, B. (2025). Exploring Biological Risk Factors in Treatment-Resistant Depression. Psychiatry International, 6(4), 134. https://doi.org/10.3390/psychiatryint6040134





