Abstract
Background: Youth and young adults with early psychosis frequently use cannabis, yet the reliability of self-reported use is uncertain in clinical practice. We examined the concordance between self-reported cannabis use and urine toxicology among patients enrolled in an Early Psychosis Intervention (EPI) program in Southeast Ontario, Canada. Methods: We conducted a cross-sectional chart review of 116 EPI patients (2016–2019). Demographics, self-reported cannabis use (yes/no), concurrent substance use, and urine toxicology results from the initial clinical assessment were extracted. Diagnostic indices (sensitivity, specificity, positive/negative predictive values, and accuracy) were calculated using urine toxicology as the reference. The clinical panel used a 50 ng/mL threshold for THC-COOH; the specific assay platform (immunoassay vs. confirmatory GC-/LC-MS) was not specified in records and is noted as a limitation. Results: Overall, 82.8% (96/116) self-reported cannabis use. Self-report showed high sensitivity (88.4%) but very low specificity (20.3%), with PPV 39.2%, NPV 75.0%, and accuracy 45.30%, indicating limited concordance with urine toxicology. Self-reported cannabis use was significantly associated with self-reported cocaine and MDMA use, while associations with methamphetamine, opioids, and benzodiazepines were not significant. Conclusions: In this EPI cohort, self-reports overestimated cannabis use relative to urine toxicology (high sensitivity, low specificity, and accuracy <50%). These findings support cautious clinical interpretation of self-report and the complementary value of biological verification, especially when use is infrequent or the testing window/threshold may miss exposure. Future work should incorporate use frequency, potency, and timing relative to testing, and clearly specify toxicology assay methods.
Keywords:
cannabis; THC; early psychosis; schizophrenia; youth; urine toxicology; self-report; diagnostic accuracy 1. Introduction
Cannabis is the most widely used drug in the world, with 4% of the population taking it during the last year [1]. Cannabis use is common among psychotic patients, with 36% of first-episode psychosis and 21% of established schizophrenia fitting cannabis use disorder criteria [2]. Its use can result in severe symptoms, higher relapse rates, longer hospital admissions, and a worse quality of life. The effects are dose-dependent, with poorer results for frequent users and high-potency strains [3]. The research discovered that cannabis causes nearly half of the damage in schizophrenic patients [4]. According to a National Institutes of Health (NIH)-funded study called Monitoring the Future, cannabis is the most often overused and abused drug among teenagers [5]. Early identification of high-risk people is critical for treating comorbid drug use disorders. Individuals are classified as cannabis users, misusers, or abusers/dependents, which helps physicians establish appropriate care levels and influences the degree of drug use disorder [6].
Between 2000 and 2016, the incidence of “cannabis-induced psychosis” increased by 67% in Norway, 115% in Denmark, and 238% in Sweden [7]. In Canada, the number of patients with “cannabis-induced psychoses” doubled between 2015 and 2019 [8]. This issue is significant as many develop psychotic disorders. In the US, the proportion of people with a psychotic disorder and daily cannabis use increased from 3% in 2001 to 8% in 2012 [9]. Canadian teens have one of the highest rates of cannabis usage, with up to 40–50% reporting use in the previous year [8]. In a recent survey, the provincial and territorial usage rates ranged from 18% to 41% in Canada. As in previous years, cannabis use was highest among 20–24-year-olds (50%), followed by 16–19-year-olds (37%), and those 25 and older (25%). Males reported higher cannabis use (30%) than females (25%) in 2021. Notably, cannabis use increased among females between 2021 and 2022 but remained unchanged among males [10]. Cannabis was once considered an illicit drug in Canada, subject to rigorous laws and criminal penalties. However, views regarding drug policy and evidence of therapeutic advantages have shifted. In October 2018, Canada formally legalised recreational cannabis use among adults, citing public health reasons, therefore limiting the supply of illicit cannabis and regulating distribution. This shift in public drug policy has created opportunities for clinical research and spurred public debate about its implications, notably for mental health outcomes [11].
Cannabis’ psychotropic properties are mainly due to delta-9-tetrahydrocannabinol (THC). It can accumulate in lipophilic compartments, producing metabolites that remain even when the drug is no longer used [12]. THC is slowly released into the bloodstream, where it is metabolised to carboxy-THC (THC-COOH) and eliminated in the urine [13]. It may take several weeks to eradicate cannabis metabolites, subject to the frequency, potency, and form of cannabis product (e.g., smoked or ingested) preparation consumed. Chronic users require up to 28 days to eliminate cannabinoids. Urinalysis is the most commonly used technique for detecting psychoactive drugs, with urine immunoassays detecting THC-COOH at quantities of roughly 50 ng/mL [14]. However, because THC-COOH has a lengthy excretion half-life, determining the timing of the last drug exposure is challenging; therefore, findings from a single urine test may not demonstrate maintained abstinence in the early post-abstinence interval. The initial results from a study with a history of substance use disorders indicate that the sensitivity of self-reported cannabis use compared with urine toxicology testing is low (58%), although somewhat higher than other types of drugs [15].
Cannabis use data is primarily based on self-report, which has limitations such as recall bias, lack of object verification, and confounding with other substance use. This approach may lead to denial or underreporting of cannabis use, especially in substance-using adolescents who fear personal consequences or legal sanctions [16]. Researchers have attempted to establish reliability between self-reporting and urine toxicology, with one study reporting an agreement of 75.4%. Other studies have highlighted the importance of measures like urine toxicology for Delta-9-Tetrahydrocannabinol (THC) and self-reported frequency of use as stronger predictors for cannabis dependency and acute psychotic-like effects. Self-report may confirm cannabis consumption, but it is a less reliable proxy for THC presence than toxicology [17].
Cannabis use among Canadian youth and young adults is a significant concern, prompting the inclusion of Early Psychosis Intervention (EPI) services [18]. These programs aim to provide timely support to those experiencing their first episode of psychosis, aiming to improve outcomes and minimise the long-term impact of the condition. Targeting at-risk individuals allows EPI services to intervene early and reduce the progression of mental health disorders, including those related to substance use, like cannabis-induced psychosis. Reducing cannabis use is crucial for EPI services to be effective. The study aimed to assess the concordance or reliability of S-R cannabis use and objective urine toxicology testing. It also examined sociodemographic differences, potential under-detection in toxicology tests, and whether under-detection varies by self-reported cannabis use among 14–35-year-olds attending an EPI program in Southeast Ontario.
2. Methods
2.1. Study Setting and Ethical Statement
This study conducted a cross-sectional chart review on a cohort of 116 individuals participating in the Southeast Ontario Early Psychosis Intervention (EPI) program in Kingston, Ontario, Canada, from 2016 to 2019. All data were obtained from existing medical records of the Southeast Ontario Early Psychosis Intervention (EPI) Program at Hotel Dieu Hospital, Kingston, Ontario, Canada. Ethical approval was obtained from the Queen’s University Health Sciences & Affiliated Teaching Hospitals Research Ethics Board (HSREB# 6025629).
2.2. Participants
The EPI program supports individuals aged 14 to 35 years who are experiencing their first episode of psychosis or have not previously received treatment for a psychotic illness within the region.
2.3. Procedure and Data Collection
Data collection involved extracting demographic information, such as age, gender, and educational status, from contemporary clinical records. Additionally, comprehensive data on self-reported cannabis use and concurrent use of other drugs were gathered during the initial assessment. Urine toxicology was also carried out as part of the initial screening panel to support diagnosis and clinical management. Urine toxicology screening was performed as part of the first evaluation panel. Clinical documentation indicated a detection threshold of 50 ng/mL for THC-COOH; however, the specific assay platform (e.g., immunoassay vs. confirmatory GC-MS/LC-MS) was not specified in available records, which represents a limitation of this study.
2.4. Statistical Analyses
Statistical analyses were performed using SPSS version 25. Descriptive statistics were calculated as frequencies and percentages for categorical variables. The Chi-square test and Fisher’s exact test evaluated the association between self-reported cannabis use and other substance abuse. Diagnostic accuracy, sensitivity, specificity, positive predictive value and negative predictive value were also calculated for self-reported Cannabis use by taking the Urine toxicology report as the gold standard. A p-value < 0.05 was considered significant.
3. Results
3.1. Participant Characteristics According to Self-Reported Cannabis Use
A total of 116 participants were included in the study, 96 of whom self-reported cannabis use, while 20 reported non-use. Of the self-reported cannabis users, 69 (71.9%) were male and 27 (28.1%) were female. “Cannabis use was significantly more prevalent among men than women. Among cannabis users, 71.9% were male, while only 28.1% were female. In contrast, the proportion of non-users was higher among women (40.0%) compared to men (60.0%). Regarding age, the majority of participants, 56 (58.3%), were between 18 and 25 years old. This indicates that young adults are the age group most likely to use cannabis. In contrast, a significant proportion of non-users are under the age of 18 (30.0%), which is significantly higher than the 15.6% of cannabis users in this age group. (Table 1) The table provides information on the simultaneous use of various substances among cannabis users and non-users.

Table 1.
Demographics of Participants According to Self-Reported Cannabis Use.
3.2. Use and Concurrent Use of Alcohol and Other Substances
Among S-R cannabis users, 37.5% also reported using cocaine, while this was not the case among non-users, resulting in a significant association with a p-value of 0.000. In contrast, the prevalence of methamphetamine use was 24.0% among cannabis users and 0.0% among non-users (p-value > 0.05). MDMA use was reported by 19.8% of cannabis users, while none of the non-users reported any use. Cannabis use was significantly associated with MDMA use (p = 0.041). Conversely, the use of opioids was reported by 13.5% of cannabis users, while no use was found among non-users (p-value > 0.05). 12.5% of cannabis users reported using benzodiazepines, while no use was found among non-users (p-value > 0.05). In summary, a significant association was found between cannabis use and the use of cocaine and MDMA However, no significant associations were observed between cannabis use and methamphetamine, opioids, or benzodiazepines. (Table 2)

Table 2.
Comparison of Self-Reported Cannabis Use in Contrast to Other Substance Use.
3.3. Concordance Between Self-Reported Substance Use and Urine Toxicology
3.3.1. Cannabis
Self-reported cannabis use demonstrated high sensitivity (88.4%), indicating that most individuals who tested positive on urine toxicology also reported use. However, specificity was extremely low (20.3%), resulting in a high proportion of false positives. The overall accuracy was only 45.3%, underscoring that self-report substantially overestimated cannabis use compared with toxicology verification (Table 3, Figure 1).

Table 3.
Concordance of Self-Reported Substance Abuse with Urine Toxicology Report.
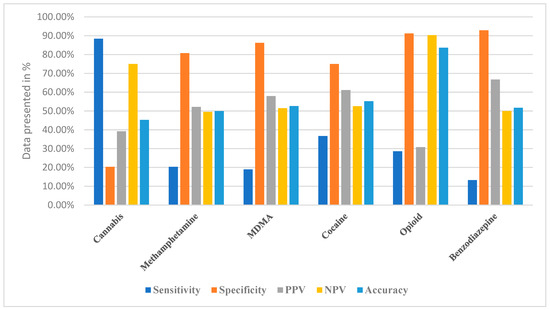
Figure 1.
Comparison of Diagnostic Accuracy of Self-Reported Substance Abuse and Urine Toxicology Report.
3.3.2. Methamphetamine
For methamphetamine, self-reporting showed very poor sensitivity (20.3%) but relatively high specificity (80.7%). This pattern indicates that many true users were not captured by self-report, although non-users were correctly excluded. The overall accuracy of 50.0% suggests that self-reporting was essentially no more reliable than chance in identifying methamphetamine use (Table 3, Figure 1).
3.3.3. MDMA
3.3.4. Cocaine
In contrast, self-reported cocaine use demonstrated moderate sensitivity (36.7%) and specificity (75.0%), producing an overall accuracy of 55.2%. Although these values remain suboptimal, they suggest that self-reports for cocaine were somewhat more reliable compared with other substances, reflecting a better—though still incomplete—alignment with urine toxicology (Table 3, Figure 1).
3.3.5. Opioids
Opioid self-reporting presented with low sensitivity (28.6%) but very high specificity (91.2%). This produced a relatively high accuracy (83.6%), suggesting that while self-report was not effective in identifying true opioid users, it was highly effective at confirming non-use. Such asymmetry underscores the selective utility of self-reporting for certain substances (Table 3, Figure 1).
3.3.6. Benzodiazepines
For benzodiazepines, self-reports had extremely low sensitivity (13.3%) but very high specificity (92.9%), resulting in an overall accuracy of 51.7%. This again illustrates that self-reporting was poor at identifying actual users but reliable in ruling out non-use (Table 3, Figure 1).
Overall, the data in Table 3 and the comparative profiles illustrated in Figure 1 reveal substantial variability in the diagnostic performance of self-reports across different substances. While cannabis self-report achieved the highest sensitivity, its specificity was unacceptably low. Conversely, opioids and benzodiazepines exhibited the opposite trend, with poor sensitivity but strong specificity, highlighting the selective strengths and weaknesses of self-report depending on the substance.
4. Discussion
The present study examined the concordance between self-reported cannabis use and urine toxicology in a Canadian Early Psychosis Intervention (EPI) cohort. Findings showed high sensitivity (88.4%) but very low specificity (20.3%), resulting in limited overall accuracy (45.3%). These results suggest that while self-report effectively identifies most users, it substantially overestimates use compared to toxicology verification.
These findings can be better understood within disclosure theory and social desirability frameworks, which propose that stigma, anticipated consequences, and social context influence individuals’ reporting of sensitive behaviours. In early psychosis populations, factors such as paranoia, insight level, or diagnostic subtype (e.g., schizophrenia vs. substance-induced psychosis) may further shape reporting accuracy. In Canada, recreational cannabis was legalised in October 2018; in this study, we cite legalisation only as a contextual background that may shape disclosure norms. We did not perform any pre- versus post-legalisation analyses, and no causal or temporal inferences are drawn from legalisation status in our data [19].
A notable finding was the exceptionally low specificity (20.3%), indicating a high rate of false positive instances where participants reported cannabis use but urine toxicology was negative. Several explanations are possible. First, there may be a mismatch in timeframes: self-report often refers to use in the past 30 days, whereas urine toxicology typically detects THC metabolites only within days to weeks, depending on frequency and potency. Second, the detection threshold used in this study (50 ng/mL) may have missed infrequent or low-dose use that participants nonetheless disclosed. Third, individual variation in cannabinoid metabolism and elimination could contribute [20].
In the present study, the peak age of cannabis use was observed between 18 and 25 years, during early age, which is a critical period of brain maturation. Other studies show that the median age of first use is around 14 years [21].
A significant correlation was found between self-reported cannabis use and positive urine toxicology results for THC, confirming the validity of self-reporting in this Canadian population. A study by Salottolo et al. conducted among trauma patients also showed a high correlation between self-reporting and positive toxicology screening [22]. However, this was not the case in another study that surveyed drivers -in this instance, self-reporting had low sensitivity but high specificity [23]. A scoping review of studies conducted among women of reproductive age showed that self-reporting was only reliable among participants with a prior known history of cannabis use [24]. With both driving and pregnancy, the illegal and socially undesirable nature of each may lead to underreporting. The urine toxicology measures help mitigate self-reported biases, such as underreporting due to legal implications or social stigma [25]. An additional benefit was the application of monitoring to the therapeutic program. These findings highlight the importance of incorporating biological measures in both clinical and research studies to validate self-reported substance use, particularly in naïve or newly initiated users.
The association between cannabis use and the use of other substances was a significant finding in this study. A substantial proportion of cannabis users reported concurrent alcohol use (56.3%), and statistically significant associations were observed between cannabis use and the use of cocaine, methamphetamine, and MDMA. Of concern, this concurrent use amplified the risk of harmful and high-risk behaviour. The significant associations between cannabis use and both cocaine and MDMA use underscore the complex substance profiles in this vulnerable population, consistent with prior evidence that polysubstance use amplifies clinical risks [26]. The co-occurrence of cannabis with other substances raises concerns regarding potential cumulative effects on mental health and overall well-being [27]. In another study, it was reported that the combined use of alcohol and cannabis is linked to compounded performance impairments, higher and more frequent consumption, increased social and behavioural consequences like impaired driving, and a greater risk of developing comorbid substance use and mental health disorders [28].
While overall numbers were small, the results also indicated a decline in the number of individuals with positive urine toxicology results in subsequent tests. This important finding is a positive indicator of the importance of psychoeducation, harm reduction and specific therapeutic interventions regarding cannabis use with patients. This suggests a decrease in cannabis use, including total abstinence with EPI intervention over time. This finding aligns with the concept of intervention effectiveness within EPI programs, which focus on addressing substance use among individuals experiencing their first episode of psychosis [27]. Such reductions in substance use are critical for improving treatment outcomes and minimising the harmful risks associated with cannabis use, including the conversion to schizophrenia [29].
In this study, a significant association between cannabis use and psychosis and SIP diagnoses was evident, signifying the potential role of cannabis in the development or exacerbation of these psychiatric conditions. Most mental illnesses may also be associated with higher reward-seeking states, prompting affected individuals to use cannabis and other substances more frequently and more heavily as a form of self-medication [30].
The current study’s findings underscore the importance of screening, identification and cannabis-related therapeutic interventions with a harm reduction or less harmful users, particularly in youth and young adults already at heightened risk attending an EPI program [31]. The co-occurrence of cannabis with other substances and its potential impact on mental health outcomes necessitate comprehensive and integrated approaches to address both substance use and mental health simultaneously.
Longitudinal studies are required to better understand the temporal relationships between cannabis use and psychosis, as well as the naturalistic history of therapeutic interventions on the course and severity of illness. Moreover, the study with a relatively small sample size was confined to a specific geographical region, limiting the generalizability of the findings to other populations. The issue of screening youths for substance use disorders is still debated in adult and child psychiatry, and the trend supporting self-report as a reliable measure in substance misuse assessment is further validated in this study. Future research should involve more diverse and representative samples to strengthen the external validity of the results. Further research is required to understand the underlying causes of this association and develop effective interventions to reduce harmful cannabis use among young adults with psychosis. By addressing cannabis use during the early stages of psychosis, not only can we improve treatment outcomes and illness trajectories of psychotic episodes, but also the overall quality of life.
The study has several limitations that should be considered. First, this was a single-site cross-sectional chart review, limiting generalizability and precluding longitudinal analysis of reporting accuracy over time. Second, cannabis use was recorded as a binary variable without details on frequency, potency, or method of consumption, restricting interpretation. Third, the toxicology assay platform was not specified in the available records, leaving uncertainty about whether only immunoassay screening was used or whether confirmatory GC-/LC-MS was conducted. Fourth, the 50 ng/mL detection threshold may have missed infrequent use, contributing to low specificity. Finally, reliance on retrospective chart data introduces potential inconsistencies in documentation. Although the cohort spans 2016–2019 (i.e., both before and after legalization), we did not stratify or compare outcomes by legalization period; the cross-sectional design and sample size do not support temporal analyses. These limitations highlight the need for prospective studies with a standardised assessment of cannabis use.
Despite these limitations, this study makes a valuable contribution by evaluating real-world concordance of self-report and toxicology in a Canadian EPI population, where cannabis use is highly prevalent. The results caution against over-reliance on self-report alone in clinical settings, emphasising the complementary role of toxicology screening. Importantly, even ‘negative’ findings such as poor specificity have clinical relevance, as they highlight the complexities of monitoring cannabis use in psychosis and underscore the need for more nuanced, multimodal assessment strategies.
5. Conclusions
This study demonstrated that self-reported cannabis use in a Canadian Early Psychosis Intervention population showed high sensitivity (88.4%) but very low specificity (20.3%), resulting in an overall accuracy below 50%. These findings indicate that while self-report captures most users, it substantially overestimates cannabis use compared to urine toxicology. Clinicians should interpret self-reported cannabis use with caution and consider biological verification, particularly when use is infrequent or the detection threshold may miss exposure. Future research should incorporate detailed measures of frequency, potency, and timing of use, alongside standardised toxicology methods, to more accurately assess substance use in youth and young adults with psychosis.
Author Contributions
Conceptualization, N.A.A. and O.A.; methodology, N.A.A.; software, N.A.A.; validation, N.A.A. and O.A.; formal analysis, N.A.A.; investigation, N.A.A.; resources, O.A.; data curation, N.A.A.; writing—original draft preparation, N.A.A.; writing—review and editing, N.A.A.; visualization, N.A.A.; supervision, O.A.; project administration, N.A.A.; funding acquisition, O.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of The Queen’s University Health Sciences & Affiliated Teaching Hospitals (protocol code HSREB # 6025629 and 14 January 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data supporting this study’s findings are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Conflicts of Interest
All authors declare no conflicts of interest.
References
- Charitos, I.A.; Gagliano-Candela, R.; Santacroce, L.; Bottalico, L. Cannabis spread throughout the continents and has been used therapeutically throughout history. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 407–417. [Google Scholar]
- Sánchez-Gutiérrez, T.; Fernandez-Castilla, B.; Barbeito, S.; González-Pinto, A.; Becerra-García, J.A.; Calvo, A. Cannabis use and nonuse in patients with first-episode psychosis: A systematic review and meta-analysis of studies comparing neurocognitive functioning. Eur. Psychiatry 2020, 63, e6. [Google Scholar] [CrossRef] [PubMed]
- Hasin, D.; Walsh, C. Cannabis use, cannabis use disorder, and comorbid psychiatric illness: A narrative review. J. Clin. Med. 2020, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Hjorthøj, C.; Posselt, C.M.; Nordentoft, M. Development over time of the population-attributable risk fraction for cannabis use disorder in schizophrenia in Denmark. JAMA Psychiatry 2021, 78, 1013–1019. [Google Scholar] [CrossRef]
- World Health Organization. The Health and Social Effects of Nonmedical Cannabis Use; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Bierhoff, J.; Haardörfer, R.; Windle, M.; Berg, C.J. Psychological risk factors for alcohol, cannabis, and various tobacco use among young adults: A longitudinal analysis. Subst. Use Misuse 2019, 54, 1365–1375. [Google Scholar] [CrossRef]
- Rognli, E.B.; Taipale, H.; Hjorthøj, C.; Mittendorfer-Rutz, E.; Bramness, J.G.; Heiberg, I.H.; Niemelä, S. Annual incidence of substance-induced psychoses in Scandinavia from 2000 to 2016. Psychol. Med. 2023, 53, 5246–5255. [Google Scholar] [CrossRef]
- Callaghan, R.C.; Sanches, M.; Murray, R.M.; Konefal, S.; Maloney-Hall, B.; Kish, S.J. Associations between Canada’s cannabis legalization and emergency department presentations for transient cannabis-induced psychosis and schizophrenia conditions: Ontario and Alberta, 2015–2019. Can. J. Psychiatry 2022, 67, 616–625. [Google Scholar] [CrossRef]
- Livne, O.; Shmulewitz, D.; Sarvet, A.L.; Wall, M.M.; Hasin, D.S. Association of cannabis use–related predictor variables and self-reported psychotic disorders: US adults, 2001–2002 and 2012–2013. Am. J. Psychiatry 2022, 179, 36–45. [Google Scholar] [CrossRef]
- Bahji, A.; Kaur, S.B.; Devoe, D.; Patten, S.M. Trends in Canadian cannabis consumption over time: A two-step meta-analysis of Canadian household survey data. Can. J. Addict. 2022, 13, 6–13. [Google Scholar] [CrossRef]
- Hall, W.; Stjepanović, D.; Dawson, D.; Leung, J. The implementation and public health impacts of cannabis legalization in Canada: A systematic review. Addiction 2023, 118, 2062–2072. [Google Scholar] [CrossRef]
- Grotenhermen, F. Clinical pharmacokinetics of cannabinoids. J. Cannabis Ther. 2003, 3, 3–51. [Google Scholar] [CrossRef]
- Dietz, L.; Glaz-Sandberg, A.; Nguyen, H.; Skopp, G.; Mikus, G.; Aderjan, R. The urinary disposition of intravenously administered 11-nor-9-carboxy-delta-9-tetrahydrocannabinol in humans. Ther. Drug Monit. 2007, 29, 368–372. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Salem, M. Cannabinoids analysis: Analytical methods for different biological specimens. In Handbook of Analytical Separations; Elsevier: Amsterdam, The Netherlands, 2000; Volume 2, pp. 163–193. [Google Scholar]
- Khalili, P.; Nadimi, A.E.; Baradaran, H.R.; Janani, L.; Rahimi-Movaghar, A.; Rajabi, Z.; Rahmani, A.; Hojati, Z.; Khalagi, K.; Motevalian, S.A. Validity of self-reported substance use: Research setting versus primary health care setting. Subst. Abus. Treat. Prev. Policy 2021, 16, 66. [Google Scholar] [CrossRef]
- Jackson, C.T.; Covell, N.H.; Frisman, L.K.; Essock, S.M. Validity of self-reported drug use among people with co-occurring mental health and substance use disorders. J. Dual Diagn. 2005, 1, 49–63. [Google Scholar] [CrossRef]
- Ruglass, L.; Shevorykin, A.; Zhao, Y.; Killeen, T.; Bauer, A.; Morgan-López, A.; Back, S.; Fitzpatrick, S.; López-Castro, T.; Norman, S. Self-report and urine drug screen concordance among women with co-occurring PTSD and substance use disorders participating in a clinical trial: Impact of drug type and participant characteristics. Drug Alcohol Depend. 2023, 244, 109769. [Google Scholar] [CrossRef] [PubMed]
- Ghelani, A. Perspectives toward cannabidiol (CBD) among youth in Early Psychosis Intervention programs: A qualitative study. Early Interv. Psychiatry 2024, 18, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Hicks, D.L.; Kedia, S.; Regmi, S.; Mou, X. Mental health problems, substance use, and perceived risk as pathways to current cannabis use among high school seniors in the United States. Child. Youth Serv. Rev. 2024, 158, 107456. [Google Scholar] [CrossRef]
- Shah, K.; Farwa, U.E.; Vanaparti, A.; Patel, S.; Kanumuri, M.; Vashishth, O.; Hossain, N.; Dahiya, R.; Banala, M.; Enamorado, F.; et al. Global epidemiology of cannabis use disorders and its trend from 1990 to 2019: Benchmarking analysis of the global burden of disease study. J. Fam. Med. Prim. Care 2024, 13, 881–889. [Google Scholar] [CrossRef]
- Kotz, D.; Kastaun, S.; Manthey, J.; Hoch, E.; Klosterhalfen, S. Cannabis use in Germany: Frequency, routes of administration, and Co-use of inhaled nicotine or tobacco products. Dtsch. Aerzteblatt Online 2024, 121, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Salottolo, K.; McGuire, E.; Madayag, R.; Tanner, A.H.; Carrick, M.M.; Bar-Or, D. Validity between self-report and biochemical testing of cannabis and drugs among patients with traumatic injury: Brief report. Cannabis Res. 2022, 4, 29. [Google Scholar] [CrossRef]
- Eichelberger, A.H.; Kelley-Baker, T. Measuring drug use among drivers: How accurate is self-reported use? J. Stud. Alcohol Drugs 2020, 81, 104–114. [Google Scholar] [CrossRef]
- Sujan, A.C.; Alexeeff, S.E.; Slama, N.E.; Goler, N.; Avalos, L.A.; Adams, S.R.; Conway, A.; Ansley, D.; Pal, A.; Gunn, R.L. Agreement between self-reports and urine toxicology measures of illicit methamphetamine and cocaine use during early pregnancy. J. Addict. Med. 2024, 18, 28–32. [Google Scholar] [CrossRef]
- Gorfinkel, L.; Stohl, M.; Shmulewitz, D.; Hasin, D. Self-reported substance use with clinician interviewers versus self-administered surveys. J. Stud. Alcohol Drugs 2024, 85, 92–99. [Google Scholar] [CrossRef]
- Binkowska, A.A.; Jakubowska, N.; Gaca, M.; Galant, N.; Piotrowska-Cyplik, A.; Brzezicka, A. Not just a pot: Visual episodic memory in cannabis users and polydrug cannabis users: ROC and ERP preliminary investigation. Front. Hum. Neurosci. 2021, 15, 677793. [Google Scholar] [CrossRef]
- Halladay, J.; Freibott, C.E.; Lipson, S.K.; Zhou, S.; Eisenberg, D. Trends in the co-occurrence of substance use and mental health symptomatology in a national sample of US post-secondary students from 2009 to 2019. J. Am. Coll. Health 2022, 72, 1911–1924. [Google Scholar] [PubMed]
- Yurasek, A.M.; Aston, E.R.; Metrik, J. Co-use of alcohol and cannabis: A review. Curr. Addict. Rep. 2017, 4, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Coles, A.S.; Kivlichan, A.E.; Castle, D.J.; George, T.P. Treating Cannabis Use in Schizophrenia and Other Psychotic Disorders. In Marijuana and Madness; Cambridge University Press: Cambridge, UK, 2023; pp. 246–266. [Google Scholar]
- Botsford, S.L.; Yang, S.; George, T.P. Cannabis and cannabinoids in mood and anxiety disorders: Impact on illness onset and course, and assessment of therapeutic potential. Am. J. Addict. 2020, 29, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Mansell, H. Selective Studies on the Challenges of Cannabis Use in Children, Youth and Young Adults. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2022. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).