Abstract
This study describes the roles of laboratory information management systems (LIMS) in multi-site genetics studies in Africa. We used the HiGeneS Africa project as a case study. The study participants were recruited in six African countries between 2019 to 2021. The Baobab LIMS, a server–client-based system (an African-led innovation) was used for the coordination of the biospecimen. The development phase of the LIMS showcased the team formation, data collection, biospecimen collection, and shipment strategies. The implementation phase showcased the biospecimen registration, processing, and quality control (QC) analytics. The sample QC was done using Nanodrop, Qubit, and PicoGreen/gDNATapestation assays. The results showed that a total of 3144 study participants were recruited from Cameroon, Ghana, Mali, Rwanda, Senegal, and South Africa. The biospecimen registration provided a comprehensive registry that included patient demographics, genetic information, and clinical and blood/saliva samples from the proband and family relatives. The QC analyzes identified 30 samples that failed QC, linked to overdue storage in the freezer before DNA extraction. The LIMS components implemented in this project formed a structure that can be upscaled to artificial intelligence-based LIMS. In conclusion, this study represents the largest and the most diverse collection of biospecimens for the genetic study of hearing impairment in Africa to date. A well-characterized LIMS should be recommended for multi-site molecular studies, particularly in Africa, to enhance African participation in global genomic medicine.
1. Introduction
A laboratory information management system (LIMS) is a software-based application used for managing samples, procedures, equipment, reagents, staff, and data analysis to achieve end-point quality data [1,2]. LIMS has evolved into complete management solutions, with technological innovations which have improved quality management and laboratory services globally [3]. The utilization of LIMS is a more common practice in various higher income countries. In 2019, North America accounted for the largest proportion of LIMS users, followed by Europe [3]. The practical application of LIMS remains limited in Africa due to inadequate access to innovative technologies and research funds, which become prohibitive [4]. The multidisciplinary approach to LIMS has contributed to the rapid development of research activities globally [4]. Research collaborations, particularly in tropical medicine, are becoming necessary to reduce the level of global disease burden in Africa [3,4].
Hearing impairment genetics study (HiGeneS) in Africa is an initiative established to investigate the genetic causes of hearing impairment in Africa. Our project has study sites in Cameroon, Ghana, Mali, Senegal, Rwanda, and South Africa. LIMS can be used to coordinate multi-site genetic research. It can be used to track biospecimen collection, shipment, storage, and other activities. LIMS can improve personnel training and compliance with global best practices for IT based biological systems [5]. Best practices must be followed for biospecimen handling. These should include optimization, pre-analysis, analytics, and postanalytical activities [6,7]. In order to achieve high standard analyzes, attention should be paid to the methods used for the recruitment, biospecimen collections, storage, and shipment [5,6,7]. Lack of organizational management practices in biomedical research can hamper the quality of biospecimens and negatively impact research [3,8]. The methods used for collecting specimens for human research may also influence the quality of the biospecimens, as well as subsequent downstream activities. The temperature system of the storage, timing of biospecimen processing, volume, storage duration, and the number of aliquots prepared over time can affect quality of research [9]. Therefore, biospecimen such as blood, saliva, urine, swap, or feces demand standard methods for collections and handling [3,10]. Deoxyribonucleic acid (DNA) sequencing studies have improved disease diagnosis and treatments [11]. Genetic studies greatly contribute to the epidemiology and treatment of various diseases [12].
It is against this background that a LIMS was implemented to manage the biospecimen metadata, collection, and research activities management in this study. The implementation of LIMS in this project can improve the quality of research collaborations among African scientists, as well as globally. Our study describes the LIMS implementation and a model to upscale it for better experience through artificial intelligence (AI). AI uses complex algorithms and software to emulate human cognition in the analysis of medical data generated from diagnostics, medical records, research, clinical trials, and so on [13].
2. Materials and Methods
2.1. LIMS Development Phase
The LIMS was developed based on the steps described in Figure 1a. We evaluated the human resources, e.g., skills, as well as the capital, equipment, materials, and LIMS software availability across the sites. The most important aspects of LIMS implementation are highlighted in Table 1. These prerequisites are needed to establish a multi-site genetic study. The project planning phase also entailed setting standards for the recruitment and the number of participants to recruit, the methods for collecting biospecimens, extracting DNA with high quality, and the types of biospecimen acceptable. This included hosting a secure portal for documenting patient data and a biorepository for biospecimens. The study also aimed to assess the level of challenges that face multi-site genetic studies in Africa.
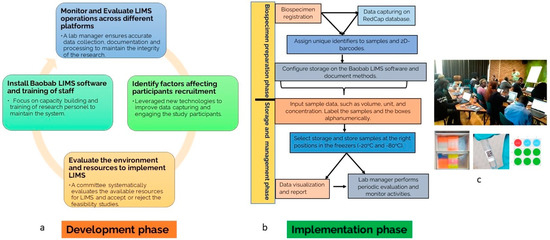
Figure 1.
(a) Overview of LIMS design development phase. (b) The overview of the implementation phase of LIMS across study sites coordinated from South Africa. (c) The training of the staff team on LIM, labeling and barcoding of sample, Baobab storage layout.

Table 1.
The LIMS components.
2.2. LIMS Implementation Phase
Biospecimen Collection and Data Capturing
The procedures for the biospecimen collection in this project followed the procedures for collection and management of biospecimens described by the (SPIDIA) consortium (http://www.spidia.eu/, accessed on 18 June 2022) and the International Society for Biological and Environmental Repositories (ISBER) (www.isber.org, accessed on 18 June 2022). A standard operating procedure (SOP) was assigned to each study site for compliance. In addition, staff were offered training on each aspect of LIMS described in the implementation phase. The selected data-capturing portal used in this project was Research Electronic Data Capture (REDCap). The study participants’ demographic information at various sites was captured adequately. Sample collections were done accordingly and were retained on dry ice, sealed, and labeled for shipments.
2.3. LIMS Data Capture and Management
The workflow for the data capturing and management was described in Figure 1b. The Baobab LIMS (https://baobablims.org/, accessed on 15 April 2019) was installed on a secured network in our laboratory. The software is an open-source tool. The Baobab LIMS storage was configured according to the project sites. The storage on the LIMS represents the actual physical storage in the laboratory, which are −20 °C freezers. Hence, we generate a code such as:
HiGeneS/Storage/UCT/Falmouth/Room4.25/Freezer1/Shelf1/Box.001/0.01.
2.4. Biospecimen Labeling and Barcoding System
The labeling of the biospecimen corresponds to short codes for the country, region, and family identification (ID) given at the point of recruitment. This method allows easy sorting of samples in case of a mix-up. Each label was assigned a quick response (QR) barcode to uniquely identify the sample during storage and shipment.
2.5. Sample Storage and Tracking
It is important to register samples batch by batch and for research personnel to cross validate. The registered and properly labeled biospecimens were stored accordingly in the compartments of the freezers. The IDs and other relevant details were imported to the already configured Baobab LIMS. The tracking of the biospecimens was monitored throughout the study by the assigned lab manager, who monitors and analyzes the temperature changes, number of volumes pipetted, change in ID, concentration of the DNA, shipment records, and describes the organizational workflow. We ensured that the research coordinators received proper training on each of the aforementioned steps (Figure 1c).
2.6. Biospecimen Quality Control Before Genomics Studies
We ensured that blood samples were extracted at the recruitment sites within three weeks of collection. Where it was not possible, the blood samples were transported through a courier service to South Africa. The DNA extraction was performed uniformly across the study site using the Qiagen DNA extraction kits or the Perkin Elmer Chemagic extraction kit. Records were kept of the extraction dates. We performed the quality control (QC) check on the DNA using the Nanodrop, Qubit, and PicoGreen/gDNATapestation assays.
2.7. Model for LIMS and Artificial Intelligence System
We proposed that AI would integrate data from both internal and external sources (e.g., genomic databases, metadata platforms) with a major big data platform and analyze it for various utilities, such as diagnosis, methods, predictions, and treatments. These treatments would be accessed by the researchers/clinicians, study participants, and collaborators via a network system, using an interface such as a phone device. The user interactions would serve as curation purposes to optimize and improve the AI system. The model could upscale how study participants and other classified end users interact with clinical and laboratory data (Figure 2).
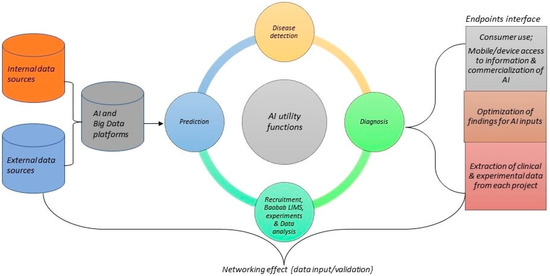
Figure 2.
An overview of AI model for data integration, accessibility, and sharing to improve global genomic medicine collaboration in Africa.
3. Results
The results of our findings showed that organizing a multi-site genetics study in Africa can be challenged by limited access to the internet, cloud-storage systems, automated LIMS and experts, data scientists, and information communication technology devices. These are listed as resource constraints. Other challenging factors include:
- Family members on the recruitment list may be living in geographically different locations;
- A need for translators to inform participants in their local language and vocabulary;
- Difficulty collecting samples, such as blood, from small children.
However, the training of staff on LIMS and following a unified SOP proved to be very useful in a multi-site project. Moreover, it formed the basis for the creation and accumulation of knowledge for the content of LIMS and AI model synchronization, described in the supplementary file S1.
The best practices outlined under the methods section of this study assisted us in achieving the project’s three-year milestones. The number of study participants recruited from 2019 to 2021 in 20 recruitment sites in the six countries was 3144 (Figure 3). All clinical and demographical data were successfully captured for genetics and epidemiology analysis and data sharing. In terms of the QC, the results showed that of the 3144 biospecimens collected, only 30 samples failed the QC criteria set for the project (≥150 ng/uL DNA). The biospecimens were successfully used in a range of analyses, such as Polymerase chain reaction (PCR), Sanger Sequencing, TaqMan® assay, qPCR, next-generation sequencing, gene expression assays, and molecular cloning over the three-year period (with up to sixteen genetics papers published that provided patient-centered information which can benefit healthcare research in the future).
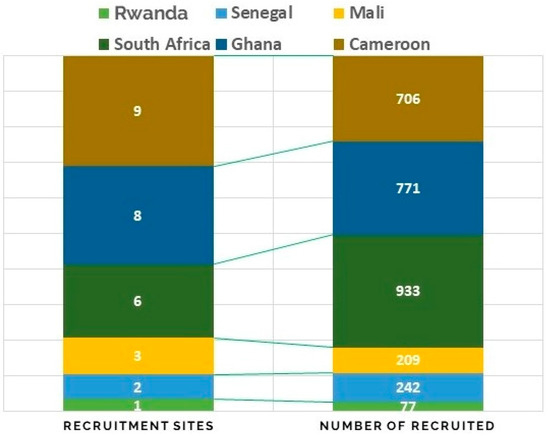
Figure 3.
Distributions of the study participants that were recruited for the HiGeneS project in the six African countries between the years 2019–2020.
The DNA samples that failed QC belonged to the category of blood samples that were not extracted over a period of six months, and compared with the blood samples that were extracted over two weeks after collection (Figure 4). Hence, the DNA quality in a blood sample can be degraded if the DNA has not been extracted, despite being stored at −20 °C over a long period (~6 months). The evaluation of the Baobab by a random questionnaire among staff members showed that most people were comfortable using the platform, and found it useful in coordinating simple tasks, such as registries and logistics.
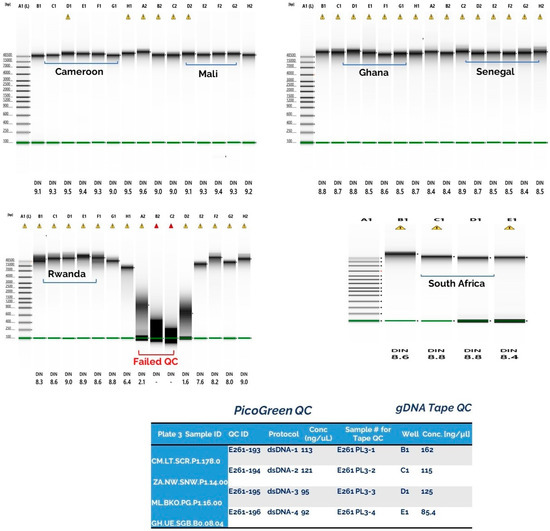
Figure 4.
The quality control result showed that close to 98.9% of the DNA samples had good concentrations, between 350–1650 ng/µL, on Nanodrop. The PicoGreen and gDNATapestation assays showed lower DNA conc (90–850 ng/µL). Approximately 30 samples (with overdue blood storage ~6 months) had degraded or had low input (<10 ng dsDNA) concentration. The red bracket indicates the low quality/degraded DNA samples which were extracted 6 months after blood collection and storage at −20 °C.
The African Academy of Sciences experts recently published a framework for implementing genomic medicine in Africa. https://www.aasciences.africa/sites/default/files/Publications/A%20Framework%20for%20the%20Implementation%20of%20Genomic%20Medicine%20for%20Public%20Health%20in%20Africa.pdf (accessed on 5 August 2022). The framework provides some information and recommendations to enable the implementation of genomic medicine. The discussions rest on a two-pronged approach to address, namely: (1) Filling the gaps in locally relevant genomics research, and implementation in clinical practice for relevant diseases. They identified clinical facilities for patient counselling, screening, treatment, and monitoring as essential parts of genomic medicine. (2) In addition, sample collection, processing and data generation facilities, data storage, curation, analysis, interpretation, and sharing infrastructure are important. They also identified the need to make available genomic medicine training programs for healthcare professionals as parts of their needs. Most importantly, they reflected on the need to advance knowledge bases with up-to-date information on the genotype–phenotype link and actionability.
Our study implemented some of the actionable plans described in the framework, by recruiting patients for genomic studies, improving storage of biospecimens, and providing adequate training in these areas for professionals.
Inadequate recruitment is known to have a significant impact on scientific and research enterprises. Our study implemented LIMS in a multi-site genetic study in Africa in order to improve recruitment and research enterprise. This knowledge can help us to maintain biospecimen management to be effective, especially in a multi-site genetic study. When developing research collaboration, it is important to include LIMS at the proposal stage. Since most decisions regarding a patient’s diagnosis and treatment are based on results of laboratory tests, it is, therefore, important to ensure that the processes of obtaining laboratory findings are error-free. When describing LIMS, it is vital that quality control systems are implemented early on [14].
From our experience on the strategies for implementing LIMS, while considering all the challenges facing multi-site research in Africa, we demonstrate the importance of customizing a workable LIMS that can fit the scope of each project, and that LIMS processes should begin with strategic meetings amongst all the stakeholders. Designing a recruitment strategy prior to study initiation can significantly improve recruitment of study participants. Additionally, as demonstrated in this study, having a trained and dedicated recruitment team can ameliorate the challenges of low recruitment that has been bewildering those who study genetics in Africa. Having a well-detailed SOP is important, as we saw it was useful in our study. In addition, during the recruitment, it is important to budget for travel compensation to individuals who are living in separate geographical locations, thereby indirectly incentivizing recruitment attendance. Furthermore, the assistance of local and sign-language interpreters allowed for more effective communication. African genetics studies are quickly becoming relevant, as Africa is highly admixed and contributes to increased genetic diversity and identification of genetic diseases [15,16,17].
Willingness to participate in studies, particularly where blood samples are collected, pose a challenge, due, in part, to anthropological and cultural perceptions of the direct and indirect impact on affected families. In order to address this, we invited parents (both parents and one unaffected child) of prospective cases, who were identified by our contacts (teachers) in the selected schools, to attend on scheduled recruitment dates.
Biospecimen collections do present challenges related to sample labeling, sample storage, and record keeping, particularly in infrastructure, as well as settings with limited resources. In order to address this, per site, at least two scientists facilitated DNA extractions and storage. Multi-site biospecimen collection for familial studies usually faces the challenges of thorough record keeping of operational metadata, such as inventory, plans/protocols, purchasing records, number of sites, number of recruiters, and number of study participants recruited. This was implemented and maintained throughout the study. Registries were retained in both hard copy and electronic formats (G-drive and RedCap database). Documented metadata additionally contributed to the epidemiological research on hearing impairment genetics in Africa [18].
The pre-analytical factors related to the collection, processing, shipment, and storage of biospecimens can introduce disparities in experimental results. Biospecimen characteristics may be impacted and changed based on the environmental factors, exposure to certain chemicals or temperature changes during collection, shipment, processing, and storage [19,20,21]; as a result, such changes may cause inaccurate determinations of the molecular and physical characteristics of the biospecimen during downstream analysis. Biospecimens collected from multi-site centers have a greater risk of exposure to heterogenous environmental factors, including temperature changes due to the geographical and resource limitations of the countries. However, the pattern of coordination, logistics, and LIMS implemented in this study prevented such changes, despite covering six African countries. We used three different types of QC analysis to validate our findings of DNA quality per sites. Each of these methods are unique. Particularly, the picogreen and gDNATape assays help to obtain the actual concentrations of double strands DNA in a sample; therefore, the DNA concentration measured is usually lower than Nanodrop and Qubit. The samples that failed QC were due to long-term storage of blood samples before extraction. Researchers may consider using blood stabilizers for biospecimens that they intend to store for longer than 6 months before extraction.
Another benefit of LIMS, as observed in our study, was that it improves human capacity development in Africa. Approximately 20 members were trained on LIMS, which improved their computational, organizational, and logistics skills. For example, the coding system used in this study was based on pedigree analysis that allowed us to easily perform relatedness analysis between the patients and kindreds. The accurate labeling of biospecimens contributes to the integrity of scientific research [22], because a code on a biospecimen may compromise the accuracy and reproducibility of a study. Labeling of biospecimens forms an integral part of the LIMS in this project, as a varying number of characters may pose challenges to downstream biostatistics and bioinformatic analyses. The risk of label repetition increases when large sample numbers are considered from the study sites. We recommend using an alphanumeric coding system, which can be used to differentiate biospecimens that were collected from each study site. In addition, the complexity in the family pedigrees revealed a challenge and opportunity for multi-site genetic studies in Africa. Hence, handwriting of labels, although cheap and convenient, is advised against, due to the significant risk of labeling error. Furthermore, handwritten labels may be difficult to read, and the ink can smear or erase, thereby further reducing the legibility of the label. In order to overcome the inherent errors associated with handwritten labels, sample label printing machines should be used. Cost-effective barcoding systems and printers are readily available, and, ultimately, costs related to data capture and error are reduced with their use.
As LIMS enters the era of artificial intelligence for automation, geneticists and other scientific researchers should utilize LIMS as part of their operational quality management processes, particularly when research activities are biocollection- and curatorship-focused [21]. Baobab LIMS have given us access to technical and operational support, as it is local. Baobab LIMS is an upgradable and secure LIMS software, with content configuration flexibility to suit individual needs. The biospecimen data which are captured within the system are made readily available to the collaborating laboratories. The use of the LIMS improved traceability and allowed for comprehensive reporting, as well as effective and efficient administration. The AI model will involve the LIMS; it is believed that the LIMS already performs pre-analytical variables which can serve as input for AI, as well as the sequencings and post-sequencing data. The knowledge of AI in health is increasing rampant. A recent study conducted on the value of AI in laboratory medicine showed that AI is already being used by 15.6% of the organizations, while 66.4% felt that they might use AI in the future [23]. AI algorithms function with reliable and accurate laboratory data, which can allow LIMS data to serve as input for AI utility for broader usage and commercialization. AI initiatives can accelerate efforts with speed and scale up optimal customer value. Africa is not quite there yet in terms of readiness for AI in health care systems. Our model still demands to be tested and validated in the future. AI with LIMS in clinical and laboratory research will build a diverse and inclusive team which will solve most of the health challenges in Africa. For example, our team developed the HigeneS ontology tool in advance [24]; a portal such as this would facilitate the implementation of LIMS with the AI system, and thus improve the experience for patients, clinicians, and researchers. Our study had some limitations. This paper is restricted to the findings in recruitment and LIMS implementation. We did evaluate other similar studies, which could have strengthened our conclusions. Other limitations include a lack of awareness about the study in rural communities. There were social and cultural issues that posed resistance to data collection. The complexity of the study protocol was also a limitation, since not everyone in the team has genetics knowledge. Additionally, there were limited resources at some recruitment sites in the remote areas compared to the recruitment sites located in the cities.
4. Conclusions
In conclusion, the implementation of LIMS provides not only an opportunity for cohesion and harmonization in multi-site research, but also allows for capacity building. Using Baobab LIMS reduced pressure on recruiters, supported the patients with robust information, and permitted uniform procedures in biospecimen collection and laboratory testing. The QC methods were also fit-for-purpose. The quality data achieved in our studies can be linked to the quality of biospecimens maintained at the project biorepository.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmp3040022/s1, Supplementary File S1: The working document for HiGeneS Africa database for the future AI LIMS.
Author Contributions
O.G.O., D.A., and M.J. organized LIMS set-up and trained staff; O.G.O. and C.O. implemented DNA extraction, data storage, and data management; S.M.A., E.T.A., E.T.W., A.Y., E.U., N.M., K.M., R.P, T.M, K.P., and Y.D. implemented on-site biospecimen and data collections; C.d.C., V.N., and A.W. performed administrative duties and supervised the projects; all authors contributed to the S.O.P. and data management framework described in the study during recruitment, and they consented to the findings in the study to be published. The study was performed according to the tenets of the Declaration of Helsinki. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Institute of Health (NIH, Bethesda, MD, USA), grant number U01-HG-009716 to A.W.; the African Academy of Science and Wellcome Trust, grant number H3A/18/001 to A.W.; the funders were not involved in data generation, analysis, or the decision to publish.
Institutional Review Board Statement
The study was approved in South Africa by the Human Research Ethics Committee of the University of Cape Town, South Africa (Ethics approval number HREC Ref: 104/2018), the Committee for Human Health of the Gynaeco-Obstetric and Paediatric Hospital of Yaoundé, Cameroon (Ethics Approval N° 723/CIERSH/DM/2018), Institutional Ethics Committee of the Faculty of Medicine and Dentistry of the University of Sciences, Techniques and Technologies of Bamako, Mali (N° 2020/129/CE/FMOS/FAPH), he Research Ethics, Committee of Cheikh Anta Diop University, Dakar, Senegal (CER/UCAD/AD/MSN/034/2020), and the Ethics Committee for Basic and Applied Science (ECBAS), University of Ghana (ECBAS 053/19-20).
Informed Consent Statement
Written informed consent was obtained from each patient during recruitment, according to the tenets of the Declaration of Helsinki, and each patient consented to the findings in the study to be published.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We acknowledge the Baobab developers and maintenance team at SANBI, University of Western Cape South Africa, for allowing us to purchase the right to use the software, and for their technical supports towards implementing the LIMS for this project. In addition, we acknowledge the Omegabioscience laboratory, Atlanta, United States for support on QC validation results.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Skobelev, D.O.; Zaytseva, T.M.; Kozlov, A.D.; Perepelitsa, V.L.; Makarova, A.S. Laboratory information management systems in the work of the analytic laboratory. Meas. Tech. 2011, 53, 1182–1189. [Google Scholar] [CrossRef]
- Isfahani, S.S.; Khajouei, R.; Jahanbakhsh, M.; Mirmohamadi, M. The evaluation of hospital laboratory information management systems based on the standards of the American National Standard Institute. J. Educ. Health Promot. 2014, 3, 61. [Google Scholar]
- Laboratory Information Management System (LIMS) Market-Global Forecast to 2025|MarketsandMarkets. Available online: https://www.marketsandmarkets.com/Market-Reports/laboratory-information-management-systems-market-250610373.html?gclid=Cj0KCQiAyJOBBhDCARIsAJG2h5edRqsMsUGxquSITQNHcu-XM3kcdnx6soJoCBBeK-q0tG6TQLsjvqsaAptZEALw_wcB. (accessed on 18 June 2022).
- Colangeli, P. Laboratory information management system: An example of international cooperation in Namibia. Vet. Ital. 2012, 48, 241–251. [Google Scholar] [PubMed]
- Nyasulu, P.S.; Paszko, C.; Mbelle, N.A. Narrative Review of the Laboratory Information System and Its Role in Antimicrobial Resistance Surveillance in South Africa. Adv. Microbiol. 2014, 4, 692–696. [Google Scholar] [CrossRef]
- Chang, A.Y.; Skirbekk, V.F.; Tyrovolas, S.; Kassebaum, N.J.; Dieleman, J.L. Measuring population ageing: An analysis of the Global Burden of Disease Study 2017. Lancet Public Health 2019, 4, e159–e167. [Google Scholar] [CrossRef]
- 2012 best practices for repositories collection, storage, retrieval, and distribution of biological materials for research international society for biological and environmental repositories. Biopreservation Biobanking 2012, 10, 79–161. [CrossRef]
- Mitchell, D. Biobanking from the patient perspective. Res. Involv. Engagem. 2015, 1, 4. [Google Scholar] [CrossRef]
- Riegman, P.H.J.; Morente, M.M.; Betsou, F.; de Blasio, P.; Geary, P. Biobanking for better healthcare. Mol. Oncol. 2008, 2, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Bell, W.C.; Sexton, K.C.; Grizzle, W.E. Organizational issues in providing high-quality human tissues and clinical information for the support of biomedical research. Methods Mol. Biol. Clifton NJ 2010, 576, 1–30. [Google Scholar]
- Engel, K.B.; Vaught, J.; Moore, H.M. National Cancer Institute Biospecimen Evidence-Based Practices: A Novel Approach to Pre-analytical Standardization. Biopreservation Biobanking 2014, 12, 148–150. [Google Scholar] [CrossRef]
- Malentacchi, F. Influence of pre-analytical procedures on genomic DNA integrity in blood samples: The SPIDIA experience. Clin. Chim. Acta Int. J. Clin. Chem. 2015, 440, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Katsanis, S.H.; Katsanis, N. Molecular genetic testing and the future of clinical genomics. Nat. Rev. Genet. 2013, 14, 415–426. [Google Scholar] [CrossRef] [PubMed]
- McGuire, A.L. The road ahead in genetics and genomics. Nat. Rev. Genet. 2020, 21, 581–596. [Google Scholar] [CrossRef]
- Janzen, W. Establishing and Maintaining a Robust Sample Management System. SLAS Technol. Transl. Life Sci. Innov. 2019, 24, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.W. A High-Density Admixture Map for Disease Gene Discovery in African Americans. Am. J. Hum. Genet. 2004, 74, 1001–1013. [Google Scholar] [CrossRef]
- Shriner, D. Overview of Admixture Mapping. Curr. Protoc. Hum. Genet. 2013, 1, 1–23. [Google Scholar] [CrossRef]
- Halder, I.; Shriver, M.D. Measuring and using admixture to study the genetics of complex diseases. Hum. Genom. 2003, 1, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Wonkam Tingang, E. Hearing Impairment Overview in Africa: The Case of Cameroon. Genes 2020, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Neumeister, V.M. A tissue quality index: An intrinsic control for measurement of effects of preanalytical variables on FFPE tissue. Lab. Investig. J. Tech. Methods Pathol. 2014, 94, 467–474. [Google Scholar] [CrossRef]
- Poste, G. Leveling the playing field: Bringing development of biomarkers and molecular diagnostics up to the standards for drug development. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 1515–1523. [Google Scholar] [CrossRef]
- Portier, B.P. Delay to formalin fixation ‘cold ischemia time’: Effect on ERBB2 detection by in-situ hybridization and immunohistochemistry. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2013, 26, 1–9. [Google Scholar] [CrossRef]
- Xie, R. Factors influencing the degradation of archival formalin-fixed paraffin-embedded tissue sections. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2011, 59, 356–365. [Google Scholar] [CrossRef]
- Morrison, A.P. Reduction in specimen labeling errors after implementation of a positive patient identification system in phlebotomy. Am. J. Clin. Pathol. 2010, 133, 870–877. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).