Abstract
The chemical and physical routes are usually used to synthesize metal nanoparticles. However, the harmful effects on the environment and human health has turned scientists into finding greener methods. We have developed the novel green method for the synthesis of flower Pd nanoparticles based on the chitosan (CS) polymer. In this method, CS can work as a stabilizer, a shape-directing agent, and a size-controllable agent for the synthesis of these nanoparticles. This study provides pioneer evidence about the multifunctional roles of natural polymers in the preparation of metal nanoparticles. Deep and extensive studies should be conducted to explore the great benefits of natural polymers in the green synthesis of metal nanoparticles.
1. Introduction
Palladium (Pd) nanoparticles are emerging as an outstanding metal material in the nanotechnology field. They have excellent physical and chemical properties such as high thermal and chemical stability, high photocatalytic activity, and low cost [1]. Pd nanomaterials have been applied in wide ranges in hydrogen storage/sensing, organic coupling synthesis, hydrogen detection, fuel cells, and sensors [2]. Pd nanostructures acting as photocatalysts, prodrug activators, and antimicrobial agents have also been discovered. The biomedicine applications of Pd nanoparticles have been discovered recently [3,4]. To reduce the cost and the number of toxic materials of the chemical and physical method, green methods have been focused on to discover recently. Herein, we reported the simple and scalable method to synthesize Pd nanoparticles. CS, which is a natural polymer, has biodegradability, biocompatibility, nontoxicity, and antibacterial activity. CS is a good stabilizer for metal nanoparticles [5]. In this work, we experimented with the effect of CS amount on the Pd nanoparticles. Vitamin C acts as the mild reducing agent in this method. Interestingly, we observed that CS not only worked as a stabilizer but also as a shape-directing and size-control agent for the synthesis of FPNPs. The sizes of FPNPs range from 25 to 150 nm. The resulting Pd nanoparticles have good biocompatibility, strong absorption in the NIR range, and good photothermal conversion.
2. Materials and Methods
2.1. Materials
Palladium chloride (PdCl2), CS (50 to 190 kDa and 75–85% degrees of deacetylation), L-ascorbic acid, and polyvinyl alcohol (PVA, 89 to 98 kDa).
2.2. One-Spot Synthesis of Pd Nanoparticles
The Pd nanoparticles were synthesized by the one-pot method. Firstly, a defined amount of CS and 50 mg of ascorbic acid were added to the beaker with 15 mL of distilled water. Next, the various amounts of CS were used to obtain different mass concentrations (C%) of CS. Then, 10 mL of HPdCl4 0.01 M was dropped into the CS solution. The nanoparticles solution was left for aging for 2 h. Finally, the resulting nanoparticles were collected by centrifugation and were washed three times with distilled water. The same procedure was used to synthesis Pd nanoparticles for the control without adding CS.
3. Result and Discussion
3.1. Chitosan as a Stabilizer for the Synthesis of Pd Nanoparticles
Two experimental conditions were set up to evaluate the effect of CS on the formation of Pd nanoparticles (CS reaction and no-CS reaction). The TEM images (Figure 1) revealed that the no-CS reaction produced big-sized nanoparticles. Meanwhile, the CS reaction produced small-sized nanoparticles. The resulting solution was left for one day. In the no-CS reaction, aggregation was observed in the bottom of the tube; meanwhile, the CS reaction solution had no aggregation. The results again proved that CS is an excellent stabilizer for metal nanoparticles.
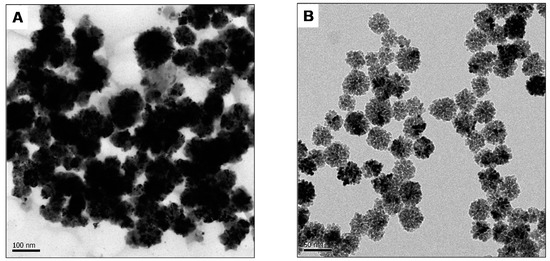
Figure 1.
Morphologies of the synthesized Pd nanoparticles without CS (A) and with CS (B).
3.2. Chitosan as a Size-Control Agent and Shape-Directing Agent for the Synthesis of Flower Pd Nanoparticles
The effect of CS on the formation of Pd nanoparticles was evaluated by setting six experimental conditions with different CS amounts. The TEM images were captured for morphology analysis. The nanoparticles have a flower shape with a range of sizes. The Pd nanoparticles, which were prepared with 0.05% (w/v) CS, have a nanoparticle size of 153.7 ± 59.7 nm. Increasing the mass concentration of CS led to a decrease in the size of the resulting nanoparticles, as shown in Figure 2.
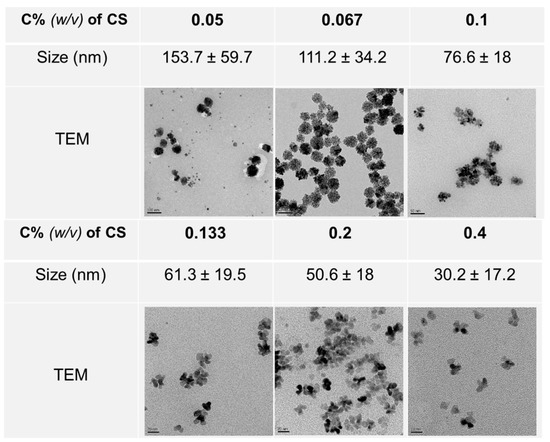
Figure 2.
Morphologies and nanoparticle size of Pd nanoparticles synthesized with different C% (w/v) of CS.
4. Conclusions
In conclusion, CS acted as a multifunctional agent in the developed green synthesis method for the preparation of Pd nanoparticles. CS acted as a stabilizer, a size-control agent, and a shape-directing agent. The Pd nanoparticles can be controlled by adjusting the CS amount. This method is useful for preparing Pd nanoparticles, with desired sizes, that are suitable for different applications.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, A.; Ostrom, C. Palladium-Based Nanomaterials: Synthesis and Electrochemical Applications. Chem. Rev. 2015, 115, 11999–12044. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Hung, H.-H.; Huang, M.H. Seed-Mediated Synthesis of Palladium Nanorods and Branched Nanocrystals and Their Use as Recyclable Suzuki Coupling Reaction Catalysts. J. Am. Chem. Soc. 2009, 131, 9114–9121. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Shin, W.; Kang, K.; Choi, M.-H.; Kim, Y.-J.; Kim, Y.-K.; Min, D.-H.; Jang, H. Revisiting of Pd Nanoparticles in Cancer Treatment: All-Round Excellence of Porous Pd Nanoplates in Gene-Thermo Combinational Therapy. ACS Appl. Mater. Interfaces 2018, 10, 13819–13828. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Youn, J.-I.; Kim, Y.-J.; Oh, H.-J. Effect of Palladium Nanoparticles on Photocatalytic Characteristics of N doped Titania Catalyst. J. Mater. Sci. Technol. 2015, 31, 664–669. [Google Scholar] [CrossRef]
- Collado-González, M.; Montalbán, M.G.; Peña-García, J.; Pérez-Sánchez, H.; Víllora, G.; Baños, F.G.D. Chitosan as stabilizing agent for negatively charged nanoparticles. Carbohydr. Polym. 2017, 161, 63–70. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).