Abstract
Microplastics (MPs) have emerged as pervasive environmental contaminants due to their widespread presence across various ecosystems, including their use in single-use plastic food ware and table salt dispensers. This issue coincides with the presence of heavy metals in water sources in Doha, Qatar. Fourier Transform Infrared (FTIR) analysis revealed that the plastic plate and spoon were composed of polyolefin, with the spoon exhibiting additional peaks that indicated oxidation or the presence of additives. Thermogravimetric Analysis (TGA) revealed that the spoon exhibited higher thermal stability, retaining approximately 10% of its mass at 700 °C, than the plate, which retained 2%, indicating the presence of complex additives or contamination. MPs in food-grade salt samples were verified through filtration and Fourier Transform Infrared (FTIR) Spectroscopy, identifying polymers such as polyethylene (PE), polypropylene (PP), and polyethylene terephthalate (PET). These MPs likely stem from exposure to packaging or environmental contaminants. FTIR spectra confirmed the integrity of the polymers after treatment. Inductively Coupled Plasma–Optical Emission Spectroscopy (ICP-OES) analysis revealed varying levels of heavy metals in bottled and tap water, with notable findings including detectable arsenic and lead in both, higher calcium and magnesium in bottled water, and the presence of copper present in tap water only, highlighting potential health and infrastructure-related concerns. These results highlight the possible risks associated with exposure to MPs and heavy metals from everyday products and water sources, underscoring the need for enhanced regulatory oversight and safer material choices to ensure protection.
1. Introduction
Microplastics (MPs), defined as plastic particles smaller than 5 mm, have emerged as pervasive environmental contaminants due to their widespread presence across various ecosystems and their potential health risks. These particles originate from primary sources, such as industrial raw materials and personal care products, as well as secondary sources, including the degradation of larger plastic debris through physical, chemical, and biological processes. Common polymers found in MPs include polyethylene (PE), polypropylene (PP), polystyrene (PS), and polyethylene terephthalate (PET), which are prevalent in packaging materials and consumer products [1,2]. Their large specific surface area and hydrophobic nature contribute to their ability to absorb various pollutants, including heavy metals [3,4]. The presence of functional groups, such as carboxyl and hydroxyl groups, on MPs enhances their ability to adsorb heavy metals [3,5]. Interestingly, the adsorption ability of MPs varies depending on the type of plastic and metal species involved. For instance, PE and PET MPs have shown higher adsorption capacities for Cu(II), Cr(III), and Pb(II) compared to PP MPs [3].
MPs have been detected in diverse environmental compartments, including marine and freshwater systems, soils, and even remote regions like Antarctica. In aquatic environments, MPs are introduced through wastewater discharge, stormwater runoff, and atmospheric deposition, with urban watersheds and wastewater treatment plants being significant contributors [6,7]. Soils act as major sinks for MPs, receiving inputs from sewage sludge, landfills, and agricultural practices [8]. Notably, the presence of MPs in Antarctic snow highlights their capacity for long-distance atmospheric transport and global distribution [9].
The environmental persistence of microplastics (MPs) poses significant risks to both ecosystems and human health. In aquatic organisms, MPs can cause physical damage, disrupt feeding and reproduction, and contribute to bioaccumulation and biomagnification within food webs [10,11]. Humans are exposed to MPs through ingestion, inhalation, and dermal contact. Dietary intake is a major exposure route, with MPs detected in seafood, fruits, vegetables, bottled water, and sea salts [12,13,14,15]. Inhalation of airborne MPs, especially in indoor environments, and occupational exposure in industries handling plastics further increase human exposure [16,17]. Although dermal exposure is considered a minor route, the potential for skin penetration and associated health effects warrants further investigation [18,19].
Health effects associated with MP exposure include inflammation, oxidative stress, and toxicity, affecting multiple physiological systems. MPs can interact with the immune system, leading to both localized and systemic effects, and have been found to accumulate in various systems, including the digestive, respiratory, cardiovascular, and reproductive systems. Their ability to absorb environmental pollutants, such as heavy metals and organic toxins, further exacerbates their toxicity and immunotoxicity [20].
Analyzing MPs requires advanced techniques to identify and characterize these particles accurately. Spectroscopic methods, such as Fourier Transform Infrared (FTIR) and Raman spectroscopy, are commonly used to determine the chemical composition of MPs [21,22]. For particles smaller than 10 µm, micro-Raman (µRaman) and scanning electron microscopy (SEM) are essential tools [22]. Mitigation strategies for (MP) pollution include innovative approaches such as catalytic and enzymatic degradation techniques, which show promise in effectively breaking down MPs [23,24]. Additionally, ensuring that MP research data adhere to the FAIR principles—Findability, Accessibility, Interoperability, and Reusability—is crucial for developing evidence-based policies and facilitating collaborative efforts [25].
Packaging materials are significant contributors to (MP) pollution, as they undergo degradation due to various environmental factors. Common packaging polymers, including PE, PP, PET, and PS, degrade through physical, chemical, and biological mechanisms, with abiotic degradation often preceding biodegradation [26,27]. Factors such as UV radiation, oxygen exposure, and mechanical stress initiate degradation processes, resulting in the formation of smaller polymer fragments and the creation of new chemical groups [26]. The degradation pathways and rates are influenced by environmental conditions, material composition, and processing techniques, underscoring the need for developing alternative, environmentally friendly packaging options to mitigate MP pollution. This research aims to enhance the understanding of potential contamination in various consumer and environmental samples through two primary pathways: the presence of MPs and heavy metal contamination. Plastic food service ware, along with commonly consumed food products, underwent examination for MPs using advanced spectroscopic techniques. Concurrently, multiple drinking water samples underwent analysis to detect and quantify toxic heavy metals, providing a comprehensive assessment of chemical contaminants in water sources. These products were selected due to their high frequency of consumption and direct contact with potential sources of MP and chemical contamination, representing a relevant exposure route for the general population.
2. Materials and Methods
2.1. Overview of Sample Collection and Analytical Approach
The investigation aimed to detect, isolate, and characterize MP particles through an integrated experimental protocol combining chemical digestion, physical separation, thermal exposure simulation, and advanced instrumental analysis to achieve this. The integrated workflow (Figure 1) outlines the stepwise procedure, from sample collection to spectroscopic and chemical analysis, ensuring the reliable detection of contaminants and supporting health risk assessments linked to packaging-related pollution.
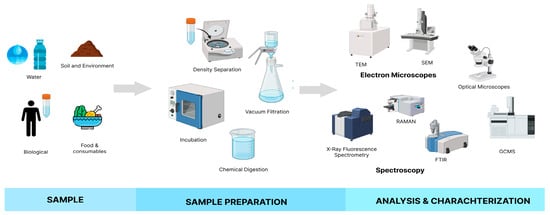
Figure 1.
Stepwise workflow for sample collection, preparation, and multimodal analysis of complex matrices.
2.2. Sample Preparation
Plastic food service ware—specifically, disposable spoons and plates—was analyzed to determine its polymeric composition and its potential role as a source of (MP) contamination. The plastic materials were cut into 2 × 2 cm pieces, cleaned with methanol, air-dried at room temperature, and then characterized using FTIR and Thermogravimetric Analysis (TGA). Multiple water samples were analyzed using ICP-OES to detect and quantify toxic heavy metals, providing a broader perspective on chemical contamination in water sources. In parallel, food-grade salt samples from five commercial brands, bought from local markets in Doha, Qatar, were examined for MP contamination. For each brand, 50 g of salt was dissolved in 500 mL of filtered, deionized water. The solution was filtered through a 0.45 μm membrane to collect possible MPs, and the filters were treated with 30% hydrogen peroxide (H2O2) under controlled conditions (65 °C for 24 h followed by 30 °C for 48 h) to remove organic matter while preserving synthetic polymers.
2.3. Analytical Methods
TGA was performed on the plastic spoons and plates to assess their thermal stability and the potential leaching of contaminants at elevated temperatures. The analysis was carried out using a PerkinElmer TGA 4000 instrument (PerkinElmer, Inc., Shelton, CT, USA). Samples were heated from 30 °C to 800 °C at a rate of 10 °C/min under a nitrogen atmosphere with a flow rate of 20 mL/min. The resulting degradation patterns were analyzed to assess the material’s thermal behavior and detect the presence of potential additives or contaminants. Additionally, FTIR was performed on the filtered particles collected from the salt samples and on the packaging materials of disposable plastics such as spoons and plates. The analysis was conducted using a Perkin Elmer Spectrum Two spectrometer (PerkinElmer, Inc., Shelton, CT, USA), with spectra collected in the range of 4000–500 cm−1. The resulting spectra were then compared against reference polymer libraries to identify the types of synthetic polymers present in the samples. Trace heavy metal analysis in water samples was performed using a PerkinElmer Optima 7300 DV ICP-OES system (PerkinElmer, Inc., Shelton, CT, USA). To preserve dissolved metal ions, samples were first diluted with ultrapure water and acidified with nitric acid (HNO3). Calibration was conducted using certified multi-element standard solutions to ensure the accuracy and reproducibility of the results. The analysis focused on several key heavy metals, with detection limits ranging from 0.0005 mg/L to 0.01 mg/L. This approach enabled the sensitive and reliable assessment of trace heavy metals in the water samples.
2.4. Statistical Analysis
All experiments were conducted in triplicate, and the data obtained are expressed as the mean ± standard deviation (SD). Statistical analyses were performed using Student’s t-test for comparisons between two groups, or via one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple group comparisons. A p-value of less than 0.05 was considered statistically significant.
3. Results and Discussion
3.1. Characterization of Packaging Materials
FTIR spectroscopy was utilized to analyze the chemical makeup and potential impurities of a plastic plate and a plastic spoon (Figure 2). Both items display significant absorption bands around 2950, 2920, and 2850 cm−1, which are indicative of asymmetric and symmetric C–H stretching in aliphatic hydrocarbons, suggesting that they are primarily composed of polyolefins such as PE or PP. Nevertheless, noticeable spectral differences reveal chemical distinctions between the two plastics. The sample exhibits a distinct absorption peak near 1740 cm−1, corresponding to C=O stretching vibrations typically associated with ester or ketone groups. This indicates the presence of additives, degradation products, or potential contamination. Additional bands in the 1250–1000 cm−1 range, associated with C–O stretching, further support the presence of processing additives or surface residues. These features are commonly associated with oxidative degradation and polymer breakdown, which are early indicators of MP formation. In contrast, the plastic plate spectrum lacks these carbonyl and ester-related peaks, suggesting a simpler composition with fewer chemical alterations. These differences indicate that the spoon has undergone more significant chemical changes and may contain oxidation products, food residues, or plasticizers, making it more susceptible to additive leaching and fragmentation into MPs under environmental stress, such as heat, light, or mechanical abrasion.
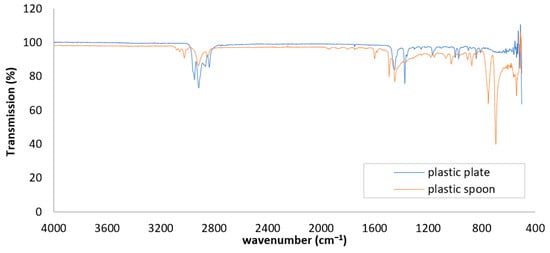
Figure 2.
The FTIR spectra of the packaging materials, specifically the plastic plates and plastic spoons.
Figure 3 displays the TGA thermograms for the plastic plate and plastic spoon, revealing their thermal degradation behaviors under a nitrogen atmosphere. Both samples exhibited a single, prominent weight loss event, corresponding to the main phase of polymer decomposition. The onset of thermal degradation for both materials began around 370 °C, consistent with the breakdown of polyolefins such as PE or PP. The plastic plate exhibited a slightly sharper and earlier degradation curve, with peak weight loss occurring just before 450 °C. In contrast, the spoon’s mass declined more gradually, suggesting the presence of enhanced thermal stability, which may be attributed to the presence of stabilizing additives. Notably, a significant difference in residual mass was observed at 700 °C. The plastic plate retained less than 2% of its original weight, whereas the spoon exhibited approximately 10% residue. This disparity suggests the presence of non-volatile inorganic fillers, pigments, or environmental contaminants in the spoon material, which may include calcium carbonate, titanium dioxide, or silicates. The higher thermal residue in the spoon also implies a more complex composition, potentially linked to manufacturing additives or post-use contamination. These thermal characteristics are essential for evaluating the materials’ behavior under high-temperature processing or disposal conditions and may also influence their propensity to generate MP residues.
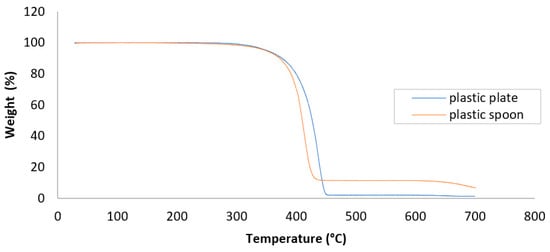
Figure 3.
TGA curves of plastic plates and plastic spoons, highlighting their thermal degradation behavior.
3.2. Heavy Metal Concentrations in Drinking Water
The levels of certain heavy metals in both bottled and tap water samples were measured using ICP-OES. Table 1 displays the concentrations (mg/L) as mean ± standard deviation. Arsenic (As) was found in bottled water (0.007 ± 0.001 mg/L) and tap water (0.003 ± 0.001 mg/L). Despite these low values, the presence of arsenic is concerning due to its well-documented carcinogenic properties. Beryllium (Be) was absent in both water types, consistent with its generally low presence in drinking water. Calcium (Ca) and magnesium (Mg), which are vital minerals contributing to water hardness, were significantly more abundant in bottled water (28.537 ± 2.153 mg/L and 19.098 ± 1.420 mg/L, respectively) compared to tap water (Ca not detected, Mg at 2.289 ± 0.112 mg/L). These minerals affect the taste of water and the likelihood of scaling in distribution systems. Chromium (Cr) appears only in bottled water (0.042 ± 0.003 mg/L), possibly due to corrosion or environmental pollution. Copper (Cu) was found solely in tap water (0.114 ± 0.008 mg/L), likely from household plumbing. Manganese (Mn) was present in bottled water (0.001 ± 0.000 mg/L) but absent in tap water. Lead (Pb) was detected in both water types (0.003 ± 0.001 mg/L in bottled and 0.004 ± 0.001 mg/L in tap), which is significant given its cumulative neurotoxic effects, even at low concentrations.

Table 1.
Mean concentrations of heavy metals in drinking water samples (mg/L).
3.3. MP Detection in Commercial Salt Samples
FTIR analysis was conducted on MP particles extracted from five different commercial salt brands to determine the polymeric makeup of the contaminants (Figure 4). The spectra displayed distinctive absorption bands that correspond to widely used synthetic polymers. The presence of PET was identified by specific peaks around 1710–1740 cm−1 (C=O stretching) and 1240–1100 cm−1 (C–O stretching). High-Density Polyethylene (HDPE) was identified by peaks at approximately 2915 and 2849 cm−1 (C–H stretching) and bending modes at 1460 and 720 cm−1. Furthermore, PP was detected, exhibiting characteristic bands near 2950, 2918, and 2838 cm−1 (C–H stretching), along with CH3 bending vibrations at 1454 and 1375 cm−1. The variation in peak intensity across the spectra indicates differences in polymer concentrations and potential compositional complexity among the brands. Overlapping absorption features in the 1000–1500 cm−1 region suggest the presence of multiple polymer types within individual samples. These results confirm that commercial salts are contaminated with MPs originating from packaging and processing materials, mainly composed of PET, HDPE, and PP.
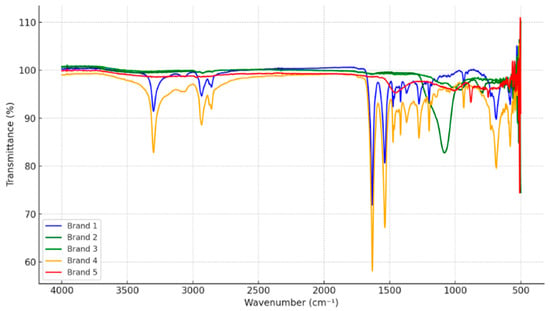
Figure 4.
Representative FTIR of MP particles isolated from commercial salt samples, showing characteristic peaks for PET, HDPE, and PP.
4. Conclusions
This study highlights the dual challenge of the presence of MP and heavy metal contamination in everyday consumables, underscoring the need for safer packaging, increased public awareness, and improved monitoring technologies. Packaging materials made from polyolefins such as PE and PP were found to undergo chemical and thermal degradation, leading to additive leaching and oxidation. These processes contribute to MP formation under environmental stress. FTIR and TGA analyses confirmed the presence of functional groups and residues indicative of early MP fragmentation, particularly in plastic spoons, which showed higher thermal residues and complex compositions. ICP-OES analysis of water samples revealed the presence of trace amounts of toxic heavy metals. Although within regulatory limits, their coexistence with MPs raises concern due to MPs’ strong adsorptive properties, potentially facilitating the transport of heavy metals into biological systems. Moreover, the presence of MPs in commercial salt products highlights the widespread nature of contamination across food chains. The consistent detection of synthetic polymers in consumer goods necessitates urgent regulatory action and enhanced waste management to mitigate pollution and human exposure. Future research should investigate the long-term health effects of chronic exposure and develop advanced filtration and degradation solutions to safeguard both ecosystem integrity and public health.
Author Contributions
Conceptualization, N.A.-Q.; methodology, M.A.-A., A.A.-A., M.K., Z.S., A.M. and M.S.; software, M.S. and N.A.-Q.; validation, M.A.-A. and N.A.-Q.; formal analysis and investigation, M.A.-A., A.A.-A., M.K., Z.S., A.M. and M.S.; resources, M.S. and N.A.-Q.; data curation, M.A.-A., A.A.-A. and M.K.; writing—original draft preparation, M.A.-A., A.A.-A., M.K., Z.S., A.M., M.S. and N.A.-Q.; writing—review and editing, M.A.-A., A.A.-A., M.K. and N.A.-Q.; visualization, N.A.-Q.; supervision, M.S. and N.A.-Q.; project administration, N.A.-Q.; funding acquisition, N.A.-Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Qatar National Research Fund (QNRF), a member of Qatar Foundation, under the Undergraduate Research Experience Program (UREP) grant number UREP31-074-1-016. Additional support was provided by Qatar University through the Student Grant Program (QUST), grant number QUST-1-CAM-2025-239. Statements made herein are solely the responsibility of the authors. Open access funding was provided by the Qatar National Library.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are not available due to privacy concerns and are part of ongoing research.
Acknowledgments
The authors would like to thank the Center for Advanced Materials (CAM) and the Central Laboratories Unit (CLU) at Qatar University for their valuable assistance in sample characterization.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fiore, L.; Serranti, S.; Bonifazi, G.; Mazziotti, C.; Benzi, M.; Riccardi, E. Classification and Distribution of Freshwater Microplastics along the Italian Po River by Hyperspectral Imaging. Environ. Sci. Pollut. Res. 2022, 29, 48588–48606. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.I.; Alena Vdovchenko, A.; Cooling, D.; Murphy, J.F.; Arnold, A.; Pretty, J.L.; Spencer, K.L.; Markus, A.A.; Vethaak, A.D.; Resmini, M. Systematic Analysis of the Relative Abundance of Polymers Occurring as Microplastics in Freshwaters and Estuaries. Int. J. Environ. Res. Public Health 2020, 17, 9304. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhai, L.; Lu, X.; Yu, X.; Feng, J.; Ma, W.; Zhu, L.; Vogt, R.D.; Wang, S. Kinetics and Size Effects on Adsorption of Cu(II), Cr(III), and Pb(II) Onto Polyethylene, Polypropylene, and Polyethylene Terephthalate Microplastic Particles. Front. Mar. Sci. 2021, 8, 785146. [Google Scholar] [CrossRef]
- Vo, H.C.; Pham, M.H. Ecotoxicological effects of microplastics on aquatic organisms: A review. Environ. Sci. Pollut. Res. 2021, 28, 44716–44725. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; An, Y.-J.; Chae, Y. Mixture Toxicity of Nickel and Microplastics with Different Functional Groups on Daphnia magna. Environ. Sci. Technol. 2017, 51, 12852–12858. [Google Scholar] [CrossRef] [PubMed]
- Birch, Q.T.; Potter, P.M.; Pinto, P.X.; Dionysiou, D.D.; Al-Abed, S.R. Sources, Transport, Measurement and Impact of Nano and Microplastics in Urban Watersheds. Rev. Environ. Sci. Bio/Technol. 2020, 19, 275–336. [Google Scholar] [CrossRef] [PubMed]
- Reza, T.; Riza, Z.H.M.; Abdullah, S.R.S.; Abu Hasan, H.; Ismail, N.‘I.; Othman, A.R. Microplastic Removal in Wastewater Treatment Plants (WWTPs) by Natural Coagulation: A Literature Review. Toxics 2023, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Omidoyin, K.C.; Jho, E.H. Effect of Microplastics on Soil Microbial Community and Microbial Degradation of Microplastics in Soil: A Review. Environ. Eng. Res. 2023, 28, 220716. [Google Scholar] [CrossRef]
- Patil, P.B.; Sarkar, A.; Maity, S. Potential Human Health Risk Assessment of Microplastic Exposure: Current Scenario and Future Perspectives. Environ. Monit. Assess. 2022, 194, 898. [Google Scholar] [CrossRef] [PubMed]
- Saha, G.; Saha, S.C. Tiny Particles, Big Problems: The Threat of Microplastics to Marine Life and Human Health. Processes 2024, 12, 1401. [Google Scholar] [CrossRef]
- Yahya, H.; Zanuari, F.I.; Karim, S.N.; Yahya, H.N.; Syed Abd Halim, S.A.S.; Yahaya, N. Occurrence and Pathways of Microplastics, Quantification Protocol and Adverse Effects of Microplastics towards Freshwater and Seawater Biota. Food Res. 2023, 7, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.-S.; Lee, Y.-C.; Walther, B.A. Microplastic Contamination of Three Commonly Consumed Seafood Species from Taiwan: A Pilot Study. Sustainability 2020, 12, 9543. [Google Scholar] [CrossRef]
- Aydın, R.B.; Yozukmaz, A.; Giannetto, D.; Şener, İ.; Temiz, F. Occurrence of Microplastics in Most Consumed Fruits and Vegetables from Turkey and Public Risk Assessment for Consumers. Life 2023, 13, 1686. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, G.; Fu, J.; Dai, X.; Peng, L. A Microscopic Survey on Microplastics in Beverages: The Case of Beer, Mineral Water and Tea. Analyst 2022, 147, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Sathish, M.N.; Patterson, J.; Jeyasanta, I. Microplastics in Salt of Tuticorin, Southeast Coast of India. Arch. Environ. Contam. Toxicol. 2020, 79, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Gaston, E.; Anderson, S.; Steele, C.; Sukumaran, S.; Woo, M. Microplastics Differ Between Indoor and Outdoor Air Masses: Insights from Multiple Microscopy Methodologies. Appl. Spectrosc. 2020, 74, 1079–1098. [Google Scholar] [CrossRef] [PubMed]
- Limsiriwong, K.; Winijkul, E. Exploring Personal Exposure to Airborne Microplastics across Various Work Environments in Pathum Thani Province, Thailand. Int. J. Environ. Res. Public Health 2023, 20, 7162. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Wang, W.-X. Human Exposure to Microplastics and Its Associated Health Risks. Environ. Health 2023, 1, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Urrutia-Pereira, M.; Solé, D.; Guidos-Fogelbach, G.; Chong-Neto, H.J. Microplastics exposure and immunologic response. Allergol. Et Immunopathol. 2023, 51, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Nong, D.-Q.; Xiang, P.; Peng, W.-G.; Bao, Y.-B. Human Accumulation and Toxic Effects of Microplastics: A Critical Review. Huan Jing Ke Xue = Huanjing Kexue 2024, 45, 1173–1184. [Google Scholar] [CrossRef]
- Guo, X.; Xu, S.; Lin, H.; He, L. Recent Advances in Spectroscopic Techniques for the Analysis of Microplastics in Food. J. Agric. Food Chem. 2022, 70, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Han, I.; Lee, C.; Shipper, A.G.; Wiens, K.E.; Belchez, C. Microplastics in Urban Ambient Air: A Rapid Review of Active Sampling and Analytical Methods for Human Risk Assessment. Environments 2024, 11, 256. [Google Scholar] [CrossRef]
- Zandieh, M.; Liu, J.; Van Cappellen, P.; Rezanezhad, F.; Waldie, A.; Li, S.; Honek, J.; Griffiths, E. Catalytic and Biocatalytic Degradation of Microplastics. Exploration 2023, 4, 20230018. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Alcaide, M.F.; Iqbal, H.M.N.; Godínez-Alemán, J.A.; Parra-Saldívar, R.; Reyna Berenice González-González, R.B. Environmental Impact and Mitigation of Micro (Nano)Plastics Pollution Using Green Catalytic Tools and Green Analytical Methods. Green Anal. Chem. 2022, 3, 100031. [Google Scholar] [CrossRef]
- Jenkins, T.; Persaud, B.D.; Cowger, W.; Szigeti, K.; Roche, D.G.; Clary, E.; Slowinski, S.; Lei, B.; Abeynayaka, A.; Nyadjro, E.S.; et al. Current State of Microplastic Pollution Research Data: Trends in Availability and Sources of Open Data. Front. Environ. Sci. 2022, 10, 912107. [Google Scholar] [CrossRef]
- Gewert, B.; Macleod, M.; Plassmann, M.M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Kadac-Czapska, K.; Knez, E.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Grembecka, M. Microplastics Derived from Food Packaging Waste-Their Origin and Health Risks. Materials 2023, 16, 674. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).