Abstract
The application of H2 to pre-reduce manganese ores is a sustainable approach to performing decarbonization in the ferroalloy industry. The process has been extensively studied and tested in a lab-to-pilot scale in the HAlMan EU project. This work presents the results of an experimental study that was conducted in a lab-scale vertical thermogravimetric furnace for the pre-reduction of a manganese ore by H2 under isothermal conditions at 500 °C, 600 °C, 700 °C, and 800 °C. The ore and reduced samples were characterized by XRF, XRD, BET and SEM techniques to outline the H2 reduction behavior of the ore from mineralogical, microstructural, and chemical points of view. The rate and extent of reduction were studied using the continuous mass changes during the reduction. It was found that the pre-reduction at a temperature of 700 °C and 800 °C yields metallic iron formation from Fe2O3 and MnO formation from MnO2/Mn2O3. The pre-reduction at lower temperatures did not show a complete reduction in Fe and MnO. The pore structure of the ore was affected by the pre-reduction temperature, and a significant porosity evolution was observed.
1. Introduction
According to the Paris agreement, 45% of CO2 emissions should be reduced by 2030 and net zero should be achieved by 2050 []. To achieve this, the roadmap of the Norwegian process industries presents the vision of “Combining growth and zero emissions by 2050” []. To approach the vision, development of technologies that have a potential to reduce the carbon footprint from the ferro-alloy process industry is needed. The commercial production of HCFeMn is through the carbothermic reduction in manganese ores, with more than 90% of their production primarily being produced in a submerged arc furnace (SAF). The SAF is charged with a constant supply of raw materials such as manganese ore, sinter, metallurgical coke, and flux blended in predetermined ratios. Metallurgical coke is the most common source of reductant and typical fluxes added to adjust the composition of the slag are dolomite and limestone []. The production of HCFeMn through a carbothermic process in the SAF is accompanied by a significant CO2 emission of 1–1.4 tCO2/t metal. On the other hand, the SAF process is an energy-intensive process, and 2000–3000 kWh of energy is utilized per ton of metal produced []. Therefore, the development of new sustainable Mn production processes is important to reduce greenhouse gas emissions in the future.
The rate of H2 reduction in Mn ores and oxides has been studied in the past, and the use of H2 and CO gas has been investigated by many researchers with varying reduction temperatures, reduction times and particle sizes [,,,,,,,]. The effect of the temperature, gas composition, Mn ore composition, and particle size has been investigated. The particle size was not found to affect the time conversion of the ore into MnO in the study conducted by De Bruijn et. al. where the sizing of particles was between 0.0675 and 0.1275 mm []. It has been found that the reduction using H2 and CO gases without a solid carbon source reduces Mn ores to MnO [,], and that H2 increases the rate of reduction []. Barner and Mantell investigated the reduction in synthetic MnO2 at different H2 partial pressures at 200–500 °C. The samples investigated were particles from 0.07 mm to 0.21 mm and compressed pellets weighing 1.5 g []. Ostrovski et al. found that the reduction in pure MnO2 in powder form starts at 305–320 °C in an Ar-H2 atmosphere. In the temperature interval studied, 200–900 °C, the complete reduction in MnO2 to MnO took place at 610–620 °C with no further reaction at higher temperatures []. The effect of H2 gas on the reduction rate was also seen by Schanche and Tangstad, where the pre-reduction of Nchwaning ore was conducted in CO/CO2/H2 gas mixtures at 600–800 °C. They found that Fe oxides reduced more slowly than manganese oxides. Mn2O3 was reduced to MnO, and Mn3O4 was not seen as a stable intermediate phase []. Cheraghi et al. studied the calcination and reduction of a low-grade manganese ore by methane gas; they found that the onset temperatures of thermal decompositions of pyrolusite and calcite are 868 K and 1056 K, respectively []. The use of H2 as a reduction agent for low-grade Mn ore was investigated by El-Gawad et al. at 800–950 °C. They found that an increase in H2 partial pressure and temperature correlated with an increase in the reduction rate. After pre-reduction at 950 °C, metallic Fe, MnO, and Mn3O4 were present []. The presence of an H2 gas mixture increased the reduction rate of Fe oxides, also found in other studies [,]. Ahamed et al. investigated the pre-reduction of the Bahariya high manganese iron ore by H2 at 800–1000 °C. They showed that the iron oxide was reduced by H2 to metallic iron, while the manganese oxides were reduced to MnO. The iron content in the reduced product increased with the increasing temperature. The apparent activation energy values were computed, leading to the conclusion that the interfacial chemical reaction controlled the reaction at the early stages, while the solid-state diffusion contributed to the interfacial chemical mechanism at the intermediate stages. At the final stages, the solid-state diffusion is the rate-determining step in the reduction process [].
The present work is based on the idea of using H2 to reduce Mn oxides to MnO in a pre-reduction step in which the chemical reactions (1) to (3) occur. Then, the aluminothermic reduction of the pre-reduced ore (or MnO-containing material) is conducted to produce Mn metal, aluminum-manganese alloys (AlMn) and ferromanganese alloys through the reaction (4) [].
2MnO2(s) + H2(g) = Mn2O3(s) + H2O(g) ΔH(25 °C) = −163.7 kJ/mol
3Mn2O3(s) + H2(g) = 2Mn3O4(s) + H2O(g) ΔH(25 °C) = −135.1 kJ/mol
Mn3O4(s) + H2(g) = 3MnO(s) + H2O(g) ΔH(25 °C) = −16.6 kJ/mol
3MnO(s) + 2Al(s) = 3Mn(l) + Al2O3(l) ΔH(25 °C) = −520 kJ/mol
2. Experimental Procedure
2.1. Methodology
The experimental procedure will be carried out by applying characterization techniques (XRD, XRF, SEM, Pycnometry, BET surface area analysis) to the materials and later H2 gas will be used for the isothermal reduction in different type of manganese ores, and the pre-reduced samples will be characterized afterwards. The method approach is summarized in Figure 1.
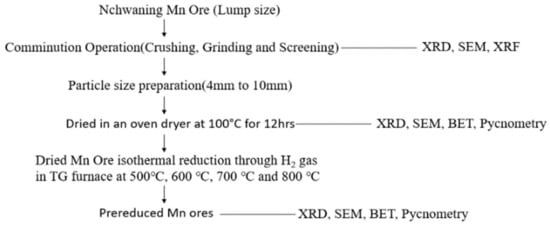
Figure 1.
Flowsheet of the process.
2.2. Materials
In this research work, Nchwaning Mn ore, provided by a partner of the HAlMan project, was used. The lump ore was crushed and screened to prepare the particle size 4 mm to 10 mm, which was further taken for the reduction study due to the TG furnace size requirements. And later, the prepared particles of 4 mm to 10 mm size were dried in an oven dryer at 100 °C for 12 h.
2.3. H2 Reduction
Heating was carried out in an argon atmosphere with a flow rate of 2 NL/min up to the targeted temperature at a heating rate of 10 °C/min and thereafter held at the targeted temperature for 10 min to obtain a uniform temperature distribution. H2 was introduced with a fixed flow rate of 4 NL/min and a constant reduction time (120 min). For all the reduction experiments, the reduction time was set to 120 min and the reduction temperatures were between 500 °C and 800 °C with 100 °C intervals. Cooling was carried out under the argon flow (2 NL/min) to prevent manganese reoxidation upon cooling. For each test, 150 g of the dried Nchwaning manganese ore sample was taken.
2.4. Methods for Characterization
Mineralogical phase analysis of the raw and pre-reduced materials was conducted with the X-ray diffraction (XRD) technique using the Bruker D8 A25 DaVinciTM, Karlsruhe, Germany, with CuKα radiation (wavelength λ of 1.54 Å), a 2θ range from 10° to 80° and a step size of 0.03°. Qualitative results were analyzed using the EVA diffraction software of version 6.0 (DIFFRAC.EVA V6.0) with a crystallographic database. Elemental analysis of raw ore was performed using the X-ray fluorescence (XRF) technique with a device from Thermo Fisher, Nemko Norlab AS, Norway. Sample preparation of testing in XRF was performed by using the flux fusion method. Microstructural examination of the products was performed via a field emission scanning electron microscope (Zeiss Ultra FESEM) equipped with a XFlash® 4010 (Bruker, Hamburg, Germany) Energy-Dispersive X-Ray Spectroscopy (EDS) detector (Bruker, Hamburg, Germany). The density of the raw ore and pre-reduced samples was measured using a Pycnometer, provided by Micromeritics (Norcross, GA, USA) named as Accupyc 1350, with helium gas of 99.99% purity. The BET surface area was measured using a 3Flex 3500 physisorption analyzer of micromeritics. Before the BET area measurement, the sample was degassed at 250 °C for 20 h to remove the moisture content of the dried and pre-reduced sample before the BET analysis.
3. Results
3.1. Chemical Analysis of the Raw Ore
The results of the XRF analysis provided by the Nemko Norlab AS for raw ore are listed in Table 1.

Table 1.
XRF analysis of dried raw Nchwaning manganese ore.
3.2. Microstructure and Minerological Characterization of the Raw Ore
The dried ore was analyzed in SEM to obtain information about the microstructure, which is shown in Figure 2. The identified minerals in the SEM image are based on the energy dispersive spectroscopy (EDS) point analysis and the ore XRD analysis results.
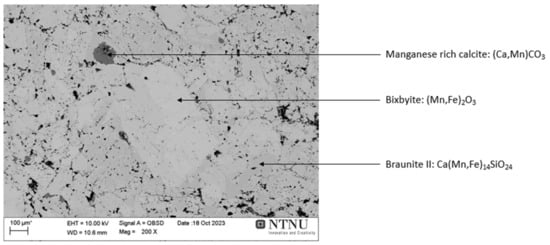
Figure 2.
Microstructure of Nchwaning manganese ore (4–10 mm), backscattered electrons imaging. X: magnification.
The result of the XRD analysis of the dried ore is shown in Figure 3 with the identified minerals, which shows six manganese-holding minerals as well as calcite, diopside, calcium silicate, quartz and hematite.
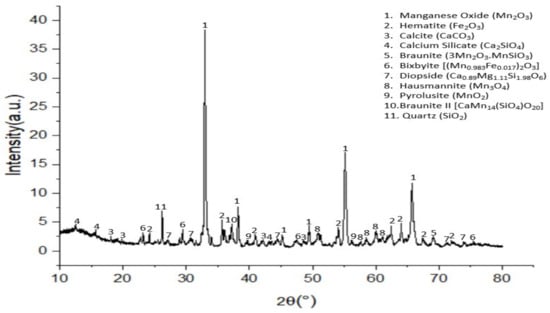
Figure 3.
X-Ray diffraction spectrum of Nchwaning manganese ore.
3.3. Physical Properties
3.3.1. Density Measurement
The true density of the dried raw ore sample and pre-reduced samples of different reduction temperatures (500 °C, 600 °C, 700 °C and 800 °C) were measured as shown in Table 2.

Table 2.
True density of the raw ore and pre-reduced samples.
3.3.2. Porosity and Surface Area Measurement
The pore volume and BET surface area measurement was carried out for the dried raw ore sample and the pre-reduced samples at different reduction temperatures (500 °C, 600 °C, 700 °C and 800 °C), and the results are shown in Table 3.

Table 3.
BET surface area and pore volume measurements for raw ore and pre-reduced samples.
3.4. Pre-Reduction Behavior
Figure 4 shows the pre-reduction behavior of dried Nchwaning manganese ore at 500 °C, 600 °C, 700 °C, and 800 °C at a fixed reduction time of 120 min for a 150 g sample weight.
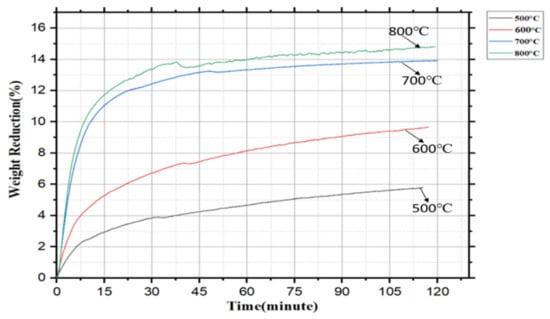
Figure 4.
Weight reduction (%) vs. time (min) plot of pre-reduced samples.
3.5. Microstructure and Phase Analysis of Pre-Reduced Samples
One pre-reduced sample of each temperature was studied by SEM through backscattered electron imaging. An overview picture of each temperature at 500× magnification is shown in Figure 5.
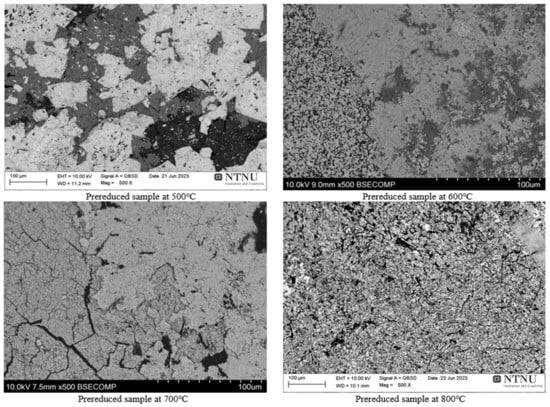
Figure 5.
Backscattered electron SEM images of pre-reduced Nchwaning manganese ore at different temperatures. X: magnification.
Figure 6 shows the XRD pattern of the pre-reduced samples reduced at different reduction temperatures of 500 °C, 600 °C, 700 °C, and 800 °C.
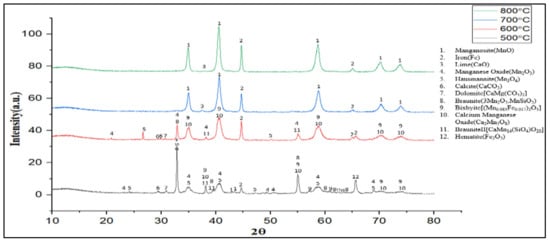
Figure 6.
XRD spectra of the pre-reduced samples reduced at different temperatures with the identified phases.
4. Discussion
4.1. Physical Properties
Table 2 shows that the true density values of the pre-reduced samples increased by increasing the pre-reduction temperature compared with the raw ore, and the highest density was found for the pre-reduced sample of 800 °C. It was also found that the BET surface area is increased with an increasing reduction temperature as compared with the raw ore up to a certain temperature of 700 °C, but then it decreased at 800 °C. The lower BET surface area for 800 °C may be due to sintering and hence porosity loss that is confirmed by the total porosity measurements (Table 3) and the SEM study (Figure 5).
4.2. Phase Evolution and Microstructural Analysis
The raw Nchwaning ore consists of six manganese-holding minerals [braunite, braunite II, pyrolusite (MnO2) manganese oxide (Mn2O3), hausmannite (Mn3O4) and bixbyite] as well as calcite, diopside, calcium silicate, and hematite (Figure 3).
The XRD analysis of the pre-reduced samples at 700 °C and 800 °C shows mainly manganosite (MnO) and iron (Fe). From the XRD spectra, it was found that manganese-holding minerals are converted to manganosite (MnO) and that hematite (Fe2O3) is converted to iron (Fe) at those temperatures through the following overall reactions regarding the ore mineralogy (Figure 6):
Mn2O3 + H2 = 2MnO + H2O
Fe2O3 + 3H2 = 2Fe + 3H2O
The SEM micrographs show that above 700 °C indicates sintering in the ore, yielding a pre-reduced ore with a lower porosity (Figure 5).
4.3. Pre-Reduction Behavior
Considering Figure 4 data, the highest weight reduction found 14.80% for the pre-reduced sample at 800 °C whereas at 700 °C it found it to be 13.90%. The weight reduction values were lower at 500 °C and 600 °C, respectively. Clearly, the reduction was not completed at these temperatures with regards to the lower BET surface area (Table 3), and an incomplete reduction in the Mn oxides (Figure 6).
5. Conclusions
The H2 reduction in dried Nchwaning manganese ore was studied using thermogravimetry methods under isothermal conditions at different reduction temperatures from 500 °C to 800 °C with a fixed reduction time of 120 min. The main conclusions of this work are summarized as follows:
- Nchwaning ore is dense and has Mn and Fe oxides in the form of Mn2O3 and Fe2O3, and it has a low porosity (0.0013 cm3/g) and BET surface area (0.49 m2/g).
- The kinetics of pre-reduction of the ore by H2 are affected by the temperature and higher temperatures show a faster and higher extent of reduction.
- Pre-reduction by H2 at temperatures of 700 °C and 800 °C within two hours showed a complete reduction and yields metallic Fe and MnO from Mn2O3 and Fe2O3 in the ore.
- The pore volume and structure of the ore are affected by the pre-reduction temperature. In addition, the BET surface area and pore volume decreased at temperatures above 700 °C, and the lowest values were obtained at 800 °C.
- Microscopic examination indicated that above 700 °C sintering in the ore occurs, yielding a pre-reduced ore with a lower porosity and higher density.
Author Contributions
Conceptualization, A.S. and T.L.S.; Methodology, A.S., T.L.S. and J.S.; Investigation, A.S. and T.L.S.; Validation, A.S., T.L.S. and J.S.; Data collection, A.S. and T.L.S.; Writing—original draft preparation, A.S. and T.L.S.; Review and editing, A.S., T.L.S. and J.S.; Visualization, A.S., T.L.S. and J.S.; Supervision, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was performed and financially supported by the European Union’s Horizon Europe program HAlMan project under the grant number of 101091936.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paris Agreement. 2015. Available online: https://www.un.org/en/climatechange/net-zero-coalition (accessed on 13 February 2023).
- The Norwegian Process Industries’ Roadmap. Norsk Industri. 2016. Available online: https://www.norskindustri.no/siteassets/dokumenter/rapporter-ogbrosjyrer/the-norwegian-process-industries-roadmap-summary.pdf (accessed on 20 June 2019).
- Olsen, S.E.; Tangstad, M.; Lindstad, T. Production of Manganese Ferroalloys; Tapir Academic Press: Trondheim, Norway, 2007. [Google Scholar]
- Safarian, J. Duplex Process to Produce Ferromanganese and Direct Reduced Iron by Natural Gas. ACS Sustain. Chem. Eng. 2021, 9, 5010–5026. [Google Scholar] [CrossRef]
- De Bruijn, J.; Soerawidjaja, T.; De Jongt, W. Modelling of the reduction of manganese oxides with H2. Chem. Eng. Sci. 1980, 35, 1591–1599. [Google Scholar] [CrossRef]
- Akdogan, G.E.R. Kinetics of the solid-state carbothermic reduction of wessel manganese ores. Metall. Mater. Trans. 1995, 26, 13–24. [Google Scholar] [CrossRef]
- Ngoy, D.; Sukhomlinov, D.; Tangstad, M. Pre-reduction Behaviour of Manganese Ores in H2 and CO Containing Gases. ISIJ Int. 2020, 60, 2325–2331. [Google Scholar] [CrossRef]
- Barner, H.E.; Mantell, C.L. Kinetics of H2 reduction of manganese dioxide. Ind. Eng. Chem. Process Des. Dev. 1968, 7, 285–294. [Google Scholar] [CrossRef]
- Kononov, R.; Ostrovski, O.; Ganguly, S. Proceedings of the 11th International Ferroalloys Congress, New Delhi, India, 18–21 February 2007; Indian Ferro Alloy Producers’ Association (IFAPA): Mumbai, India, 2007. [Google Scholar]
- Schanche, T.; Tangstad, M. Prereduction of Nchwaning Ore in CO/CO2/H2 Gas Mixtures. Minerals 2021, 11, 1097. [Google Scholar] [CrossRef]
- Cheraghi, A.; Yoozbashizadeh, H.; Ringdalen, E.; Safarian, J. Kinetics and Mechanism of Low-Grade Manganese Ore Reduction by Natural Gas. Metall. Mater. Trans. B 2019, 50, 1566–1580. [Google Scholar] [CrossRef]
- El-Gawad, H.H.A.; Ahmed, M.; El-Hussiny, N.A.; Shalabi, M.E.H. Reduction of Low-Grade Egyptian Manganese Ore via H2 at 800 °C–950 °C. Open Access Libr. J. 2014, 1, 1–11. [Google Scholar]
- Pineau, A.; Kanari, N.; Gaballah, I.I. Kinetics of Reduction of Iron Oxides by H2: Part I: Low Temperature Reduction of Hematite. Thermochim. Acta 2006, 447, 89–100. [Google Scholar] [CrossRef]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of Reduction of Iron Oxides by H2: Part II. Low Temperature Reduction of Magnetite. Thermochim. 2007, 456, 75–88. [Google Scholar] [CrossRef]
- Ahmed, A.M.; El-Geassy, A.A.; Misherky, M.L. Crude steel directly from pre-reduced high manganese containing iron ore. Ironmak. Steelmak. Process. Prod. Appl. 2015, 42, 161–168. [Google Scholar] [CrossRef]
- Safarian, J. A Sustainable Process to Produce Manganese and Its Alloys through H2 and Aluminothermic Reduction. Processes 2022, 10, 27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).