Abstract
Zinc oxide nanostructures have real potential in different scientific fields. In the case of these nanostructures, it was found that the morphology of ZnO plays an essential role in developing further applications, but it is necessary a rigorous control of the main factors which influence the size, shape, agglomeration tendency, uniformity, and orientation of the nanostructures. In the present paper, our efforts are oriented to synthesize different types of ZnO nanostructures by chemical method, and optimization was achieved with varying parameters, such as the concentration of precursors, types of solvents, pH, time, or temperature, as well as the parameters required for thermal treatment. To obtain the characteristic structural and morphological information, ZnO nanostructures were investigated using Fourier transform infrared spectrometry (FTIR), scanning electron microscopy (SEM), and X-ray diffraction (XRD). SEM analysis confirms that the morphology and size of the ZnO nanostructures depend on the process parameters. The XRD results reveal that the synthesized samples have a wurtzite crystalline structure, and FTIR spectra show the presence of Zn-O bonding. The wetting capacity of continuous ZnO surfaces with different morphologies was studied by measuring the contact angle, indicating that the wetting and percolation capacity, depend on the orientation of the synthesized nanostructures.
1. Introduction
Current efforts in the field of nanotechnology have led to the orientation of research towards transition metal oxide nanostructures, due to their characteristics, such as composition, size, shape, high surface-to-volume ratio, thermal and chemical stability, low toxicity, and the ability to be modified with specific sensitive elements [1].
Among transition oxides, zinc oxide is a versatile material with unique properties due to its advantages, such as special properties, cost efficiency, low toxicity, good biocompatibility and biodegradability, adjustable band-gap, different shapes, and a broad size distribution range, many other features, making it applicable in a wide range of scientific fields (e.g., optoelectronic devices, textile industry, food packaging, luminescent materials, drug delivery, bioimaging, medical device, cancer diagnostics, agriculture, cosmetic, etc.). Additionally, it is well known that the morphological diversity and particle size distribution of the nanoparticles have a great influence on properties and technological applications, and therefore, rigorous control over the process parameters for the synthesis of ZnO particles, become necessary [2,3].
Depending on the approached method, chemical intermediates, and the conditions involved in the synthesis process, ZnO nanostructures can be obtained in different shapes and sizes, which determines various physico-chemical properties. The synthesis methods for ZnO nanostructures have been widely developed in recent years, through the sol-gel method, direct precipitation, solid-state method, mechano-chemical process, hydrothermal and solvothermal techniques, thermal decomposition of organic precursor, biological approach, etc. [4,5,6].
By using known methods, ZnO can be synthesized as nanoparticles, nanoflowers, nanorods, nanowires, nanobelts, nanorings, nanotubes, nanoplates, or quantum dots and its oxide provides one of the greatest selections of varied structures with special properties among all known materials. Therefore, many researchers have focused on investigating the main factors, which influence the particle size, morphology, phase, and surface area of the ZnO. Among the factors that influence the final structure can be mentioned: the type of raw materials (zinc acetate dehydrate (zinc acetate dehydrate (Zn(C2H3O2)2·2H2O)), zinc nitrate hexahydrate (Zn(NO3)2·6H2O), zinc sulfate heptahydrate (Zn(SO4)2·7H2O), zinc chloride (ZnCl2)), the concentration of raw materials, molar ratio, pH of the reaction mixture, time and temperature reaction, types of solvent, nature of additives dopants, capping agents, or other parameters used during the steps of thermal treatment, etc. The use of varying precursors implies the development of materials with different morphological, textural, and optical properties. The pH value has a significant influence on the properties, morphology, and crystallite size of the ZnO. Capping agents and surfactants are responsible for the control of growth rate, particle size, and prevention of particle aggregation. Moreover, sintering temperature also significantly influences the morphology, structures, and photoluminescence properties of ZnO nanostructures [7,8,9,10,11,12].
In the present paper, we obtained ZnO nanostructures by the sonochemical method, using different zinc precursors (such as zinc acetate dihydrate and zinc sulfate heptahydrate), and establish the optimum thermal treatment temperature at 550 °C. The synthesized ZnO nanostructures have been investigated by using Fourier transform infrared spectrometry (FTIR), scanning electron microscopy (SEM), and X-ray diffraction (XRD). The wetting and percolation capacity of ZnO surfaces with different morphologies was studied by measuring the contact angle. Therefore, with the final goal to tailor the properties of ZnO structures according to a specific application, it is necessary to understand the steps and the parameters of the process, and the relationship between the physico-chemical properties of the synthesized materials.
2. Experimental Detail
2.1. Synthesis of ZnO Nanostructures
For the synthesis of ZnO, zinc salt, such as Zinc acetate dihydrate [Zn(CH3COO2)2 2H2O] and zinc sulfate heptahydrate [ZnSO4 7H2O], was used as the source for Zn2+ cations, and sodium hydroxide [NaOH] as precipitator material. Sodium hydroxide solution [NaOH] (0.5 M) was added to the aqueous solution of zinc acetate [Zn(CH3COO2)2 2H2O] (0.05 M), respectively the solution of zinc sulfate heptahydrate [ZnSO4 7H2O] (0.05 M), drop by drop under continuous stirring, until the formation of white precipitates. The vessels with the formed precipitations were sealed and left under magnetic stirring for 2 h; then, the solutions were ultrasonication for 1 h, at a temperature of 40 °C, with a frequency of 45 kHz. After ultrasonication, the formed precipitates were filtered and washed with deionized water and ethanol at least three times to remove impurities and other unreacted compounds. The samples were left overnight in the desiccator, under vacuum, followed by heat treatment at 550 °C, with an oven heating rate of 7 °C/min and maintained for 3 h.
2.2. Characterization
The ZnO samples were characterized by Fourier transform infrared spectroscopy (FTIR) using a Tensor 27 FTIR spectrometer (Bruker Optics, Germany), in the spectral range 4000–370 cm−1, by averaging 64 scans and with a resolution of 4 cm−1 at room temperature, using an ATR platinum holder.
The morphology and particle size of the ZnO samples were investigated by a field emission scanning electron microscope (FEI Company, Hillsboro, OR, USA) with an operating voltage of 10 kV. The as-synthesized ZnO samples were analyzed using a Rigaku Smartlab diffractometer, operating at 75 mA and 40 kV with Cu-Kα radiation. The diffraction spectra were recorded 2θ between 20° and 90°.
Contact angles were estimated with a goniometer (Theta Optical Tensiometer, KSV Instruments, Finland) equipped with the CAM 101 camera, light source, lens, and the 1394 firewire interface for fast image acquisition. The mean contact angle was determined with the help of Attension Theta software, using polar liquid water with the volume of the drop varied between 1 and 1.5 μL.
3. Results and Discussion
3.1. FTIR Analysis
Figure 1 shows the comparative ATR-FTIR spectra drawn for ZnO nanoparticles obtained from precursors of (a) acetate and (b) sulfate (Figure 1A), but also the detail of the main band’s characteristic of the Zn-O bond (Figure 1B). The spectra of the oxide are characterized by absorption bands of high intensity below 500 cm−1. The absorption bands centered at about 385 cm−1 can be attributed to the vibrational mode of the Zn-O bond in the wurtzite structure of ZnO. In the case of the obtained oxide from the sulfate precursor, the existence of the second peak at 410 nm, associated with the Zn-O bond, indicates the coexistence of an oxide with different morphologies.
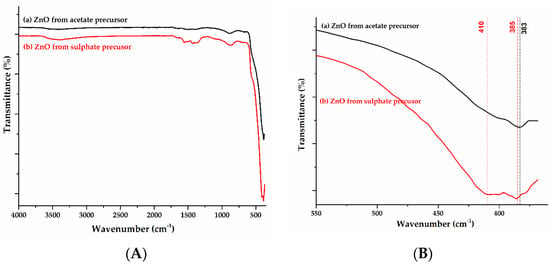
Figure 1.
ATR-FTIR spectra for ZnO samples obtained from (a) zinc acetate; (b) zinc sulfate (A); detail of Zn-O bands (B).
3.2. SEM Analysis
Figure 2 shows the morphology of ZnO samples obtained from (A) zinc acetate and (B) zinc sulfate. From the examination of the SEM micrograph in Figure 2A it can be seen that the particles have spherical formations, with a slight tendency to agglomerate, and with sizes varying between 25–70 nm. Figure 2B of the ZnO sample obtained from zinc sulfate shows irregular morphologies with different shapes, spherical nanoparticles with a tendency to aggregate, but also of the type of sheets/plates of various sizes maintained in the nanometric range. As can be seen, the morphology of the samples depends strongly on the nature of the used precursors.
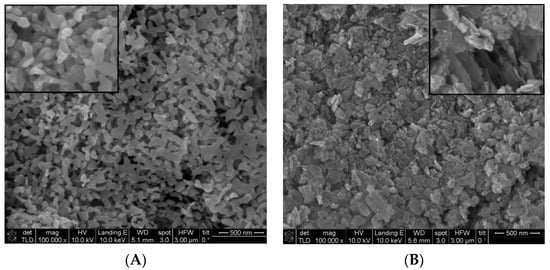
Figure 2.
SEM images for ZnO samples obtained from zinc acetate (A); zinc sulfate (B).
3.3. XRD Analysis
The XRD patterns of the ZnO samples synthesized using the two precursors (zinc acetate and zinc sulfate) are shown in Figure 3 (a and b). For both samples, it was found that the diffraction peaks correspond to the wurtzite phase of ZnO, which belongs to the space group P63mc, according to the International Center for Diffraction Data (ICDD), card no. 36-1451 and other data that exist in the literature [12].
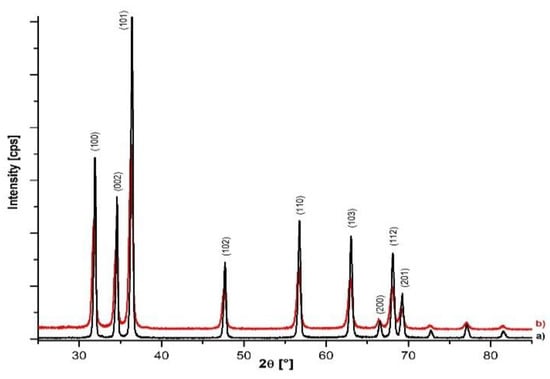
Figure 3.
XRD diffraction patterns for ZnO samples obtained from (a) zinc acetate; (b) zinc sulfate.
No peaks characteristic of other phases of ZnO or corresponding to any impurity were detected, which confirmed the high purity of the synthesized samples. The sharp and narrow diffraction peaks revealed the high crystallinity of the ZnO nanostructures. Based on the diffraction peak positions and their full width at half maximum (FWHM) the values of the unit cell (a and c) and the mean crystallite size were calculated (Table 1). From the analysis of the parameters, it was found that the value of the lattice constant shows a negligible variation, and the average size of the crystallites calculated using the Debye–Scherer equation varies between 16–32 nm, depending on the type of precursor used.

Table 1.
XRD data analysis and crystallite size of ZnO samples obtained from different zinc precursors.
3.4. Wetting Capacity (Contact Angle)
Water contact angle (WCA) measurements were performed to investigate the surface wettability of ZnO nanostructures. Figure 4 shows the value of the contact angle (WCA) of the water drop in contact with the surface of the ZnO nanostructures deposited on the Si substrate, observing a hydrophilic character regardless of the type of precursor used in the synthesis. The contact angle for the ZnO samples indicates high hydrophilicity with a value of 52° (in the case of using zinc acetate) and 82° (in the case of using zinc sulfate). The decrease in the hydrophilic character of the ZnO obtained from sulfate can be associated with the existence of sheets/plates. The surface of nanostructured ZnO exhibits hydrophilic wetting behavior and good percolation capacity, which can be attributed to the surface morphology, size, and structure form.
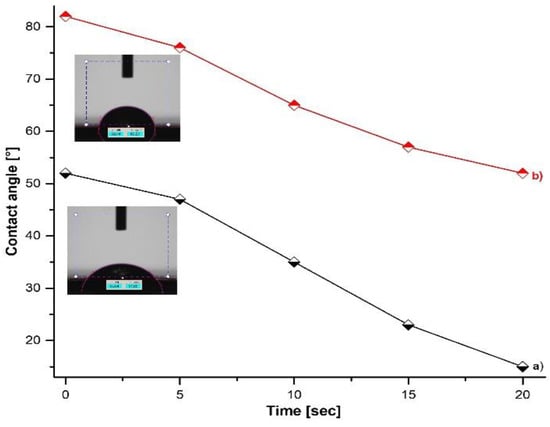
Figure 4.
The variation of contact angle depends on time of the contact of the water droplet with surface of ZnO samples obtained from (a) zinc acetate; (b) zinc sulfate.
4. Conclusions
ZnO nanostructures with two types of zinc precursors have been synthesized by the sonochemical method. The FTIR spectra of ZnO nanostructures showed the characteristic absorption of the Zn–O bond, regardless of the type of precursor used in their synthesis. The FESEM morphology of the analyzed samples indicates the formation of some spherical particles when using acetate and the coexistence of different morphologies for sulfate oxide, with a tendency to agglomerate and keep the dimensions in the nanometric range. The XRD results show that the synthesized powders possess crystalline, wurtzite hexagonal phases of ZnO for both samples, and the crystallite size is strongly influenced by morphology, and, respectively by the type of precursor used. According to the water contact angle measurements, ZnO nanostructures exhibit hydrophilic wetting character and good percolation properties, better for ZnO synthesized from an acetate precursor than sulfate. Based on the obtained results, ZnO could be considered a functional material with applicability in multidisciplinary fields.
Author Contributions
A.M. conceived, planned, carried out the experiments for the synthesis of ZnO nanostructures; FTIR characterization, V.T.; XRD characterization, C.R.; SEM characterization, O.B.; wettability studies, A.M.; writing—original draft preparation, A.M.; writing—review and editing, A.M., V.T., C.R. and O.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Core Program within the National Research Development and Innovation Plan 2022–2027, carried out with the support of MCID, project no. 2307 (µNanoEl).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Core Program within the National Research Development and Innovation Plan 2022–2027, carried out with the support of MCID, project no. 2307 (µNanoEl).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hedlund Orbeck, J.K.; Hamers, R.J. Surface properties and interactions of transition metal oxide nanoparticles: A perspective on sustainability. J. Vac. Sci. Technol. 2020, 38, 031001. [Google Scholar] [CrossRef]
- Borysiewicz, M.A. ZnO as a Functional Material, a Review. Crystal 2019, 9, 505. [Google Scholar] [CrossRef]
- Matei, A.; Tucureanu, V.; Dumitrescu, L. Aspects regarding synthesis and applications of ZnO nanomaterials. Bull. Transilv. Univ. Brasov. Ser. I Eng. Sci. 2014, 7, 45–52. [Google Scholar]
- Raha, S.; Ahmaruzzaman, M. ZnO nanostructured materials and their potential applications: Progress, challenges and perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pi, J.; Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Matei, A.; Dumitrescu, L.; Cernica, I.; Tucureanu, V.; Mihalache, I.; Bita, B.; Danila, M.; Manciulea, I. Study of the influence of capping agents on the structural and optical properties of ZnO nanostructures. J. Optoelectron. Adv. Mater. 2015, 17, 952–957. [Google Scholar]
- Mousavi, S.M.; Behbudi, G.; Gholami, A.; Hashemi, S.A.; Nejad, Z.M.; Bahrani, S.; Chiang, W.H.; Wei, L.C.; Omidifar, N. Shape-controlled synthesis of zinc nanostructures mediating macromolecules for biomedical applications. Biomater. Res. 2022, 26, 4. [Google Scholar] [CrossRef] [PubMed]
- Gatou, M.A.; Lagopati, N.; Vagena, I.A.; Gazouli, M.; Pavlatou, E.A. ZnO Nanoparticles from Different Precursors and Their Photocatalytic Potential for Biomedical Use. Nanomaterials 2023, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Shaba, E.Y.; Jacob, J.O.; Tijani, J.O.; Suleiman, M.A.T. Critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment. Appl. Water Sci. 2021, 11, 48. [Google Scholar] [CrossRef]
- Sambath, K.; Saroja, M.; Rajendran, K.; Muthukumarasamy, N. Morphology controlled synthesis of ZnO nanostructures by varying pH. J. Mater. Sci. Mater. Electron. 2012, 23, 431–436. [Google Scholar] [CrossRef]
- Rathnasekara, R.; Hari, P. Impedance spectroscopy of nanostructured ZnO morphologies. J. Mater. Res. 2021, 36, 1937–1947. [Google Scholar] [CrossRef]
- Matei, A.; Tucureanu, V.; Popescu, C.M.; Romanitan, C.; Mihalache, I. Influence of Cu dopant on the morpho-structural and optical properties ZnO nanoparticles. Ceram. Int. 2019, 45, 10826–10833. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).