Nafion-Based Layer-by-Layer Coatings with Antimicrobial Activity †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
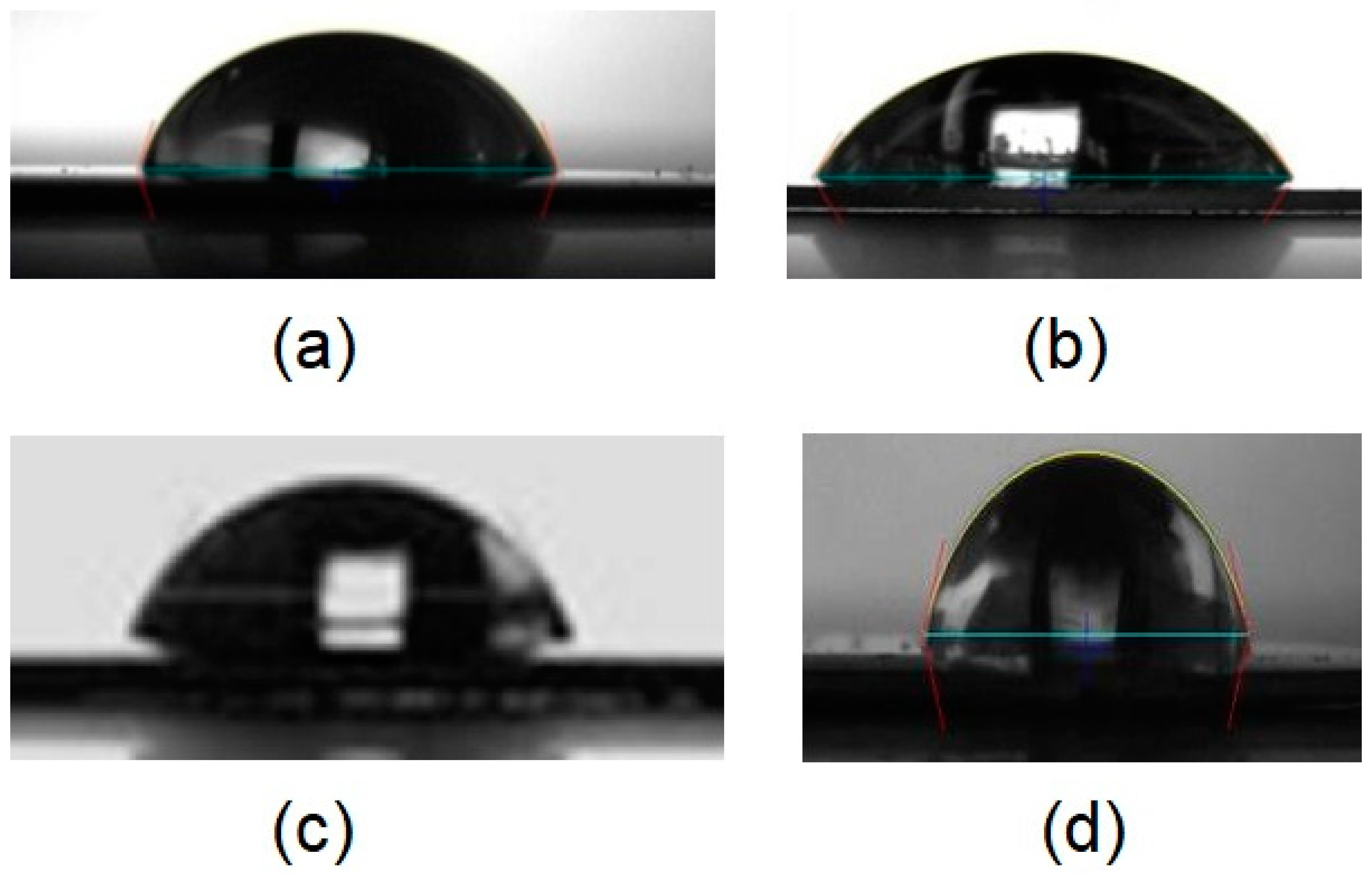
2.2. Contact Angle Measurements
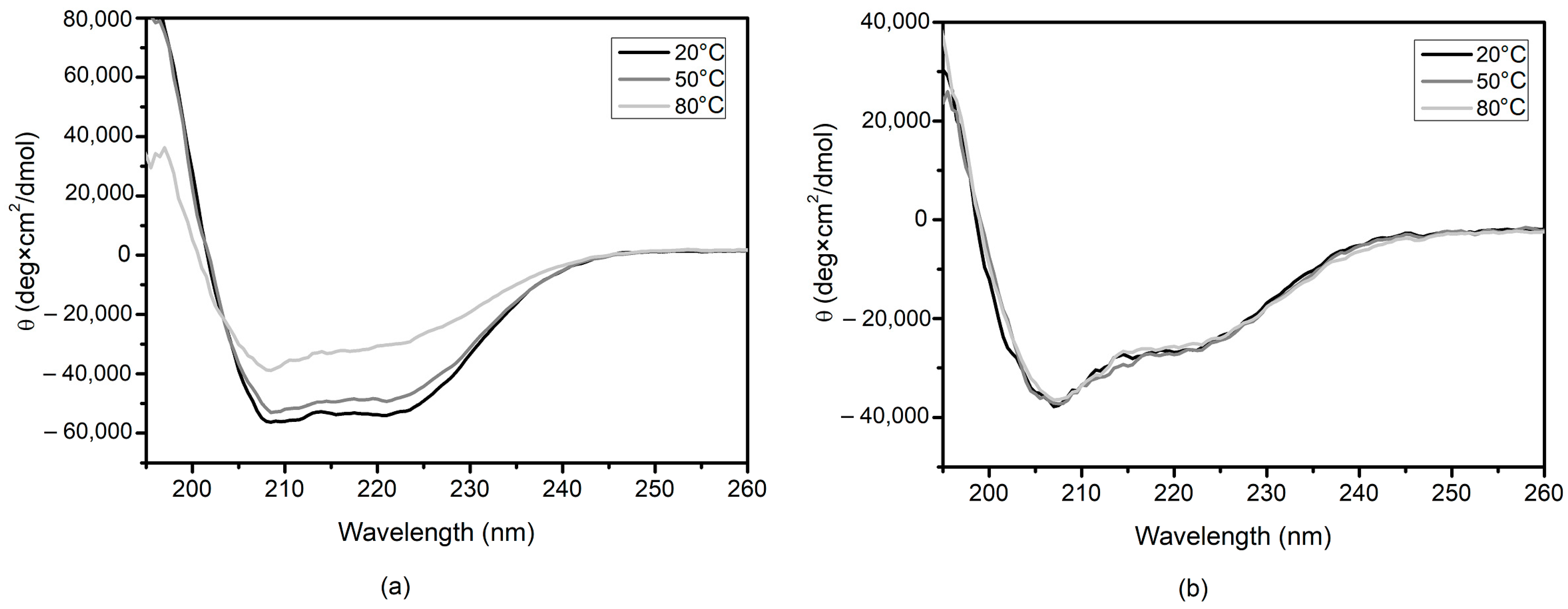
2.3. Circular Dichroism Spectrometry (CD)
2.4. Atomic Force Microscopy (AFM)
2.5. Antimicrobial Testing
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, J.F.; Johnston, A.M.; Johnson, B.; Huntington, M.K.; Mackenzie, C.D. Microbial contamination of Dental Unit waterlines: Prevalence, intensity and microbiological characteristics. JADA 1993, 124, 59–65. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Adlhart, C.; Verran, J.; Azevedo, N.F.; Olmez, H.; Keinänen-Toivola, M.M.; Gouveia, I.; Melo, L.F.; Crijns, F. Surface modifications for antimicrobial effects in the healthcare setting: A critical overview. JHI 2018, 99, 239–249. [Google Scholar] [CrossRef]
- Michel, M.; Toniazzo, V.; Ruch, D.; Ball, V. Deposition mechanisms in layer-by-layer or step-by-step deposition methods: From elastic and impermeable films to soft membranes with ion exchange properties. ISRN Mat. Sci. 2012, 2012, 701695. [Google Scholar] [CrossRef]
- Hammond, P.T. Form and Function in Multilayer Assembly: New Applications at the Nanoscale. Adv. Mater. 2004, 16, 1271–1293. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, H.; Hu, N. Fabrication of Electroactive Layer-by-Layer Films of Myoglobin with Gold Nanoparticles of Different Sizes. J. Phys. Chem. B 2006, 110, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Albright, V.; Zhuk, I.; Wang, Y.; Selin, V.; van de Belt-Gritter, B.; Busscher, H.J.; van der Mei, H.C.; Sukhishvili, S.A. Self-defensive antibiotic-loaded layer-by-layer coatings: Imaging of localized bacterial acidification and ph-triggering of antibiotic release. Acta Biomater. 2017, 61, 66–74. [Google Scholar] [CrossRef]
- Zhong, L.J.; Pang, L.Q.; Che, L.M.; Wu, X.E.; Chen, X.D. Nafion coated stainless steel for anti-biofilm application. Colloids Surf. B 2013, 111, 252–256. [Google Scholar] [CrossRef]
- Cheng, Y.; Moraru, C.I. Long-range interactions keep bacterial cells from liquid-solid interfaces: Evidence of a bacteria exclusion zone near Nafion surfaces and possible implications for bacterial attachment. Colloids Surf. B 2018, 162, 16–24. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, C.; Zhang, X.; Wen, X.; Xiao, Y.; Li, L.; Xu, Q.; Fu, F.; Diao, H.; Liu, X. Layer-by-layer coating of carboxymethyl chitosan-gelatin-alginate on cotton gauze for hemostasis and wound healing. Surf. Coat. Technol. 2021, 406, 126644. [Google Scholar] [CrossRef]
- Chen, L.; Shi, W.; Zhang, T.; Zhou, Y.; Zhao, F.; Ge, W.; Jin, X.; Lin, W.; Guo, W.; Yin, D. Antibacterial activity of lysozyme-loaded cream against MRSA and promotion of scalded wound healing. Int. J. Pharm. 2022, 627, 122200. [Google Scholar] [CrossRef]
- Kelarakis, A. Graphene quantum dots: In the crossroad of graphene, quantum dots and carbogenic nanoparticles. COCIS 2015, 20, 354–361. [Google Scholar] [CrossRef]
- Kelarakis, A. From highly graphitic to amorphous carbon dots: A critical review. MRS Energy Sustain. 2014, 1, E2. [Google Scholar] [CrossRef]
- Krysmann, M.J.; Kelarakis, A.; Dallas, P.; Giannelis, E.P. Formation mechanism of carbogenic nanoparticles with dual photoluminescence emission. J. Am. Chem. Soc. 2012, 134, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Stachowska, J.D.; Murphy, A.; Mellor, C.; Fernandes, D.; Gibbons, E.; Krysmann, M.; Kelarakis, A.; Burgaz, E.; Moore, J.; Yeates, S.G. A rich gallery of carbon dots based photoluminescent suspensions and powders derived by citric acid/ urea. Sci. Rep. 2021, 11, 10554. [Google Scholar] [CrossRef]
- Krysmann, M.J.; Kelarakis, A.; Giannelis, E.P. Photoluminescent carbogenic nanoparticles directly derived from crude biomass. Green Chem. 2012, 14, 3141–3145. [Google Scholar] [CrossRef]
- Fernandes, D.; Krysmann, M.J.; Kelarakis, A. Carbon dot based nanopowders and their application for fingerprint recovery. Chem. Commun. 2015, 51, 4902–4905. [Google Scholar] [CrossRef]
- Verhagen, A.; Kelarakis, A. Carbon dots for forensic applications: A critical review. Nanomater. 2020, 10, 1535. [Google Scholar] [CrossRef]
- Abu Rabe, D.I.; Mohammed, O.O.; Dong, X.; Patel, A.K.; Overton, C.M.; Tang, Y.; Kathariou, S.; Sun, Y.; Yang, L. Carbon dots for highly effective photodynamic inactivation of multidrug-resistant bacteria. Mater. Adv. 2020, 1, 321–325. [Google Scholar] [CrossRef]
- Sreerama, N.; Woody, R.W. Estimation of protein secondary structure from circular dichroism spectra: Comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem. 2000, 287, 252–260. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. Protein secondary structure analyses from circular dichroism spectroscopy: Methods and reference databases. Biopolymers 2008, 89, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Ilker, M.F.; Nüsslein, K.; Tew, G.N.; Coughlin, E.B. Tuning the hemolytic and antibacterial activities of amphiphilic polynorbornene derivatives. J. Am. Chem. Soc. 2004, 126, 15870–15875. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, E.N.; Winder, C.; Barron, E.; Fernandes, D.; Krysmann, M.J.; Kelarakis, A.; Parry, A.V.; Yeates, S.G. Layer by layer antimicrobial coatings based on Nafion, lysozyme, and Chitosan. Nanomaterials 2019, 9, 1563. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.T.; Blanch, H.W.; Radke, C.J. Direct imaging of lysozyme adsorption onto mica by atomic force microscopy. Langmuir 2002, 18, 5841–5850. [Google Scholar] [CrossRef]
- James, P.J.; Elliott, J.A.; McMaster, T.J.; Newton, J.M.; Elliott, A.M.; Hanna, S.; Miles, M.J. Hydration of Nafion® studied by AFM and X-ray scattering. J. Mater. Sci. 2000, 35, 5111–5119. [Google Scholar] [CrossRef]
- da S. Pinto, T.; Alves, L.A.; de Azevedo Cardozo, G.; Munhoz, V.H.O.; Verly, R.M.; Pereira, F.V.; de Mesquita, J.P. Layer-by-layer self-assembly for Carbon Dots/Chitosan-based multilayer: Morphology, thickness and molecular interactions. Mater. Chem. Phys. 2017, 186, 81–89. [Google Scholar]
- Kirby, A. The lysozyme mechanism sorted—After 50 years. Nat. Struct. Mol. Biol. 2001, 8, 737–739. [Google Scholar] [CrossRef]
- Jiang, S.; Qin, Y.; Yang, J.; Li, M.; Xiong, L.; Sun, Q. Enhanced antibacterial activity of lysozyme immobilized on chitin nanowhiskers. Food Chem. 2017, 221, 1507–1513. [Google Scholar] [CrossRef]
- Omura, Y.; Shigemoto, M.; Akiyama, T.; Saimoto, H.; Shigemasa, Y.; Nakamura, I.; Tsuchido, T. Antimicrobial Activity of Chitosan with Different Degrees of Acetylation and Molecular Weights. Biocontrol. Sci. 2003, 8, 25–30. [Google Scholar] [CrossRef]
- Zheng, L.; Zhu, J. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Li, X.; Feng, X.; Yang, S.; Fu, G.; Wang, T.; Su, Z. Chitosan kills Escherichia coli through damage to be of cell membrane mechanism. Carbohydr. Polym. 2010, 79, 493–499. [Google Scholar] [CrossRef]
- Coma, V.; Deschamps, A.; Martial-Gros, A. Bioactive packaging materials from edible chitosan polymer—Antimicrobial activity assessment on dairy-related contaminants. J. Food Sci. 2003, 68, 2788–2792. [Google Scholar] [CrossRef]
- Chung, Y.C.; Su, Y.P.; Chen, C.C.; Jia, G.; Wang, H.L.; Wu, J.C.; Lin, J.G. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharm. Sin. B 2004, 25, 932–936. [Google Scholar]
- Li, H.; Huang, J.; Song, Y.; Zhang, M.; Wang, H.; Lu, F.; Huang, H.; Liu, Y.; Dai, X.; Gu, Z.; et al. Degradable carbon dots with broad-spectrum antibacterial activity. ACS Appl. Mater. Interfaces. 2018, 10, 26936–26946. [Google Scholar] [CrossRef]
- Bing, W.; Sun, H.; Yan, Z.; Ren, J.; Qu, X. Programmed Bacteria Death Induced by Carbon Dots with Different Surface Charge. Small 2016, 12, 4713–4718. [Google Scholar] [CrossRef]
- Klyuzhin, I.; Symonds, A.; Magula, J.; Pollack, G.H. New Method of Water Purification Based on the Particle-Exclusion Phenomenon. Environ. Sci. Technol. 2008, 42, 6160–6166. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.J.; Sharifian, G.; Wu, T.; Li, Y.; Chang, C.; Ma, J.; Dai, H. Determination of bacterial surface charge density via saturation of adsorbed ions. Biophys. J. 2021, 120, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Terada, A.; Okuyama, K.; Nishikawa, M.; Tsuneda, S.; Hosomi, M. The effect of surface charge property on escherichia coli initial adhesion and subsequent biofilm formation. Biotechnol. Bioeng. 2012, 109, 1745–1754. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibbons, E.; Krysmann, M.; Gavalas, S.; Heslop, K.; Kelarakis, A. Nafion-Based Layer-by-Layer Coatings with Antimicrobial Activity. Mater. Proc. 2023, 14, 10. https://doi.org/10.3390/IOCN2023-14471
Gibbons E, Krysmann M, Gavalas S, Heslop K, Kelarakis A. Nafion-Based Layer-by-Layer Coatings with Antimicrobial Activity. Materials Proceedings. 2023; 14(1):10. https://doi.org/10.3390/IOCN2023-14471
Chicago/Turabian StyleGibbons, Ella, Marta Krysmann, Spyridon Gavalas, Kira Heslop, and Antonios Kelarakis. 2023. "Nafion-Based Layer-by-Layer Coatings with Antimicrobial Activity" Materials Proceedings 14, no. 1: 10. https://doi.org/10.3390/IOCN2023-14471
APA StyleGibbons, E., Krysmann, M., Gavalas, S., Heslop, K., & Kelarakis, A. (2023). Nafion-Based Layer-by-Layer Coatings with Antimicrobial Activity. Materials Proceedings, 14(1), 10. https://doi.org/10.3390/IOCN2023-14471






