Abstract
Titania nanosheets (TNS) represent 2D photocatalytic material, which can strongly bind with metal nanoparticles, and, therefore, materials based on it are promising in the development of reusable substrates for reproducible SERS. In the present research, titania nanosheets were obtained during cesium titanate exfoliation. Silver nanoparticles were deposited on the surface of nanosheets via AgNO3 solution reduction. The synthesis of the substrates with optimized parameters allows achieving an enhancement coefficient of up to 1.9 × 106 and the ability to detect molecules of rhodamine 6G with concentration of 10−8 M. Moreover, the obtained substrates show highly reproducible signals throughout the surface. Due to the photocatalytic properties of titania, the surface of the substrates can be cleaned after SERS measurement by UV irradiation, and the substrates can be used repeatedly.
1. Introduction
Surface-enhanced Raman Spectroscopy (SERS) is a fast, highly selective, and sensitive method of organic molecule detection [1,2]. SERS analysis is essential for early disease detection [3] and food control [4]. In such analysis, substrates based on metal nanoparticles such as silver, which should be close to each other, are used. In order to obtain good reproducibility of the signal on the surface of a substrate, uniform distribution of silver nanoparticles should be achieved. Being a 2D material, titania nanosheets can be suggested as a component of such substrates and strongly bind with metal nanoparticles. It has been shown that titania can enhance Raman signal caused by a charge transfer [5]. Despite prospective use in an increase of Raman signal, materials based on titania and silver nanoparticles are shown to demonstrate photocatalytic properties under ultraviolet (UV) irradiation [6]. That property could be used to clean the surface of the substrate after analysis, which makes it possible to use it multiple times. Previously, reusable substrates were reported to be fabricated; however, they either have complicated production process [7] or require additional substances to clean the surface [8].
The present work demonstrates an easy to fabricate reusable substrates based on titania nanosheets and silver nanoparticles (Ag/titania nanosheets). Silver nanoparticles were deposited on the surface of titania nanosheets by a reduction in silver nitrate solution. The obtained substrates show an enhancement coefficient of up to 1.9 × 106 and great signal reproducibility on the surface. When exposed to UV irradiation, the substrates can be cleaned after the analysis and be used for the second time.
2. Methods
2.1. Synthesis of Titania Nanosheets
Titania nanosheets were synthesized by cesium titanate exfoliation process. Firstly, cesium titanate was synthesized using two-step calcination of cesium nitrate (analytical grade, Rushim, Moscow, Russia) with titania (Hombikat, Sigma-Aldrich, Saint Louis, MO, USA) at 800 °C for 20 h. The following step included titanates treatment in HCl (35% solution, Rushim) solution at pH = 1 for 4 days under stirring. Then, to remove chloride ions from the suspension the dialysis process was carried out, and after that tetrabutylammonium hydroxide (TBAOH, 40% solution, Sigma-Aldrich, Saint Louis, MO, USA) solution was added to the molar ratio ν(Ti):ν(TBA+) was 1:2. After 2 weeks the suspension obtained was purified with dialysis, and then was centrifuged several times to separate the desired TNS fraction as the final product. Finally, titania nanosheets suspension was dripped onto cover glass substrates and dried at 50 °C.
2.2. Silver Nanoparticles Deposition
Deposition of silver nanoparticles on the surface of TNS was carried out by the following technique. Solutions of AgNO3 (analytical grade, Rushim) with defined concentrations (2.5 mM, 5 mM, 10 mM, or 20 mM) were used for the synthesis. Once 100 µL of the solution was dripped on the surface of the TNS substrate, 170 µL of freshly prepared sodium borohydride (analytical grade, Rushim) solution with a concentration of 0.15 M was added. Five minutes after, the obtained Ag/titania nanosheets substrates were washed with deionized water, dried, and used for SERS measurements.
2.3. Material Characterization
Scanning transmission electron microscopy (STEM) images were obtained using an Amber GMH (Tescan, Brno, Czech Republic) microscope operated at an accelerating voltage of 30 kV using bright field (BF) and high-angle dark field (HADF) detectors.
SERS spectra were acquired using Raman microscope-spectrometer Enspectr (Enspectr, Moscow, Russia) with a solid-state laser (wavelength—532 nm, source power—50 MW, power neutral density filter of 30%). The spectra were acquired for 30 s using a ×40,000 lens with a focal length set to 250 mm. The device was adjusted using single-crystal silicon wafers as a standard. The measurements were performed with model molecule rhodamine 6G (R6G) with different concentrations. The enhancement coefficient (EC) of a substrate was calculated using the following equation:
EC = (ISERS/IRS) × (CRS/CSERS)
For the experiments regarding the reusability of the substrates, a UV lamp was used with a power of 20 W. After rhodamine 6G was objected to SERS analysis, the substrate was UV-irradiated for 4 h, and then another portion of R6G was placed on the substrate for the second analysis.
3. Results and Discussion
In the process of cesium titanate exfoliation, titania nanosheets were obtained, which then were examined using STEM analysis (Figure 1). The obtained titania nanosheets have lateral sizes up to 1 µm, although there are nanosheets with a size of about 100 nm. The thickness of nanosheets was estimated to be less than 5 nm according to STEM studying.
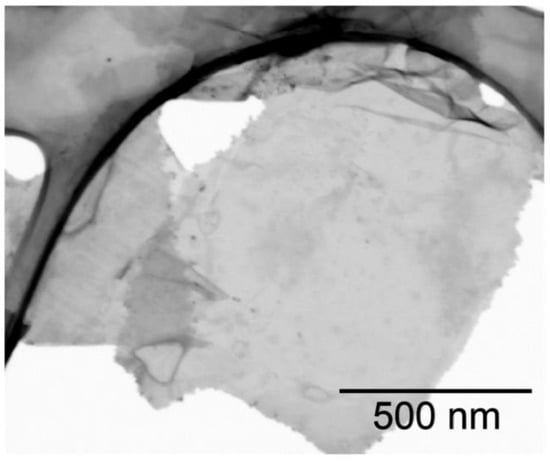
Figure 1.
BF STEM image of the obtained titania nanosheets.
In the fabrication of substrates, the concentration of silver nitrate solution was optimized to achieve a higher value of enhancement coefficient and greater reproducibility of the signal throughout the surface of the substrate. Silver nanoparticles’ distribution uniformity over the substrates was analyzed using optical microscopy (Figure 2).
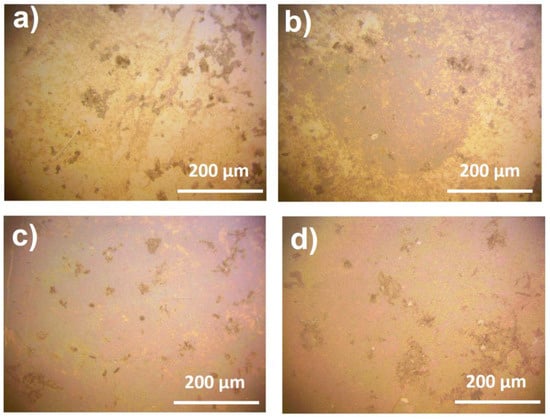
Figure 2.
Optical microscopy images of the substrates obtained with AgNO3 solutions of different concentrations: (a) 1 mM; (b) 5 mM; (c) 10 mM; (d) 20 mM. In the images, silver nanoparticles are represented by the darker areas, whereas lighter areas of the substrate represent titania with no silver nanoparticles attached.
Using an AgNO3 solution with low concentration (1 mM), silver nanoparticles do not deposit uniformly on the surface of titania with only little number of aggregates being observed (Figure 2). Nevertheless, focusing on such areas, the effect of the signal enhancement can be detected, though rather weak (Table 1, Figure 3a).

Table 1.
Enhancement coefficients for Ag/titania nanosheets substrates obtained using silver nitrate solutions with different concentrations.
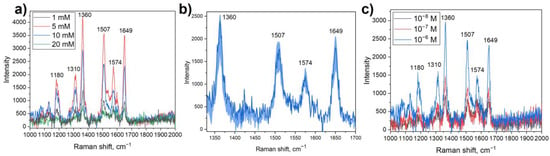
Figure 3.
(a) SERS spectra of R6G on Ag/titania nanosheets substrates obtained with silver nitrate solutions of various concentrations; (b) The stability of SERS signal throughout the surface of Ag/titania nanosheets substrate obtained using 10 mM AgNO3 solution: dark blue line indicates mean signal, light blue area represents the deviation from the mean signal; (c) SERS spectra of R6G with different concentrations on Ag/titania nanosheets (10 mM AgNO3).
The highest signal enhancement can be detected on the substrate obtained with silver nitrate solution of 5 mM: the enhancement coefficient reaches (3.8 ± 3.0) × 105. However, the signal is not stable when measuring different points of the substrate. The distribution of silver nanoparticles is not uniform, though more advantageous than this of the substrate obtained with 1 mM AgNO3 solution, with large light areas present on the substrate. This observation limits the application of the substrate.
On the other hand, the substrate obtained with 10 mM AgNO3 solutions, shows the most beneficial distribution of silver nanoparticles with no large light areas without them being attached to titania. The SERS signal on this substrate appears to be a little less than the signal on the substrate obtained with 5 mM AgNO3 solution. Nevertheless, the SERS signal is almost constant at different parts of the substrate, which makes such substrate a reliable one for the qualitative detection of organic molecules. It is worth noting that at this concentration of AgNO3, there are some nanoparticle aggregations, which represent darker areas on the images of optical microscopy. However, the SERS signal at these points is not changed compared to the other points.
Using silver nitrate solutions with higher concentrations (20 mM) the distribution of metal nanoparticles stays constant with little more silver aggregations. However, the signal enhancement decreases significantly, which could be explained by the formation of large nanoparticles, which are not suitable for SERS analysis. Therefore, Ag/titania nanosheets substrates obtained with an AgNO3 solution of 10 mM were considered to be optimal for use in SERS analysis and investigated more thoroughly thereafter.
To characterize the sensor abilities of Ag/titania nanosheets substrates R6G solutions with different concentrations were chosen for SERS analysis. It is shown that when using the substrates, it is possible to detect rhodamine 6G solutions with concentrations down to a level of 10−8 M (Figure 3c), which corresponds to an enhancement coefficient of (2.5 ± 0.6) × 106.To show the possibility to use Ag/titania nanosheets substrates multiple times, experiments with UV cleaning were conducted (Figure 4). It is shown that after the irradiation of the substrate, there is no rhodamine 6G signal to be detected. Such observation can be explained by the photodegradation of organic molecules in the presence of titania and materials based on it. Moreover, a strong binding between silver nanoparticles and titania nanosheets allows substrate pre-cleaning by deionized water. After UV treatment, which decomposes absorbed organic molecules, a new portion of the analyte can be placed onto the substrate and detected with the same efficiency. With that, the intensity of the peaks corresponding to R6G remains at the same level. It is worth noting that there is an increase in the signal intensity in the range of 1510–1560 cm−1 when comparing the first and second analyses. That can be explained by the oxidation of silver nanoparticles during the analysis, which, however, does not prevent from qualitative detection of R6G molecules by this method.
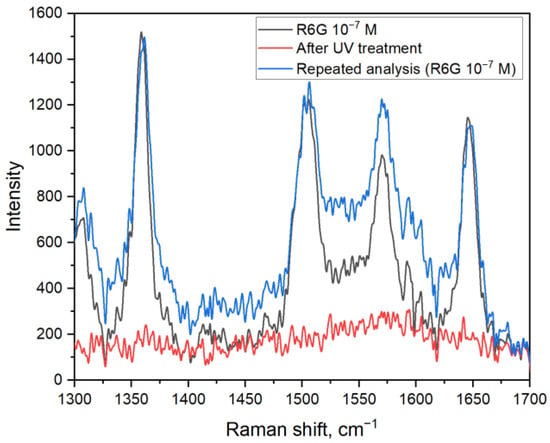
Figure 4.
Reusability of Ag/titania nanosheets substrates for the detection of rhodamine 6G molecules of 10−7 M concentration. The SERS spectra represent the ones before and after UV irradiation as well as the spectra of the second analysis with the same concentration of R6G.
4. Conclusions
Substrates based on titania nanosheets and silver nanoparticles were investigated for use in SERS spectroscopy. During the fabrication of the substrates, conditions of silver deposition are essential to achieve a high enhancement coefficient and signal reproducibility on the surface of the substrate. It is shown that AgNO3 solution with a concentration of 10 mM is optimal with an enhancement coefficient up to (2.5 ± 0.6) × 106 and the possibility to acquire SERS signal of the same level on all of the substrate’s surface. Lower concentrations of AgNO3 solutions lead to a bad distribution of silver nanoparticles with large areas of titania nanosheets without silver nanoparticles attached. On the other hand, when the concentration is more than 10 mM, the SERS signal decreases significantly, which might be explained by the silver particles being too large for the analysis. The Ag/titania nanosheets substrates can be used for repetitive analysis, which was shown for rhodamine 6G molecules. Due to the photocatalytic properties of titania, organic analytes can be decomposed when treated with UV irradiation, and the substrates can be used for the second analysis. Therefore, Ag/titania nanosheets substrate can be used as a reusable substrate with a reproducible SERS signal, which can significantly simplify the process of SERS analysis and decrease its cost.
Author Contributions
Investigation, data curation, A.O.R.; investigation, software, methodology, D.A.K.; writing—original draft preparation—A.O.R. and D.A.K.; writing—review and editing, validation, conceptualization, supervision, A.V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Foundation for Assistance to Small Innovative Enterprises in Science and Technology (FASIE), Russia, Grant No. 17423ГУ/2022, 22 April 2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The research was carried out using the equipment of MSU Shared Research Equipment Centre “Technologies for obtaining new nanostructured materials and their complex study” and purchased by MSU in the frame of the Equipment Renovation Program (National Project “Science and Universities”). SEM experiments was performed using the equipment of the JRC PMR IGIC RAS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, S.; Wu, C.; Wang, Y.; Yang, X.; Yuan, R.; Chai, Y. Ag/TiO2 nanocomposites as a novel SERS substrate for construction of sensitive biosensor. Sens. Actuators B Chem. 2021, 339, 129843. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Ozaki, Y.; Xu, Z.; Zhao, B. Effect of TiO2 on Altering Direction of Interfacial Charge Transfer in a TiO2-Ag-MPY-FePc System by SERS. Angew. Chem. Int. Ed. 2019, 58, 8172–8176. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Formoso, M.; Alvarez-Puebla, R.A. Cancer Diagnosis through SERS and Other Related Techniques. Int. J. Mol. Sci. 2020, 21, 2253. [Google Scholar] [CrossRef] [PubMed]
- Balbinot, S.; Srivastav, A.M.; Vidic, J.; Abdulhalim, I.; Manzano, M. Plasmonic biosensors for food control. Trends Food Sci. Technol. 2021, 111, 128–140. [Google Scholar] [CrossRef]
- Wang, X.; Shi, W.; Wang, S.; Zhao, H.; Lin, J.; Yang, Z.; Chen, M.; Guo, L. Two-Dimensional Amorphous TiO2 Nanosheets Enabling High-Efficiency Photoinduced Charge Transfer for Excellent SERS Activity. J. Am. Chem. Soc. 2019, 141, 5856–5862. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xu, W.; Yu, L. Photocatalytic Decomposition of Gaseous HCHO over Ag Modified TiO2 Nanosheets at Ambient Temperature. Nanomaterials 2019, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, Z.; Zhao, Y.; Zhangsun, H.; Bu, T.; Zhang, C.; Wang, X.; Wang, L. Bio-inspired self-cleaning carbon cloth based on flower-like Ag nanoparticles and leaf-like MOF: A high-performance and reusable substrate for SERS detection of azo dyes in soft drinks. Sens. Actuators B Chem. 2021, 329, 129080. [Google Scholar] [CrossRef]
- Wu, J.; Du, Y.; Wang, C.; Bai, S.; Zhang, T.; Chen, T.; Hu, A. Reusable and long-life 3D Ag nanoparticles coated Si nanowire array as sensitive SERS substrate. Appl. Surf. Sci. 2019, 494, 583–590. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).