Abstract
Nanotechnology is used today in a wide range of industries. Weakly water-soluble medications have better solubility and bioavailability when delivered by nano-specific drug delivery methods, such as nanocrystals. Another name for ziprasidone is 5-[2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one, and it is a brand-new “atypical” or “second-generation” antipsychotic drug. Its multipotent G-protein-coupled (GPCR) receptor binding profile is distinctive. It is used to treat bipolar-disorder-related acute manic or mixed episodes as well as schizophrenia. Schizophrenia is a serious mental condition in which a person can experience reality in a strange or different way. Ziprasidone is a highly lipophilic and unstable drug. Ziprasidone nanoparticles, another incarnation of this drug, are used to treat diseases. When ziprasidone is present in the form of particles with an effective average crystal size of less than or equal to 100 nm, the term “nanoparticle” is frequently used to characterize them. A colloidal submicron dispersion of ziprasidone particles is what ziprasidone nanosuspensions and nanoemulsions are made of. One formulation that makes use of solubilization technology is a nanosuspension of a crystalline ziprasidone free base. In order to get around the drug’s solubility issue and investigate its potential for nose-to-brain delivery, a buffered nanoemulsion of ziprasidone HCl has been created. We discuss numerous ziprasidone nanoformulations used to treat psychotic illnesses in this review.
1. Introduction
Today, nanotechnology is a crucial method for making poorly water-soluble pharmaceuticals more soluble. Because of the increase in surface area and saturation caused by the reduction in these medications’ particle sizes to the nanometer range, they can dissolve more quickly and have greater bioavailability [1,2]. About 40% of recently developed medicines have poor water solubility [3]. Drugs’ poor bioavailability is due to their poor water solubility [4]. To make weakly water-soluble medications more soluble, there are numerous methods such as using cosolvents, surfactants, and complexing to prepare pharmaceuticals as salts [5]. Additionally, it has been claimed that particle size reduction medicines can make them more soluble [6]. Applications of nanotechnology for pharmacists include medications with active components that are nanoscale in size [7]. As a result of the smaller drug delivery systems, drugs can now be deposited in previously inaccessible body parts, which has relevance in the identification and treatment of specific illnesses such as cancer. Target therapy and advancements in medical devices and diagnostic tests are new discoveries in medication delivery [8]. The science underlying nanotechnology is still in its infancy, which raises certain concerns about these advancements.
The National Nanotechnology Initiative (NNI) defines nanotechnology as the study of all particles with a diameter of less than 100 nanometers. One nanometer equals one-billionth of a meter [9]. Crucially, the ratio of the smaller particle size to surface molecules or atoms as a percentage causes the benefits to increase. They therefore have substantial surface areas which cause them to become more active on their surface and produce modifications to their biological and physical characteristic properties.
The benefits of nanoparticles can be summed up as follows: (i) increased bioavailability; (ii) less toxicity; (iii) sustained and controlled release; (iv) targetability; (v) provide efficient intracellular and brain delivery compartments; (vi) improved permeability; (vii) faster illness diagnosis that is more secure and accurate; (viii) surgery is accurate and less intrusive; (ix) expensive; (x) production on a large scale is doable; (xi) smaller dosages; (xii) stable dosage formulations, e.g., a smaller pill; and (xiii) a greater dissolving speed, particularly in internal aqueous fluid. In general, a quicker disintegration results in larger bioavailability, lower dosages, and less toxicity. The drugs’ capacity to remain stable in biological fluids helps to avoid allergic reactions, discomfort following an injection, and/or drug precipitation due to its dilution in the environmental blood [10,11].
2. Drug Profile
The IUPAC name of ziprasidone is 5-{2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl}-6-chloro-1,3-dihydro-2H-indol-2-one.The molecular formula is C21H21ClN4OS, and it has a molecular weight of 412.94 g/mol. It is an atypical antipsychotic drug and is slightly soluble in DMSO and methanol. Its melting point is about 213–215 °C. The pKa value is 13.34 ± 0.20 (predicted). It is a brown to dark-brown solid. The mechanism of action of ziprasidone, as with other drugs with efficacy in schizophrenia, is unknown. However, it has been proposed that this drug’s efficacy in schizophrenia is mediated through a combination of dopamine type 2 (D2) and serotonin type 2 (5HT2) antagonism. As with other drugs having efficacy in bipolar disorder, the mechanism of action of ziprasidone in bipolar disorder is unknown [12,13,14]. Figure 1 shows the structure of ziprasidone.
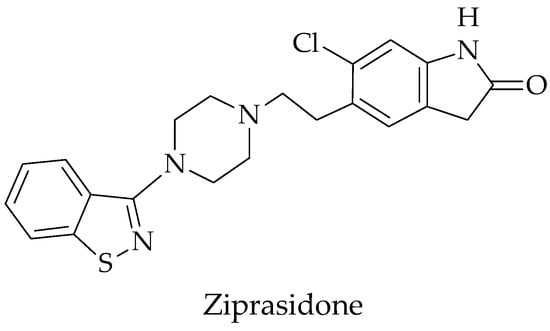
Figure 1.
Structure of ziprasidone.
3. Various Nanoformulations of Ziprasidone
Nanoformulations are a novel method of drug administration because they are easy to manipulate, are approved by the US FDA, nontoxic, and selectively and specifically degraded in the colon region. These qualities make them promising materials for application in a colon-specific drug delivery system. As the drug particle size is lowered to the nanoscale, there is an improvement in the dissolving properties and an increase in saturation solubility, which may be due to an increase in the particle surface area. This results in the drug’s saturation solubility significantly rising as the particle size is reduced. Because of their numerous advantages, including their very small particle size, nanosuspensions and nanoemulsions have become a viable method for the effective administration of hydrophobic medications. There are various nanoformulations of ziprasidone, such as nanosuspensions and nanoemulsions.
3.1. Ziprasidone Nanosuspensions
The term “nanosuspensions” refers to colloidal dispersions of pharmaceutically active component particles smaller than 1 micrometer in diameter in a liquid phase, being stabilized without the need of any matrix material polymers and surfactants [15]. A nanosuspension can be produced by an appropriate size reduction method and stabilized by a suitable stabilizer [16,17,18]. The Noyes–Whitney and Ostwald–Freundlich principles state that particles with a size in the nanometer range may have higher dissolution rates and saturation solubilities for a nanosuspension, which often comes with an enhancement of bioavailability [16,19,20]. Nanosuspensions differ from nanoparticles and solid lipid nanoparticles with respect to the fact that nanoparticles are polymeric colloidal carriers of drugs, while solid lipid nanoparticles are lipid carriers of drugs. More recently created medications have poor solubility; frequently, pharmaceuticals have poor solubility in both nanoparticles and solid lipid nanoparticles. Aside from conventional media, aqueous and organic methods for resolving these solubility issues lead to issues with bioavailability. Producing drug nanoparticles (also known as nanosuspensions) is an alternate and promising strategy to combat various challenges. As a new technology, nanosuspensions are a promising approach for effective delivery due to their wide range of properties, hydrophobic medicines, and distinctive advantages. The specific characteristics of nanosuspensions have made it possible for them to be used in a variety of dosage forms, including in customized delivery systems such as hydrogels that adhere to mucous membranes. The important general benefits of this technique include simplicity and applicability to the majority of medications [21].
All medications that are insoluble in water can be prepared as nanosuspensions, which is a straightforward process. Nanosuspensions are prepared by using wet mill, emulsion solvent, high-pressure homogenizer, supercritical fluids, melt emulsification, and evaporation fluid methods. The delivery of nanosuspensions is possible through pulmonary, ocular, parenteral, and oral routes. When included in ocular inserts and mucoadhesive hydrogels, nanosuspensions can also be employed for targeted medication administration. At the moment, efforts are being specifically aimed towards expanding their uses in site-particular medication delivery. Rapid advancements have been made in the parenteral, preoral, ophthalmic, and pulmonary delivery of nanosuspensions. Strictly speaking, nanosuspension preparations are a less complex alternative to liposomes and other types of conventional colloidal drug carriers, and they are said to be more economically sensible. They are very beneficial for people who are struggling with soluble medications, as they provide a physiologically more stable product. For making nanosuspensions, there are two opposite approaches: “Top-down procedure technology” and “Bottom-up process technology” [22].
One formulation that makes use of solubilization technology is a nanosuspension of a crystalline ziprasidone free base. Atypical antipsychotic drugs include ziprasidone hydrochloride. It is a white or slightly pink powder that is essentially insoluble in water, but is slightly soluble at the melting point of methylene chloride and methanol, which is 300 °C. It is regarded as a BCS Class II medication due to its strong permeability and low solubility. Under fed conditions, the 20 mg dose’s absolute bioavailability was reported to be about 60%. Ziprasidone hydrochloride is well absorbed from the gastrointestinal tract with peak plasma concentrations being reached 6 to 8 h after the oral dose. Ziprasidone hydrochloride is extensively metabolized by aldehyde oxidase (about 66% of a dose) and by the cytochrome P450 iso-enzyme CYP3A4. Less than 5% of a dosage is eliminated primarily as metabolites in the urine (20%) and feces (about 66%), meaning the medicine is unchanged. The drug binds to plasma proteins in 99% of cases. According to reports, terminal elimination occurs after 7 h, and the volume of distribution is 1.5 L/kg. The peak plasma levels of ziprasidone hydrochloride are about 2 to 3 h after an oral dose, when 89 ng/mL is reached [23,24,25,26,27,28].
3.1.1. Advantages
- i.
- Most cost-effective;
- ii.
- Useful for poorly soluble drugs;
- iii.
- Physically more stable than liposomes;
- iv.
- Provides ease of manufacture and scaling up for large-scale production;
- v.
- Rapid dissolution and tissue targeting;
- vi.
- Reduction in tissue irritation;
- vii.
- Higher bioavailability in ocular and inhalational drug delivery [29,30].
3.1.2. Disadvantages
- i.
- Compaction, sedimentation, and physical stability can all be problematic;
- ii.
- Because of its weight, extra caution must be used while handling and transporting;
- iii.
- Unsuitable dosage [31].
3.2. Ziprasidone Nanoemulsions
Nanoemulsions/Submicron emulsions (SMEs)/Mini-emulsions are thermodynamically stable transparent or translucent dispersions of oil and water stabilized by an interfacial film of surfactant and cosurfactant molecules that have a globule size of less than 100 nm. Nanoemulsions are now commonly employed to deliver vaccines, DNA-encoded drugs, antibiotics, and other medications, while cosmetic and topical products are advertised via a variety of channels, including oral, pulmonary, transdermal, intranasal, ocular, etc. As a type of multiphase colloidal dispersion, nanoemulsions are distinguished by their stability and clarity. When they are scattered, they generally contain tiny particles or droplets with very little oil/water interaction, and they have strain on the face. Nanoemulsions can sometimes develop naturally, spontaneously, and readily without high-energy input. There are several occurrences where a cosurfactant or cosolvent is utilized, such as in the oil phase, in addition to the surfactant, and in the water phase.
Three types of nanoemulsions are formed depending on the composition:
- Oil in water (o/w): nanoemulsions wherein oil droplets are dispersed in the continuous aqueous phase;
- Water in oil (w/o): nanoemulsions wherein water droplets are dispersed in the continuous oil phase;
- Bi-continuous: nanoemulsions wherein micro-domains of oil and water are interspersed within the system.
An adequate mixture of surfactants and/or cosurfactants stabilizes the interface in all three forms of nanoemulsions. Emulsions and nanoemulsions have a significant difference in that the former, while they may exhibit great stability of kinetic processes, is thermodynamically unstable and likely to change and separate over time. Emulsions and nanoemulsions both have distinct visual characteristics; emulsions are hazy, whereas nanoemulsions are transparent [32,33,34,35,36].
The goal is to develop a method that will effectively deliver the medicine to the target spot in the brain by avoiding the first-pass metabolism while also increasing bioavailability by an invasive procedure. Such strategies will not only decrease the dose while minimizing ancillary side effects. The intranasal (i. n.) route has been shown in studies to be a useful, noninvasive, and alternate mechanism for quick medication delivery to the brain [37], and thus, for many medications and vaccines, nasal administration has been used as an alternative to oral delivery and injection [38]. The highly vascularized and immunogenic nasal mucosa offers potential advantages in terms of quick action, improved bioavailability, and patient compliance [39]. According to reports, exogenous materials penetrate the blood–brain barrier (BBB) on their way directly from the nose to the brain via the olfactory and trigeminal nerve pathways [40,41,42]. In order to administer medications to the central nervous system, the nasal cavity’s olfactory area can be used as a conduit between the nose and the brain [43,44]. Based on these considerations, a buffered nanoemulsion of ZP HCl was developed to overcome its solubility limitation and to explore its potential for nose-to-brain delivery. As the target site of the ZP HCl for antipsychotic activity is the temporal and prefrontal area that constitutes the limbic system and mesocardial area in the brain, the intranasal route might be a better approach for the rapid attainment of an effective drug concentration in brain [45].
3.2.1. Advantages
- i.
- Removes variations in absorption;
- ii.
- Increases the rate of absorption;
- iii.
- Supports lipophilic drug solubilization;
- iv.
- Offers aqueous dosage forms for medications that are not water soluble;
- v.
- Enhances bioavailability;
- vi.
- Several delivery methods, including topical, oral, and intravenous routes, can be used to administer the product;
- vii.
- Effective and quick penetration of the drug substance aids in flavor muffling;
- viii.
- Offers defense against hydrolysis and drug oxidation in the oil phase of the o/w emulsion;
- ix.
- Less energy is necessary;
- x.
- Liquid dose forms promote patient compliance;
- xi.
- Nanoemulsions are thermally stable systems that are stable and ensure that a system’s self-emulsifying characteristics do not rely on the process that was used;
- xii.
- Nanoemulsions transport both lipophilic and hydrophilic substances;
- xiii.
- The use of nanoemulsions as a delivery system increases a drug’s effectiveness, and reduces the overall dose; hence, side effects are reduced [46].
3.2.2. Disadvantages
- i.
- The use of a high concentration of cosurfactants and surfactants is required for stabilizing nanodroplets;
- ii.
- A low solubilizing ability for high melting substances;
- iii.
- The surfactant must not be harmful in pharmaceutical uses;
- iv.
- The stability of nanoemulsions is affected by factors including environmental aspects such as temperature and pH, and due to these specifications, when a nanoemulsion is delivered, it transforms the patients [47].
4. Significance of Ziprasidone Nanoparticles
Another name for ziprasidone is 5-[2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one, and it is a brand-new “atypical” or “second-generation” antipsychotic drug. Its multipotent G-protein-coupled (GPCR) receptor binding profile is distinctive. It is used to treat bipolar-disorder-related acute manic or mixed episodes as well as schizophrenia. Schizophrenia is a serious mental condition in which a person experiences reality in a strange or different way. Ziprasidone is a highly lipophilic and unstable drug. Ziprasidone nanoparticles, as another incarnation of this drug, are used to treat diseases. When ziprasidone is present in the form of particles with an effective average crystal size of less than or equal to 100 nm, the term “nanoparticle” is frequently used to characterize it. Cognitive disorders that include hallucinations, delirium, dementia, schizophrenia, and delusions are clinical manifestations of psychoses, which are brain illnesses. Significant side effects of antipsychotic medications include dystonia, tardive dyskinesia, uncontrollable muscular movement, and metabolic abnormalities. Moreover, due to the blood–brain barrier, the antipsychotics that are currently on the market have low bioavailability, drug-related side effects, poor therapeutic efficacy, and inadequate brain delivery. Traditional dose forms, which release the medications into the bloodstream, are ineffective at efficiently delivering the drugs to the brain. As a result, a logical strategy based on nanotherapeutics may be able to circumvent these restrictions; such strategies can be employed to transport medication molecules to their intended spot. Nanotherapeutics are colloidal systems made up of particles in the nanosize range with special physicochemical characteristics, such as plasticity, biodegradability, and bioacceptability. They also have a variety of surface modification capabilities and can protect drug molecules from degradation. Various nanoformulations for the delivery of antipsychotic drugs to the brain include nanoparticles, solid lipid nanoparticles, nanostructured lipid carriers, nanoemulsions, and nanosuspensions. Additionally, these formulations are able to improve drug bioavailability and targeting affinity, as well as improve their ability to circumvent the first-pass metabolism [46,47].
5. Conclusions
Nanotechnology used by pharmacists includes medications with active components that are nanoscale in size. This review focused on ziprasidone nanoparticles, which are used in the treatment of psychotic disorders. Ziprasidone hydrochloride is an atypical antipsychotic drug. Its multipotent G-protein-coupled (GPCR) receptor binding profile is distinctive. It is used to treat bipolar-disorder-related acute manic or mixed episodes as well as schizophrenia. Ziprasidone nanosuspensions and nanoemulsions are submicron colloidal dispersions of ziprasidone particles. A nanosuspension of a crystalline ziprasidone free base is already one formulation utilizing solubilization technology. Additionally, the buffered nanoemulsion of ziprasidone HCl has been developed to overcome its solubility limitation and to explore its potential for nose-to-brain delivery. These formulations have the ability to improve drug bioavailability and targeting affinity.
Author Contributions
Conceptualization, M.P. and J.P.; methodology, K.P.; software, A.T.; validation, D.P., J.P., and M.P.; formal analysis, A.T.; investigation, J.P.; resources, M.P.; data curation, J.P.; writing—original draft preparation, M.P.; writing—review and editing, M.P.; visualization, D.P.; supervision, J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to convey our thanks to Management and Principal, P.S.G.V.P. Mandal’s College of Pharmacy, Shahada, Dist. Nandurbar, for furnishing all the essential facilities to accomplish the review work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhalekar, M.R.; Upadhaya, P.G.; Reddy, S.; Kshirsagar, S.J.; Madgulkar, A.R. Formulation and evaluation of acyclovir nanosuspension for enhancement of oral bio-availability. Asian J. Pharm. 2014, 8, 110–118. [Google Scholar] [CrossRef]
- Mokale, V.; Patil, K.; Khatik, T.; Sutar, Y. Glyburide nanosuspeNCion: Influence of processing and formulation parameter on solubility andin vitro dissolution behavior. Asian J. Pharm. 2013, 7, 111–117. [Google Scholar] [CrossRef]
- Lipincki, C. Poor aqueous solubility-an industry wide problem in drug discovery. Am. Pharm. Rev. 2002, 5, 82–85. [Google Scholar]
- Muller, R.H.; Peters, K. Nanosuspension for the formulation of poorly soluble drugs: I. Preparation by a size reduction technique. Int. J. Pharm. 1998, 160, 229–237. [Google Scholar] [CrossRef]
- Chaudhary, A.; Nagaich, U.; Gulati, N.; Sharma, V.K.; Khosa, R.L. Enhancement of solubilization and bio-availability of poorly soluble drugs by physical and chemical modification: A recent review. J. Adv. Pharm. Educ. Res. 2012, 2, 32–67. [Google Scholar]
- Jinno, J.; Kamada, N.; Miyake, M.; Yamada, K.; Mukai, T.; Odomi, M.; Toguchi, H.; Liversidge, G.G.; Higaki, K.; Kimura, T.; et al. Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogs. J. Control. Release 2006, 111, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Nanotechnology: Applications, Market and Companies; Jain Pharma Biotech Publications: Basel, Switzerland, 2005. [Google Scholar]
- Torchilin, V.P. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS 2007, 9, 128–147. [Google Scholar] [CrossRef]
- Nagavarma, B.V.N.; Hement, K.S.Y.; Ayaz, A.; Vasudha, L.S.; Shivakumar, H.G. Different techniques for preparation of polymeric nanoparticles—A review. Asian J. Pharm. Clin. Res. 2012, 5, 17–23. [Google Scholar]
- Abhilash, M. Potential applications of Nanoparticles. Int. J. Pharm. Biol. Sci. 2010, 1, 1–12. [Google Scholar]
- Harikrishna, D.; Ananthsrinivas, C.; Mansoor, M.A. Role of nanotechnology in pharmaceutical product development. J. Pharm. Sci. 2007, 96, 2547–2565. [Google Scholar]
- Ziprasidone-Wikipedia. Available online: https://en.wikipedia.org/wiki/Ziprasidone (accessed on 14 March 2023).
- Ziprasidone. Available online: https://m.chemicalbook.com/ (accessed on 14 March 2023).
- Ziprasidone (Geodon): Drug Monograph. Available online: https://www.ebmconsult.com/articles/ziprasidone-geodon#jump_ss_103734 (accessed on 14 March 2023).
- Geetha, G.; Poojitha, U.; Arshad Ahmed, K. Various techniques for preparation of nanosuspension—A review. Int. J. Pharma Res. Rev. 2014, 3, 30–37. [Google Scholar]
- Kocbek, P.; Baumgartner, S.; Kristl, J. Preparation and evaluation of nanosuspensions for enhancing the dissolution of poorly soluble drugs. Int. J. Pharm. 2006, 312, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bohm, B.H.L.; Müller, R.H. Lab-scale production unit design for nanosuspensions of sparingly soluble cytotoxic drugs. Pharm. Sci. Technol. Today 1999, 2, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Patravale, V.B.; Date, A.A.; Kulkarni, R.M. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef]
- Hintz, R.J.; Johnson, K.C. The effect of particle size distribution on dissolution rate and oral absorption. Int. J. Pharm. 1989, 51, 9–17. [Google Scholar] [CrossRef]
- Rabinow, B.E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 2004, 3, 785–796. [Google Scholar] [CrossRef]
- Kumari, K.; Rao Srinivasa, Y. Nanosuspensions: A Review. Int. J. Pharm. 2017, 7, 77–89. [Google Scholar]
- Vaneerdenbrugh, B.; Vandenmooter, G.; Augustijns, P. Top-down production of drug nanocrystals: Nanosuspension stabilization, miniaturization and transformation into solid products. Int. J. Pharm. 2008, 364, 64–75. [Google Scholar] [CrossRef]
- O’ Neil, M.J.; Heckelman, P.E.; Koch, C.B.; Roman, K.J.; Kenny, C.M.; D’Arecca, M.R. (Eds.) Ziprasidone Hydrochloride. In The Merck Index—An Encyclopedia of Chemicals, Drugs and Biological, 14th ed.; Merck Research Laboratory, Division of Merck & Co., Inc.: Whitehouse Station, NJ, USA, 2006; p. 10307. [Google Scholar]
- Sweetman, S.C. (Ed.) Ziprasidone Hydrochloride. In Martindale—The Complete Drug Reference, 36th ed.; Pharmaceutical Press: London, UK, 2019; p. 1036. [Google Scholar]
- Miceli, J.J.; Wilner, K.D.; Swan, S.K.; Tensfeldt, T.G. Pharmacokinetics, safety, and tolerability of intramuscular Ziprasidone in healthy volunteers. J. Clin. Pharmacol. 2005, 45, 620–630. [Google Scholar] [CrossRef]
- Preskorn, S.H. Pharmacokinetics and therapeutics of acute intramuscular ziprasidone. Clin. Pharmacokinet. 2005, 44, 1117–1133. [Google Scholar] [CrossRef]
- Miceli, J.J.; Smith, M.; Robarge, L.; Morse, T.; Laurent, A. The effects of ketoconazole on ziprasidone pharmacokinetics—A placebo-controlled crossover study in healthy volunteers. Br. J. Clin. Pharmacol. 2000, 49, 71–76. [Google Scholar] [CrossRef]
- Martini, L.G.; Crowley, P.J. Controlling drug release in oral product development programs: An industrial Perspective. In Controlled Release in Oral Drug Delivery; Springer: New York, NY, USA, 2011; pp. 45–70. [Google Scholar]
- Shid, R.L.; Dhole, S.N.; Kulkarni, N.; Shid, S.L. Nanosuspension: A review. Int. J. Pharm. Sci. Rev. 2013, 22, 98–106. [Google Scholar]
- Yadav, M.; Dhole, S.; Chavanet, P. Nanosuspension: A Novel Techniques In Drug Delivery System. World J. Pharm. Pharm. Sci. 2014, 3, 410–433. [Google Scholar]
- Nishi, T.; Garima, G.; Sharma, P.; Nitin, K. Nanoemulsions: A Review on Various Pharmaceutical Applications. Glob. J. Pharmacol. 2012, 6, 222–225. [Google Scholar]
- Theaj Ravi, U.P.; Thiagaraja, P. Irritancymake it a suitable carrier for the Nanoemulsions for drug delivery through different transdermal delivery of the drugs in the routes. Res. Biotech. 2011, 2, 1–13. [Google Scholar]
- Sharma, N.; Bansal, M.; Visht, S. Nanoemulsion: A new concept of delivery applicationofnanoemulsion. System 2010, 1, 2–6. [Google Scholar]
- Devarajan, V.; Ravichandran, V. Nanoemulsions: As Modified Drug Delivery Tool. Int. J. Comp. Pharm. 2011, 4, 1–6. [Google Scholar]
- Shah, P.; Bhalodia, D. Nanoemulsion: A Pharmaceutical Review. Syst. Rev. Pharm. 2010, 1, 24–32. [Google Scholar] [CrossRef]
- Vyas, T.K.; Babbar, A.K.; Sharma, R.K.; Singh, S.; Mishra, A. Intranasal mucoadhesive microemulsion of clonazepam preliminary studies on brain targeting. J. Pharm. Sci. 2006, 95, 570–580. [Google Scholar] [CrossRef]
- Khan, S.; Patil, K.; Yeole, P.; Gaikwad, R. Brain targeting studies on buspirone hydrochloride after intranasal administration of mucoadhesive formulation in rats. J. Pharm. Pharmacol. 2009, 61, 669–675. [Google Scholar] [CrossRef]
- Luppi, B.; Bigucci, F.; Abruzzo, A.; Corace, G.; Cerchiara, T.; Zecchi, V. Transport of drugs from nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 2009, 11, 1–18. [Google Scholar]
- Illum, L. Nasal drug delivery: New developments and strategies. Drug Disc. Today 2002, 7, 1184–1189. [Google Scholar] [CrossRef]
- Illum, L.; Hinchelife, M.; David, S.S. The effect of blood sampling site and physicochemical characteristics of drugs on bioavailability of drugs on administration in the sheep model. Pharm. Res. 2003, 27, 1474–1485. [Google Scholar] [CrossRef]
- Mistry, A.; Stolnic, S.; Illum, L. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pham. 2008, 379, 146–157. [Google Scholar] [CrossRef]
- Ugwoke, M.; Agu, R.; Verbeke, N.; Kinget, R. Nasal mucoadhesive drug delivery: Bachground, applications, trends and future perspectives. Adv. Drug Deliv. Rev. 2005, 57, 1640–1665. [Google Scholar] [CrossRef]
- Gavini, E.; Hegge, A.B.; Rassu, G. Nasal administration of carbamazepine using chitosan microspheres: In-vitro/in-vivo studies. Int. J. Pharm. 2006, 307, 9–15. [Google Scholar] [CrossRef]
- Pires, A.; Fortuna, A.; Alves, G.; Falcao, A. Intranasal drug delivery; how, why and what for? J. Pharm. Pharm. Sci. 2009, 12, 288–311. [Google Scholar] [CrossRef]
- Trotta, M. Influence of phase transformation on indomethacin release from microemulsions. J. Control. Release 1999, 60, 399–443. [Google Scholar] [CrossRef]
- Figueroa Alvarez, M.J.; Blanco-Méndez, J. Transdermal delivery of methotrexate: Iontophoretic delivery from hydrogels and passive delivery from microemulsions. Int. J. Pharm. 2001, 215, 57–65. [Google Scholar] [CrossRef]
- Patil, J.; Sayyed, H.; Suryawanshi, H.; Patil, B. Formulation and Evaluation of Verdant Tablets Containing Saponin-Coalesc-21 ed Silver Nanoparticles Got from Fenugreek Seed Extract. Chem. Proc. 2022, 8, 56. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).