A Mini-Review on Graphene: Exploration of Synthesis Methods and Multifaceted Properties †
Abstract
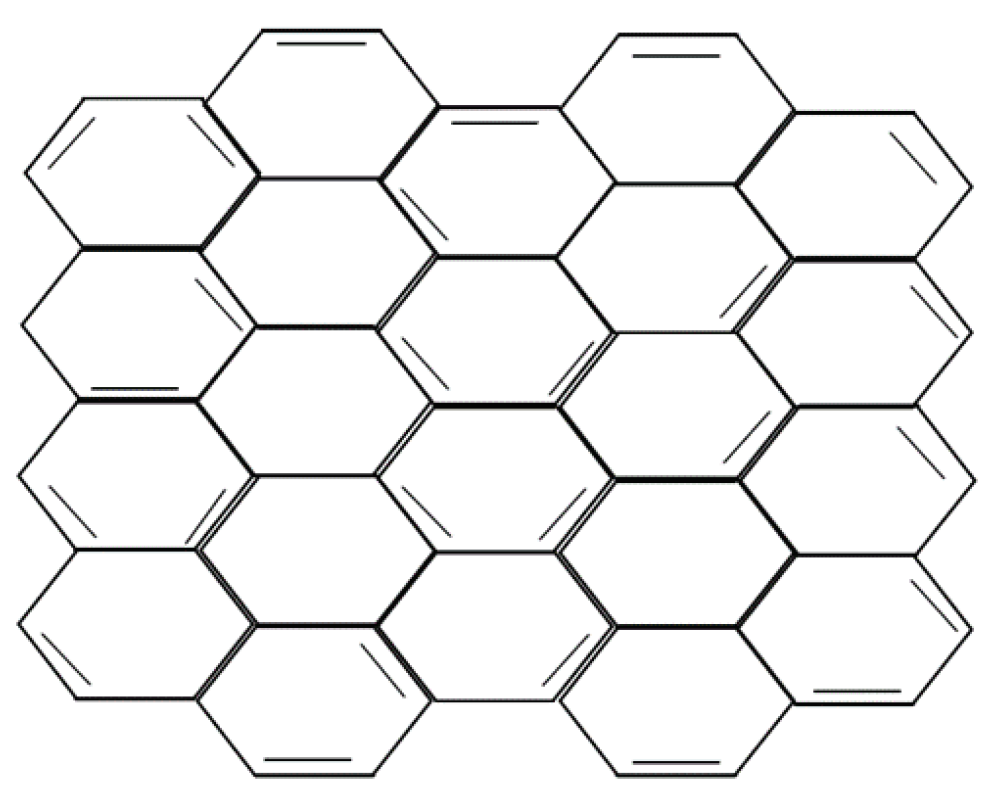
1. Introduction
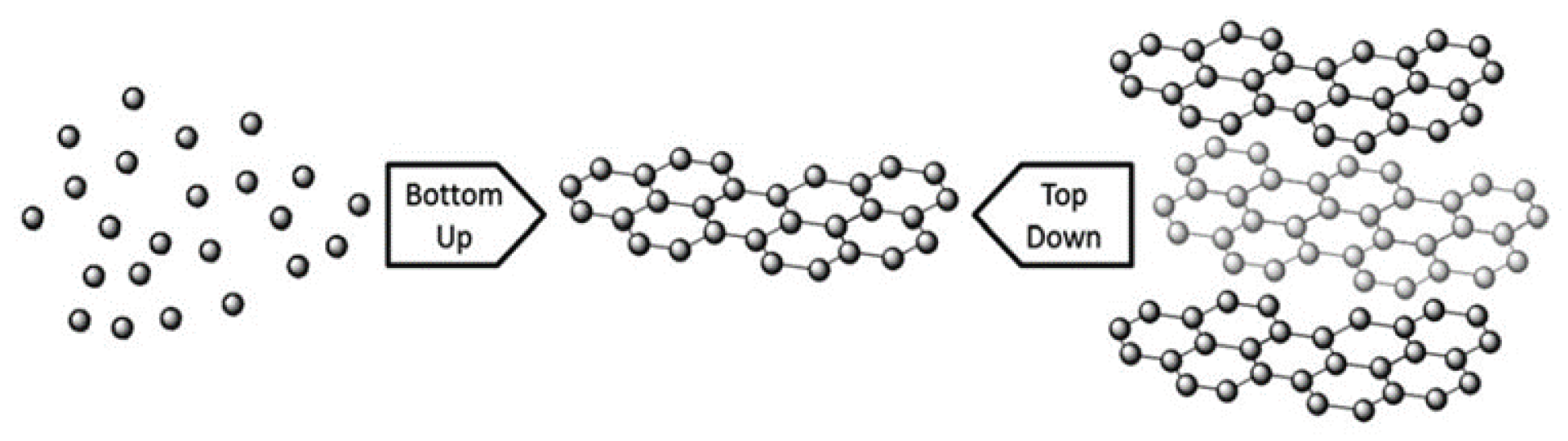
2. Synthesis of Graphene
2.1. First Approach: Bottom-Up Methods
2.2. Second Approach: Top-Down Methods
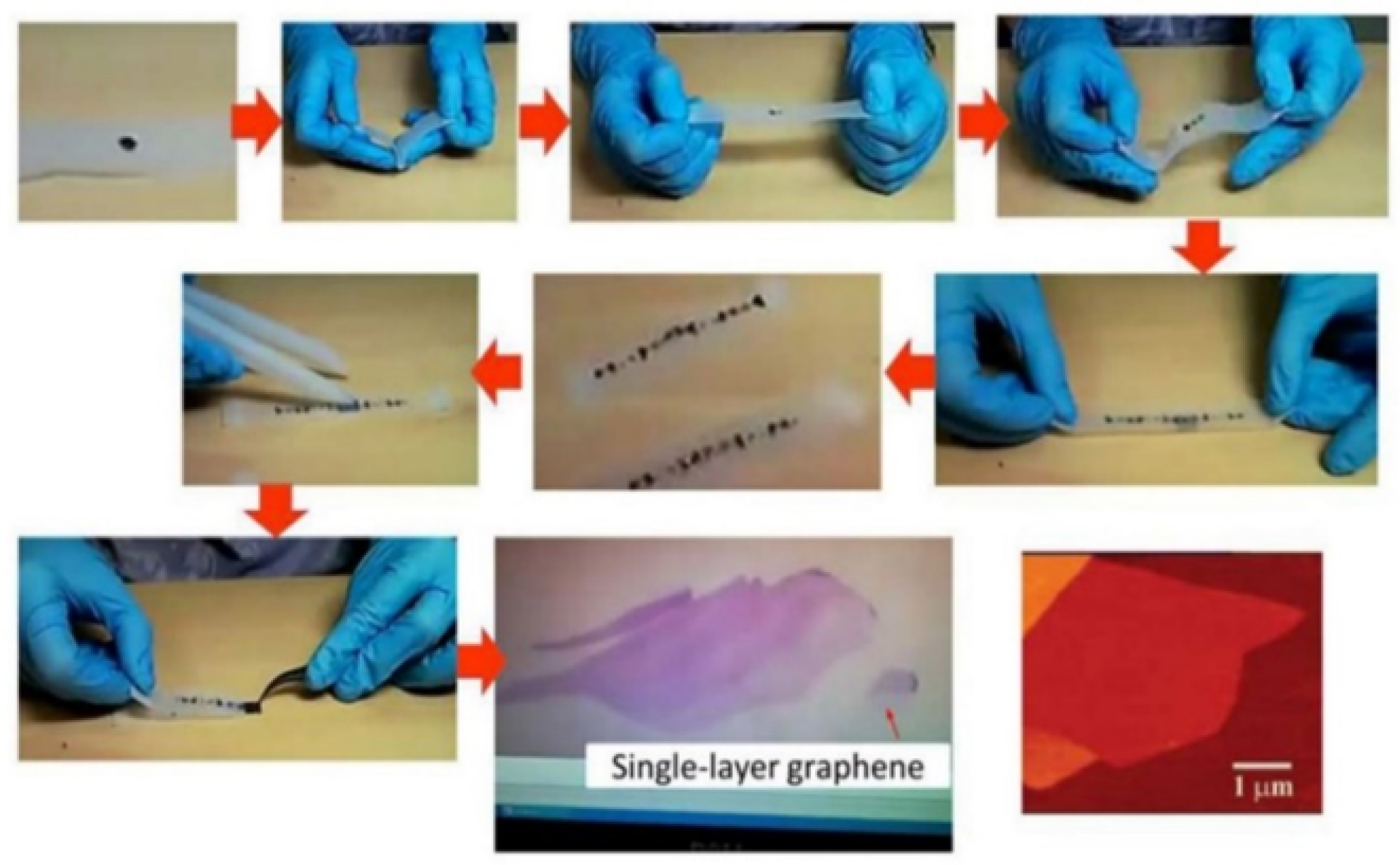
- Mechanical cleavage (adhesive tape) [29]
- Liquid-phase exfoliation [30]
- Electrochemical exfoliation of graphite [31]
- Solvothermal synthesis combined with pyrolysis [32]
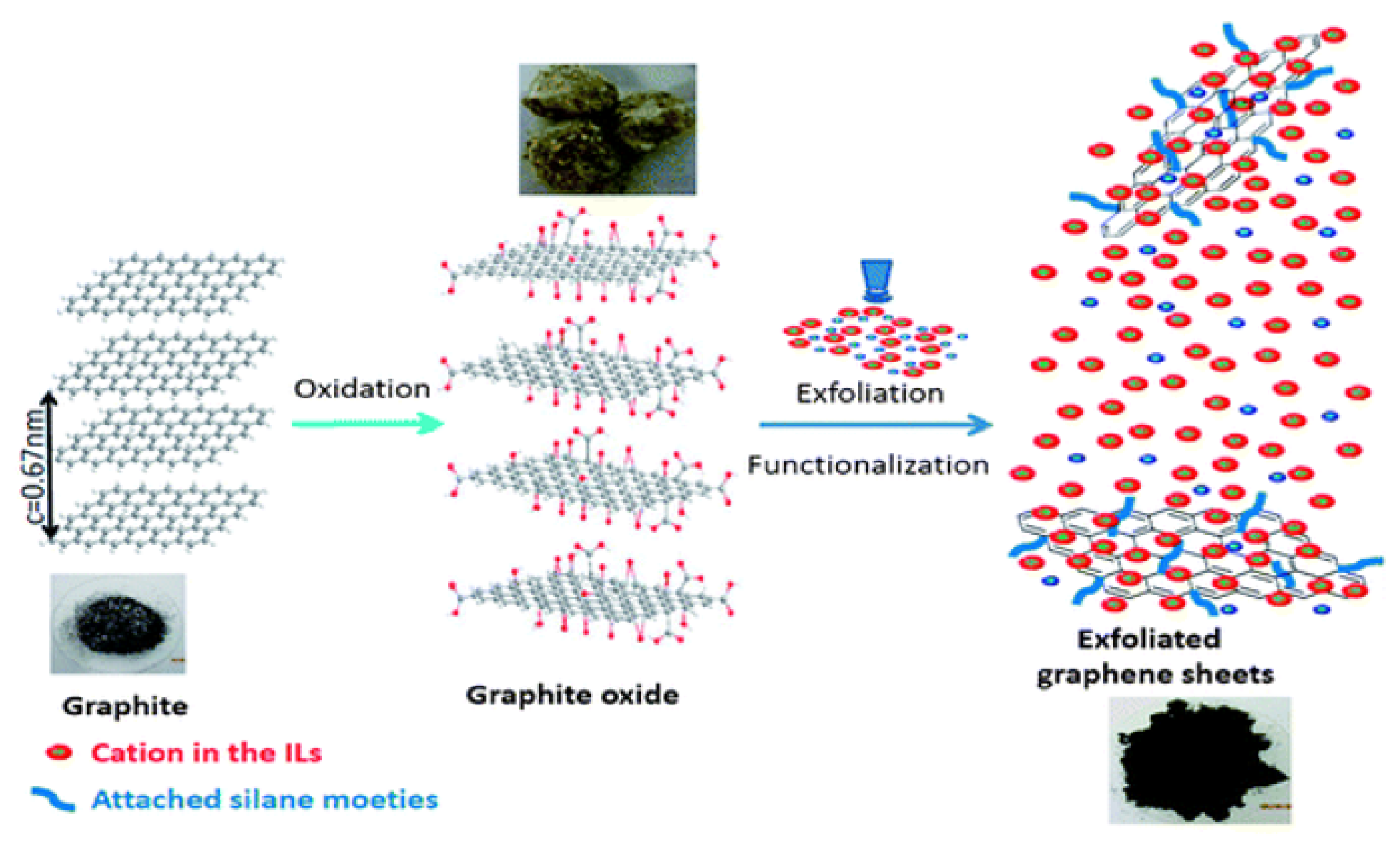
- Exfoliation of graphite intercalation compounds (GICs) [33]
- Chemical reduction of GO [34]
- Photothermal reduction of GO [35]
- Thermal exfoliation of graphite oxide [36]
- Electrochemical method to reduce GO [37]
3. Chemical Vapor Deposition (CVD)
4. Epitaxial Growth on SiC
5. Mechanical Cleavage
6. Exfoliation of Graphite Oxide
7. Properties of Graphene
7.1. Electronic Properties
7.2. Mechanical Properties
7.3. Optical Properties
7.4. Thermal Properties
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, L.-X.; Chen, Q. Properties, synthesis, and characterization of graphene. Front. Mater. Sci. China 2010, 4, 45–51. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-Dimensional Gas of Massless Dirac Fermions in Graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-M.; Dimitrakopoulos, C.; Jenkins, K.A.; Farmer, D.B.; Chiu, H.-Y.; Grill, A.; Avouris, P. 100-GHz Transistors from Wafer-Scale Epitaxial Graphene. Science 2010, 327, 662. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, X.; Chen, P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 2012, 41, 2283–2307. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Huang, M.; Pascal, T.A.; Kim, H.; Goddard, W.A., III; Greer, J.R. Electronic-Mechanical Coupling in Graphene from In Situ Nanoindentation Experiments and Multiscale Atomistic Simulations. Nano Lett. 2011, 11, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Hass, J.; Heer, W.D.; Conrad, E. The growth and morphology of epitaxial multilayer graphene. J. Phys. Condens. Matter 2008, 20, 323202. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Liu, J.; Xue, Y.; Zhang, M.; Dai, L. Graphene-Based Materials for Energy Applications. MRS Bull. 2012, 37, 1265–1272. [Google Scholar] [CrossRef]
- Yoo, E.; Okata, T.; Akita, T.; Kohyama, M.; Nakamura, J.; Honma, I. Enhanced Electrocatalytic Activity of Pt Subnanoclusters on Graphene Nanosheet Surface. Nano Lett. 2009, 9, 2255–2259. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, Y.; Cai, W.; Borysiak, M.; Han, B.; Chen, D.; Piner, R.D.; Colombo, L.; Ruoff, R.S. Transfer of Large-Area Graphene Films for High-Performance Transparent Conductive Electrodes. Nano Lett. 2009, 9, 4359–4363. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.; Tsao, N.; Tomović, Ž.; Li, J.; Müllen, K. Transparent Carbon Films as Electrodes in Organic Solar Cells. Angew. Chem. Int. Ed. 2008, 47, 2990–2992. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef]
- Yoo, E.; Kim, J.; Hosono, E.; Zhou, H.-S.; Kudo, T.; Honma, I. Large Reversible Li Storage of Graphene Nanosheet Families for Use in Rechargeable Lithium Ion Batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tao, H.; Yang, K.; Feng, L.; Cheng, L.; Shi, X.; Li, Y.; Guo, L.; Liu, Z. A Functionalized Graphene Oxide-Iron Oxide Nanocomposite for Magnetically Targeted Drug Delivery, Photothermal Therapy, and Magnetic Resonance Imaging. Nano Res. 2012, 5, 199–212. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Hussein, F.H.; Alkaim, A.F. Removal of textile dye (methylene blue mb) from aqueous solution by activated carbon as a model (corn-cob source waste of plant): As a model of environmental enhancement. Plant Arch. 2019, 19, 906–909. [Google Scholar]
- Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-Layered Graphene Oxide Nanosheets as Superior Sorbents for Heavy Metal Ion Pollution Management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef] [PubMed]
- Khurana, I.; Saxena, A.; Bharti; Khurana, J.M.; Rai, P.K. Removal of Dyes Using Graphene-Based Composites: A Review. Water Air Soil Pollut. 2017, 228, 1–17. [Google Scholar] [CrossRef]
- Gandhi, M.R.; Vasudevan, S.; Shibayama, A.; Yamada, M. Graphene and Graphene-Based Composites: A Rising Star in Water Purification—A Comprehensive Overview. ChemistrySelect 2016, 1, 4358–4385. [Google Scholar] [CrossRef]
- Alwan, S.H.; Alshamsi, H.A. In situ synthesis NiO/F-MWCNTs nanocomposite for adsorption of malachite green dye from polluted water. Carbon Lett. 2022, 32, 1073–1084. [Google Scholar] [CrossRef]
- Edwards, R.S.; Coleman, K.S. Graphene synthesis: Relationship to applications. Nanoscale 2013, 5, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Peng, Z.; Tour, J.M. Chemical vapor deposition of graphene single crystals. Accounts Chem. Res. 2014, 47, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.I.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. Graphene Oxide Dispersions in Organic Solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef]
- Tour, J.M. Top-down versus bottom-up fabrication of graphene-based electronics. Chem. Mater. 2013, 26, 163–171. [Google Scholar] [CrossRef]
- Li, D.; Kaner, R.B. Graphene-based materials. Nat. Nanotechnol. 2008, 3, 101. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, C.; Hao, R.; Hou, Y. Liquid-Phase Exfoliation, Functionalization and Applications of Graphene. Nanoscale 2011, 3, 2118–2126. [Google Scholar] [CrossRef]
- Wang, G.; Wang, B.; Park, J.; Wang, Y.; Sun, B.; Yao, J. Highly Efficient and Large-Scale Synthesis of Graphene by Electrolytic Exfoliation. Carbon 2009, 47, 3242–3246. [Google Scholar] [CrossRef]
- Choucair, M.; Thordarson, P.; Stride, J.A. Gram-Scale Production of Graphene Based on Solvothermal Synthesis and Sonication. Nat. Nanotechnol. 2009, 4, 30. [Google Scholar] [CrossRef]
- Vallés, C.; Drummond, C.; Saadaoui, H.; Furtado, C.A.; He, M.; Roubeau, O.; Ortolani, L.; Monthioux, M.; Pénicaud, A. Solutions of Negatively Charged Graphene Sheets and Ribbons. J. Am. Chem. Soc. 2008, 130, 15802–15804. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Samulski, E.T. Synthesis of Water Soluble Graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-Graphene Nanocomposites. UV-Assisted Photocatalytic Reduction of Graphene Oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Schniepp, H.C.; Li, J.-L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized Single Graphene Sheets Derived from Splitting Graphite Oxide. J. Phys. Chem. B 2006, 110, 8535–8539. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, X.; Zhang, J.; Boey, F.; Zhang, H. Direct Electrochemical Reduction of Single-Layer Graphene Oxide and Subsequent Functionalization with Glucose Oxidase. J. Phys. Chem. C 2009, 113, 14071–14075. [Google Scholar] [CrossRef]
- Somani, P.R.; Somani, S.P.; Umeno, M. Planar Nano-Graphenes from Camphor by CVD. Chem. Phys. Lett. 2006, 430, 56–59. [Google Scholar] [CrossRef]
- Bader, A.T.; Aljeboree, A.M.; Alkaim, A.F. Removal of Methyl Violet (MV) from Aqueous Solutions by Adsorption Using Activated Carbon from Pine Husks (Plant Waste Sources). Plant Arch. 2019, 19, 898–901. [Google Scholar]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Coraux, J.; N‘Diaye, A.T.; Busse, C.; Michely, T. Structural Coherency of Graphene on Ir (111). Nano Lett. 2008, 8, 565–570. [Google Scholar] [CrossRef]
- Sutter, P.; Sadowski, J.T.; Sutter, E. Graphene on Pt (111): Growth and substrate interaction. Phys. Rev. B 2009, 80, 245411. [Google Scholar] [CrossRef]
- Varykhalov, A.; Rader, O. Graphene Grown on Co (0001) Films and Islands: Electronic Structure and Its Precise Magnetization Dependence. Phys. Rev. B 2009, 80, 035437. [Google Scholar] [CrossRef]
- Kwon, S.-Y.; Ciobanu, C.V.; Petrova, V.; Shenoy, V.B.; Bareno, J.; Gambin, V.; Petrov, I.; Kodambaka, S. Growth of Semiconducting Graphene on Palladium. Nano Lett. 2009, 9, 3985–3990. [Google Scholar] [CrossRef]
- Miniussi, E.; Pozzo, M.; Baraldi, A.; Vesselli, E.; Zhan, R.R.; Comelli, G.; Menteş, T.O.; Niño, M.A.; Locatelli, A.; Lizzit, S.; et al. Thermal Stability of Corrugated Epitaxial Graphene Grown on Re (0001). Phys. Rev. Lett. 2011, 106, 216101. [Google Scholar] [CrossRef]
- Sutter, P.W.; Flege, J.-I.; Sutter, E.A. Epitaxial Graphene on Ruthenium. Nat. Mater. 2008, 7, 406. [Google Scholar] [CrossRef]
- Hass, J.; Feng, R.; Millán-Otoya, J.E.; Li, X.; Sprinkle, M.; First, P.N.; De Heer, W.A.; Conrad, E.H.; Berger, C. Structural Properties of the Multilayer Graphene/4H-SiC(000) System as Determined by Surface X-ray Diffraction. Phys. Rev. B 2007, 75, 214109. [Google Scholar] [CrossRef]
- Mosaa, Z.A.; Bader, A.T.; Aljeboree, A.M.; Alkaim, A.F. Adsorption and Removal of Textile Dye (Methylene Blue MB) from Aqueous Solution by Activated Carbon as a Model (Apricot Stone Source Waste) of Plant Role in Environmental Enhancement. Plant Arch. 2019, 19, 910–914. [Google Scholar]
- Hicks, J.; Shepperd, K.; Wang, F.; Conrad, E.H. The Structure of Graphene Grown on the SiC Surface. J. Phys. D Appl. Phys. 2012, 45, 154002. [Google Scholar] [CrossRef]
- Hannon, J.; Tromp, R. Pit Formation During Graphene Synthesis on SiC (0001): In Situ Electron Microscopy. Phys. Rev. B 2008, 77, 241404. [Google Scholar] [CrossRef]
- Coleman, J.N. Liquid Exfoliation of Defect-Free Graphene. Acc. Chem. Res. 2012, 46, 14–22. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. A Review on Mechanical Exfoliation for the Scalable Production of Graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Alqaragully, M.B.; Al-Gubury, H.Y.; Aljeboree, A.M.; Karam, F.F.; Alkaim, A.F. Monoethanolamine: Production Plant. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1287–1296. [Google Scholar]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-Yield Production of Graphene by Liquid-Phase Exfoliation of Graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Eur. J. Inorg. Chem. 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Lei, Y.; Madalena, L.d.S.; Ossonon, B.D.; Junior, F.E.B.; Chen, J.; Lanza, M.R.V.; Tavares, A.C. One-Step Synthesis of Aminobenzoic Acid Functionalized Graphene Oxide by Electrochemical Exfoliation of Graphite for Oxygen Reduction to Hydrogen Peroxide and Supercapacitors. Molecules 2022, 27, 7629. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Alkaim, A.F. Comparative Removal of Three Textile Dyes from Aqueous Solutions by Adsorption: As a Model (Corn-Cob Source Waste) of Plants Role in Environmental Enhancement. Plant Arch. 2019, 19, 1613–1620. [Google Scholar]
- Fu, Y.; Zhang, J.; Liu, H.; Hiscox, W.C.; Gu, Y. Ionic Liquid-Assisted Exfoliation of Graphite Oxide for Simultaneous Reduction and Functionalization to Graphenes with Improved Properties. J. Mater. Chem. A 2013, 1, 2663–2674. [Google Scholar] [CrossRef]
- Chen, X.M.; Wu, G.H.; Jiang, Y.Q.; Wang, Y.R.; Chen, X. Graphene and Graphene-Based Nanomaterials: The Promising Materials for Bright Future of Electroanalytical Chemistry. Analyst 2011, 136, 4631–4640. [Google Scholar] [CrossRef]
- Alkaim, A.F.; Ajobree, A.M. White Marble as an Alternative Surface for Removal of Toxic Dyes (Methylene Blue) from Aqueous Solutions. Int. J. Adv. Sci. Technol. 2020, 29, 5470–5479. [Google Scholar]
- Zhang, Y.; Tan, Y.W.; Stormer, H.L.; Kim, P. Experimental Observation of the Quantum Hall Effect and Berry’s Phase in Graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef]
- Liu, F.; Ming, P.; Li, J. Ab Initio Calculation of Ideal Strength and Phonon Instability of Graphene Under Tension. Phys. Rev. B 2007, 76, 064120. [Google Scholar] [CrossRef]
- Gao, Y.; Hao, P. Mechanical Properties of Monolayer Graphene Under Tensile and Compressive Loading. Phys. E Low-Dim. Syst. Nanostruct. 2009, 41, 1561–1566. [Google Scholar] [CrossRef]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; Van Der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable Atomic Membranes from Graphene Sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef]
- Mak, K.F.; Sfeir, M.Y.; Wu, Y.; Lui, C.H.; Misewich, J.A.; Heinz, T.F. Measurement of the Optical Conductivity of Graphene. Phys. Rev. Lett. 2008, 101, 196405. [Google Scholar] [CrossRef] [PubMed]
- Dawlaty, J.M.; Shivaraman, S.; Chandrashekhar, M.; Rana, F.; Spencer, M.G. Measurement of Ultrafast Carrier Dynamics in Epitaxial Graphene. Appl. Phys. Lett. 2008, 92, 042116. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene Photonics and Optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Seol, J.H.; Jo, I.; Moore, A.L.; Lindsay, L.; Aitken, Z.H.; Pettes, M.T.; Li, X.; Yao, Z.; Huang, R.; Broido, D.; et al. Two-Dimensional Phonon Transport in Supported Graphene. Science 2010, 328, 213–216. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alwan, S.H.; Omran, A.A.; Naser, D.K.; Ramadan, M.F. A Mini-Review on Graphene: Exploration of Synthesis Methods and Multifaceted Properties. Eng. Proc. 2023, 59, 226. https://doi.org/10.3390/engproc2023059226
Alwan SH, Omran AA, Naser DK, Ramadan MF. A Mini-Review on Graphene: Exploration of Synthesis Methods and Multifaceted Properties. Engineering Proceedings. 2023; 59(1):226. https://doi.org/10.3390/engproc2023059226
Chicago/Turabian StyleAlwan, Salam Hussein, Alaa A. Omran, Dalya K. Naser, and Montather F. Ramadan. 2023. "A Mini-Review on Graphene: Exploration of Synthesis Methods and Multifaceted Properties" Engineering Proceedings 59, no. 1: 226. https://doi.org/10.3390/engproc2023059226
APA StyleAlwan, S. H., Omran, A. A., Naser, D. K., & Ramadan, M. F. (2023). A Mini-Review on Graphene: Exploration of Synthesis Methods and Multifaceted Properties. Engineering Proceedings, 59(1), 226. https://doi.org/10.3390/engproc2023059226




