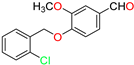
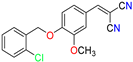
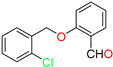
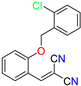
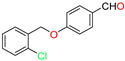
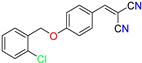
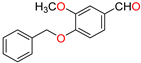
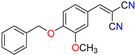
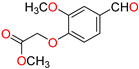
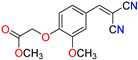
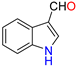
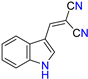
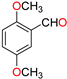
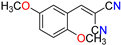
Abstract
In the present study, we investigated the catalytic power of boric acid used for the synthesis of 2-alkylidene/arylidene derivatives resulting from active methylene compounds and 4-chlorobenzaldehyde in the presence of 10 mol% of boric acid in ethanol under conventional conditions. We achieved good-to-excellent yields of synthesized products and then characterized them using conventional spectroscopic techniques.
1. Introduction
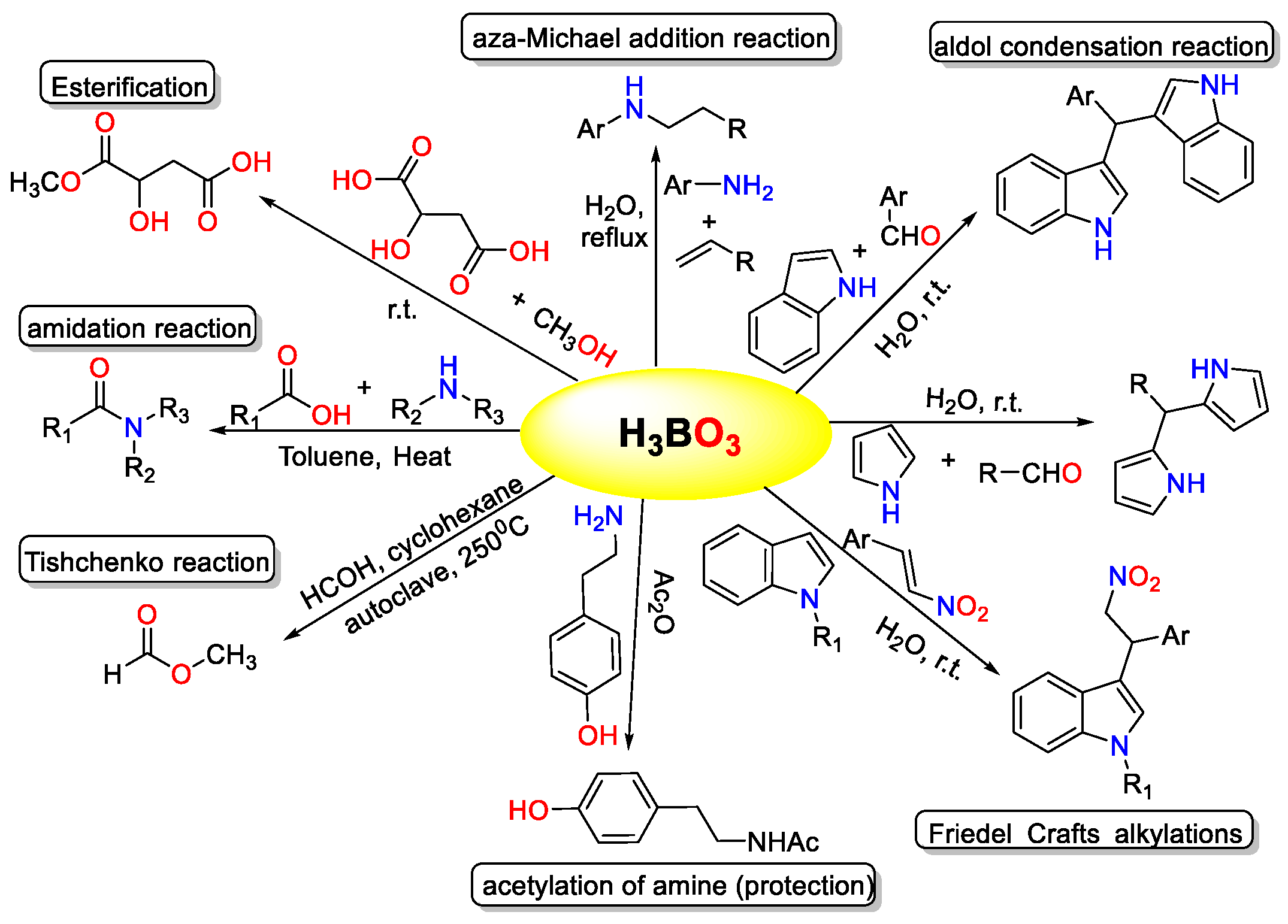
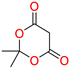
The Knoevenagel condensation, which involves the reaction of aldehydes with active methylene compounds, is a highly significant chemical conversion with broad application potential in organic synthesis [1]. It serves as a fundamental method for creating carbon–carbon bonds, playing a crucial role in the production of pharmaceutically and biologically active compounds [2,3]. In various organic transformations, boric acid has proven to be an effective catalyst (Figure 1) [4,5,6,7,8,9,10]. Its catalytic properties have been exploited in numerous reactions, including decarboxylation, bromination, amidation, esterification, trans-esterification, β-acetamido ketone synthesis, condensation reactions, ipso-hydroxylation, Mannich reactions, aza-Michael addition, and Biginelli reactions, among others. The versatility of boric acid as a catalyst underscores its importance in facilitating diverse synthetic processes [4,5,6,7,8,9,10].
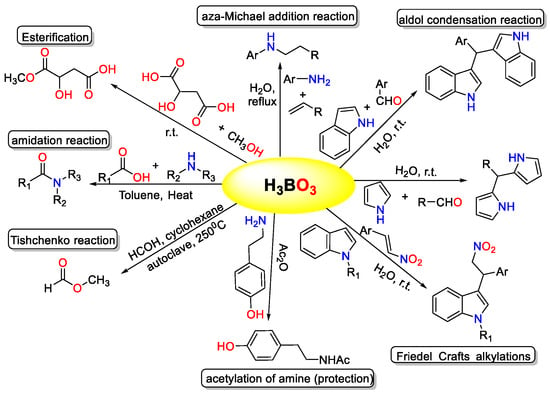
Figure 1.
Different chemical conversion catalyzed by boric acid.
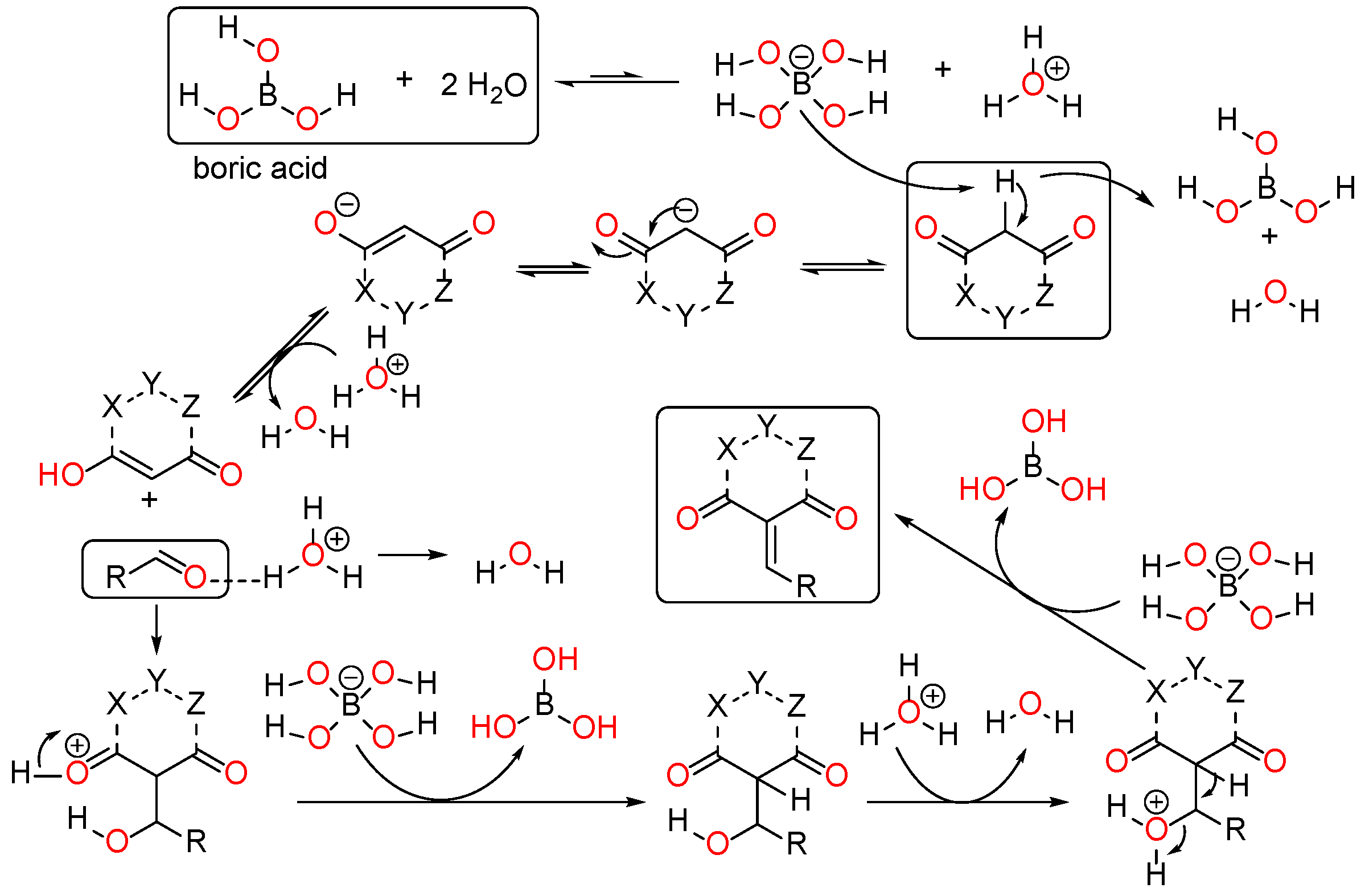
Multiple mechanisms involving Bronsted acid catalysis have been proposed for the Knoevenagel condensation process (Figure 2) [4]. The commonly utilized active methylene compounds include acyclic 1,3-dicarbonyls, as well as analogous compounds such as malononitrile, acetonitrile, acetyl acetone, acetoacetates, malonates, and others. Various cyclic compounds, such as oxazepanediones, Meldrum’s acid, etc., were also found to be employed. In certain instances, isolating the Knoevenagel product becomes challenging due to the rapid Michael addition of adduct with a second molecule of the active methylene compound. β, γ-unsaturated products were often observed as part of the isomerization of α, β-unsaturated products. Various catalysts have been reported, including phase transfer catalysts (PTCs), KF, Bronsted acids, Lewis acids, and amines and their corresponding ammonium salts, among others. Further, we did not notice any utilization of metal salts of such methylene compounds. Consequently, numerous mechanisms have been proposed to explain the reaction (Figure 2). In addition, our lab has recently explored some biologically active compounds with the use of various catalysts and theoretical methods [11,12].
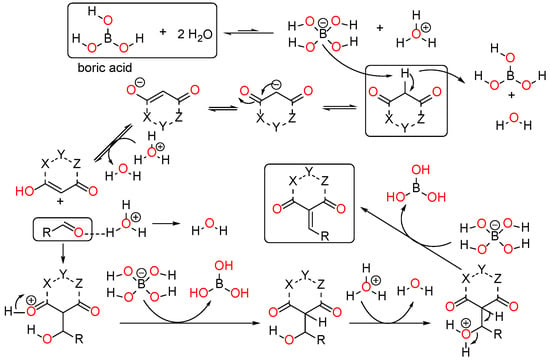
Figure 2.
Possible mechanism of Knoevenagel condensation in the presence of boric acid catalysts in ethanol.
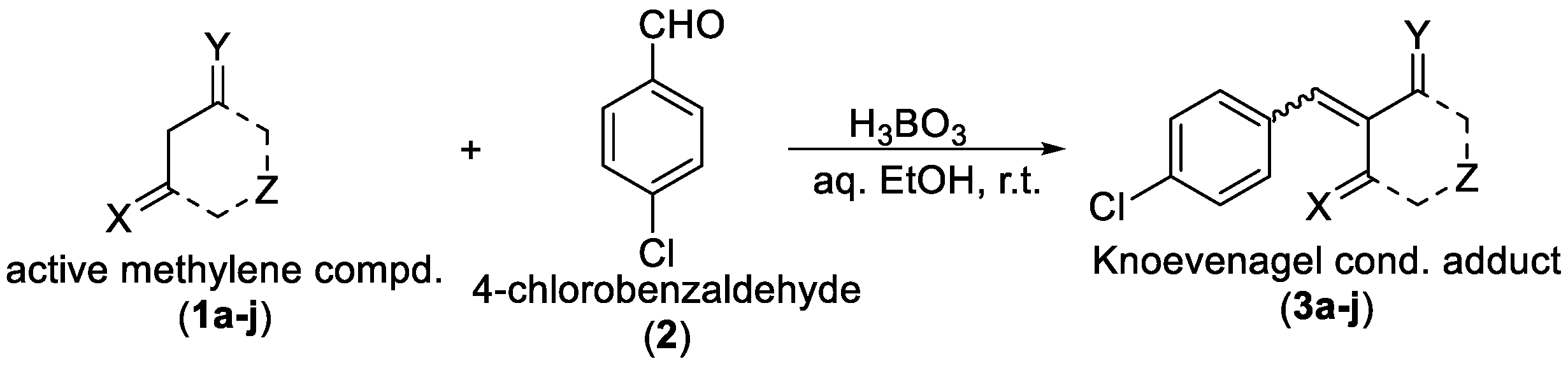
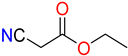
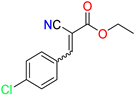
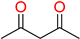
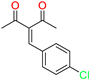
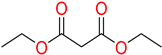
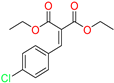
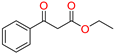
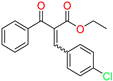
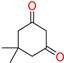
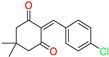
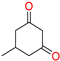
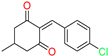
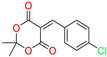
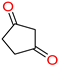
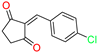
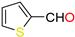
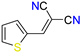
The ethanol used to contain small amounts of water reacted with boric acid B(OH)4- following the release of H+ in the solution. Based on this, the possible mechanism is shown in Figure 2. Initially, an enolate of the activated methylene compound was added to a carbonyl group of aldehydes, initially activated by H+, which led to the formation of tetrahedral intermediates. It further underwent acid-catalyzed dehydration in response to the condensed unsaturated product. The reaction was carried out in a limited amount of water; otherwise, under aqueous conditions, the starting material or product would dissolve in water or in absence of water, as boric acid does not act as catalyst. Considering the potential of boric acid as a catalyst, we developed a new methodology for the synthesis of Knoevenagel condensation products (3a–j) (Scheme 1). This methodology utilizes boric acid as a catalyst and involves the condensation of active methylene compounds (1a–j) with 4-chlorobenzaldehyde (2) in aqueous ethanol at room temperature.
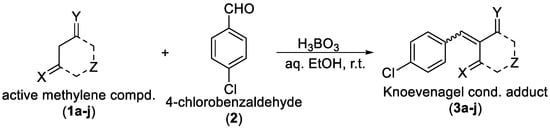
Scheme 1.
Synthesis of Knoevenagel condensation adduct (3a–j) from active methylene compounds (1a–j) and 4-chlorobenzaldehyde (2).
2. Materials and Methods
The synthesis experiments were conducted using commercially available p-Chlorobenzaldehyde, active methylene compounds, and solvents obtained from commercial chemical sources. These chemicals were utilized in their as-purchased state without undergoing any purification procedures. The reactions took place in a reaction vessel equipped with a condenser under atmospheric pressure and magnetic stirring. Melting points reported herein were uncorrected and checked using traditional methods. All synthesized compounds were tested for their 1H-NMR spectra, with CDCl3 as a solvent on a Bruker NMR machine. Shimatzu GCMS was used to analyze the molecular masses of synthesized compounds. A Bruker Tensor 2 model was used to record the Fourier transform infrared spectroscopy (FTIR) of compounds.
Synthesis
To perform the synthesis, 1 mmol of p-chlorobenzaldehyde and 1 mmol of active methylene compounds were dissolved in 5 mL of aqueous ethanol at room temperature. Then, 10 mol% of boric acid catalyst was added and the solution was stirred on a magnetic stirrer until the reaction was complete (reaction time recorded in the following table). The completion of the reaction was monitored by performing TLC in a mixture of 10 parts of ethyl acetate and 1 of part hexane. After the reaction was complete, the contents were cooled in an ice bath; the solid product was filtered and then it was either washed with cold ethanol or the product was extracted in ethyl acetate. The yield and melting point were recorded, and the product was characterized by performing spectral analysis.
3. Results and Discussion
For the synthesis of various heterocyclic compounds from active methylene compounds, the Knoevenagel condensation product was obtained as an intermediate or it was deemed one of the major impurities formed during the reaction due to a slight excess of reagents [4,5,6,7,8,9,10]. Therefore, synthesizing such an intermediate is crucial. To identify a suitable catalyst for the Knoevenagel condensation between 4-chlorobenzaldehyde and malononitrile in aqueous ethanol at room temperature, we conducted the reaction in the presence of various organic compounds and salts, including diethylamine, morpholine, ammonium chloride, sodium bicarbonate, copper sulfate, ferric chloride, nickel chloride, nanomaterials, sodium bicarbonate, boric acid, L-proline, etc.
Our research unveiled boric acid as a highly efficient catalyst, demonstrating remarkable catalytic activity, a high product yield, and facile purification via cold ethanol washing. To enhance the scope of boric acid’s catalytic applications, we conducted Knoevenagel condensation reactions between 4-chlorobenzaldehyde and various acyclic and cyclic active methylene compounds. The results unequivocally established boric acid as a potent Bronsted acid catalyst for this reaction. In the characterization of unknown compounds, infrared spectroscopy emerged as a valuable tool, particularly for identifying functional groups. However, extracting comprehensive structural information solely from an infrared spectrum can be challenging due to the presence of multiple absorption bands.
Notably, the carbonyl group, indicative of a carbon–oxygen double bond, manifested distinct and localized vibrations in numerous interacting compounds. Within esters and ketones, the absorption range for the carbonyl group was observed between 1753 and 1674 cm−1. Furthermore, the C-O stretching vibrations of esters and ethers were evident within the 1300–1100 cm−1 region. Characterizing aromatic compounds frequently revealed strong bands below 1000 cm−1. Regarding the aromatic ring’s C=C bonds, absorption bands were observed in the range of 1600–1500 cm−1. Moreover, the cyanide group of compounds 3a-b exhibited prominent stretching vibrations at 2222–2225 cm−1, while the newly formed olefinic bond was characterized by stretching vibrations in the range of 1564–1609 cm−1. The formed olefinic bond showed a strong absorption band around 1485 cm−1 in 3i-3r. The product formation was confirmed by recording the GC-MS of the molecules. In the mass spectra of (3a) and (3f), two peaks (M and M + 2) were observed with an intensity ratio of 3:1. The yield of the product depends on the reactivity and stability of the active methylene group, rather than the electronic nature of the group attached to the aldehydes (Table 1) (Please refer Supplementary Material for spectral data).

Table 1.
Reaction time, yield, color, physical constant, and IR stretching wavenumbers of Knoevenagel condensation adduct (3a–j).
4. Conclusions
In conclusion, the synthesis of heterocyclic compounds using the Knoevenagel condensation reaction involving active methylene compounds is a valuable method in organic chemistry. The Knoevenagel condensation product serves as an intermediate or a major impurity in the reaction due to the excess use of reagents, highlighting the importance of synthesizing and understanding such intermediates. Through extensive experimentation, boric acid has been identified as an effective catalyst for Knoevenagel condensation, exhibiting good catalytic activity and high yields, and facilitating the easy purification of products. The reaction between 4-chlorobenzaldehyde and various acyclic and cyclic active methylene compounds has demonstrated the versatility and efficiency of boric acid as a Bronsted acid catalyst. The characterization of synthesized compounds through spectral analysis techniques, including proton magnetic resonance (PMR) spectroscopy, mass spectrometry (GC-MS), and infrared spectroscopy (FTIR), has provided insights into the structural features and functional groups present in the products. Overall, the findings of this study help to improve our understanding of boric acid and its application as a catalyst in the synthesis of heterocyclic compounds using the Knoevenagel condensation reaction.
Supplementary Materials
The spectral data can be downloaded at: https://www.mdpi.com/article/10.3390/ASEC2023-15366/s1.
Author Contributions
Conceptualization, S.N.M., B.R.T. and S.D.T.; methodology, D.M. and B.R.T.; software, S.N.M.; writing—review and editing, S.N.M. and B.R.T.; visualization, S.N.M. and B.R.T.; supervision, B.R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors are grateful to the Principal and Head, Department of Chemistry, Government College of Arts and Science, Chhatrapati. Sambhajinagar, Maharashtra, India for his constant encouragement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Knoevenagel, E. Condensation of malonic acid with aromatic aldehydes via ammonia and amines. Chem. Ber. 1898, 31, 2596–2619. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Toyao, T.; Fujiwaki, M.; Dohshi, S.; Kim, T.; Matsuoka, M. Zeolitic imidazolate frameworks as heterogeneous catalysts for a one-pot P–C bond formation reaction via Knoevenagel condensation and phospha-Michael addition. RSC Adv. 2015, 5, 24687–24690. [Google Scholar] [CrossRef]
- Sobhani, S.; Hasaninejad, A.; Maleki, M.F.; Parizi, Z.P. Tandem Knoevenagel–Michael Reaction of 1-Phenyl-3-methyl-5-pyrazolone with Aldehydes Using 3-Aminopropylated Silica Gel as an Efficient and Reusable Heterogeneous Catalyst. Synth. Commun. 2012, 42, 2245–2255. [Google Scholar] [CrossRef]
- Shelke, K.F.; Badar, A.D.; Devhade, J.B. An Efficient Synthesis of 5-Arylidene-2,4- Thiazolidinedione Catalyzed by Boric acid in Aqueous media under Ultrasound-Irradiation. Chem. Biol. Interface 2016, 6, 410–415. [Google Scholar]
- Yamauchi, S.; Sakai, Y.; Watanabe, Y.; Kubo, M.K.; Matsue, H. Distribution of boron in wood treated with aqueous and methanolic boric acid solutions. J. Wood Sci. 2007, 53, 324–331. [Google Scholar] [CrossRef]
- Shelke, K.F.; Sapkal, S.B.; Kakade, G.K.; Shinde, P.V.; Shingate, B.B.; Shingare, M.S. Boric acid as an efficient catalyst for the synthesis of 1,1-diacetate under solvent-free condition. Chin. Chem. Lett. 2009, 20, 1453–1456. [Google Scholar] [CrossRef]
- Luo, B.; Li, R.; Shu, R.; Wang, C.; Chen, Y.J. Boric Acid as a Novel Homogeneous Catalyst Coupled with Ru/C for Hydrodeoxygenation of Phenolic Compounds and Raw Lignin Oil. Ind. Eng. Chem. Res. 2020, 59, 17192–17199. [Google Scholar] [CrossRef]
- Pathan, S.; Mahaparale, P.; Deshmukh, S.; Une, H.; Arote, R.; Sangshetti, J. Boric Acid: A Versatile Catalyst in Organic Synthesis. Applications of Nanotechnology for Green Synthesis, 3rd ed.; Springer: Cham, Switzerland, 2020; pp. 457–483. [Google Scholar]
- Makkee, M.; Kieboom, A.; Bekkum, H.V. Studies on borate esters III. Borate esters of D–mannitol, D–glucitol, D–fructose and D–glucose in water. Recueil Travaux Chimiques Pays-Bas 2015, 104, 89–95. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Winandy, J. Chemical mechanism of fire retardance of boric acid on wood. Wood Sci. Technol. 2004, 38, 375–389. [Google Scholar] [CrossRef]
- Mali, S.N.; Sawant, S.; Chaudhari, H.K.; Mandewale, M.C. In silico appraisal, synthesis, antibacterial screening and DNA cleavage for 1, 2, 5-thiadiazole derivative. Curr. Comput.-Aided Drug Des. 2019, 15, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.N.; Pandey, A. Multiple QSAR and molecular modelling for identification of potent human adenovirus inhibitors. J. Indian Chem. Soc. 2021, 98, 100082. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).