Abstract
A heterocyclic compound of S and N with cyclic structures, like Furans, thiophenes and related azole analogs, is important as a ligand because of it is readily available, stable and easily functionalized. Various types of heterocyclic molecules quinazolines and their derivatives contain important chromophores with desirable electrochemical properties to be applied in the sensor field. Metal complexes of these compounds have demonstrated significant electrochemical properties as ionophore or electroactive materials for the fabrication of ISEs with different polymeric membranes. R. Selva Kumar et al. 2019 reported the use of dibutyl(8-hydroxyquinolin-2-yl)methylphosphonate as ionophore in a PVC matrix for the fabrication of a potentiometric thorium(IV) ion-selective electrode These quinazoline-based membranes with other additives and plasticizers are very useful for the development of a potential difference across the membrane at membrane-solution interface in the required proportions . Analytes, such as Butralin, Hydroxylamine, and Nitrite, and heavy metal ions, like Fe3+ and Th4+, have also been determined using quinazoline-based membrane sensors. ISE-based electrochemical sensors are very useful in the analysis of food products, drinking water, beverages, fertilizers, soil industrial effluents, etc. They also are applied in potentiometric titration as indicator electrodes.
1. Introduction
An ion-selective electrode (ISE) based on a polyvinyl chloride (PVC) membrane is a type of electrochemical sensor used to measure the concentration of specific ions in a solution. The PVC membrane serves as a selective barrier that allows only the target ion to pass through and interact with the internal electrode. This type of electrode is widely used in various fields, including environmental monitoring, medical diagnostics, and industrial processes. Preparing an ion-selective electrode (ISE) involves several steps, including the creation of a PVC membrane, assembling the electrode, and performing calibration [1]. A general overview for the preparation of an ISE based on PVC membrane is given.
2. Materials and Equipment
- PVC polymer;
- Ionophore specific to the target ion;
- Plasticizer (e.g., dioctylphthalate);
- PVC solvent (e.g., tetrahydrofuran);
- Internal electrode (usually a silver or silver/silver chloride electrode);
- Reference electrode (e.g., Ag/AgCl electrode).
2.1. Preparation of PVC Membrane
- Dissolve the PVC polymer in the solvent (tetrahydrofuran) to create a solution.
- Add the ionophore specific to the target ion into the PVC solution. The ionophore concentration should be carefully chosen based on the desired sensitivity and selectivity of the electrode.
- Add a plasticizer (e.g., tributylphthalate) to improve the flexibility and permeability of the membrane. The plasticizer helps to ensure that the membrane is more responsive to ion changes.
- Mix the PVC solution thoroughly to ensure the uniform distribution of the ionophore and plasticizer.
- Use membrane solution casting equipment to apply a thin layer of the PVC membrane solution onto the surface of the internal electrode.
- Allow the membrane to dry, creating a stable PVC membrane layer on the electrode.
The fabrication of ion-selective electrodes (ISEs) allows the selective sensing of particular ions utilizing a variety of ionophores. One such class of ionophore that can be used to make ISEs for the specific identification of particular ions is the heterocyclic quinazoline compound. This compound has structural characteristics that enable it to interact with certain ions in a selective manner [1,2]. Due to their capacity to selectively detect particular ions, ion-selective electrodes (ISEs) using quinazoline compounds as ionophores have found uses in a variety of sectors. High selectivity, sensitivity, and stability for ion detection can be provided by quinazoline-based ISEs [3]. Their chemical structure can be modified to impart selectivity for certain ions, making them suitable candidates for ion-selective membrane development. However, the use of quinazoline compounds as ionophores in ISEs require careful design, synthesis, and characterization (Figure 1).
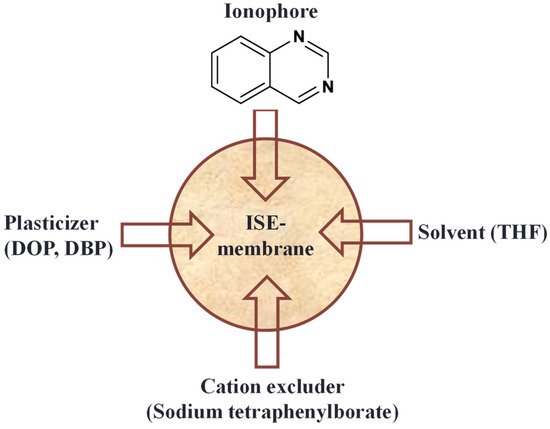
Figure 1.
Ion-selective electrode (ISE) fabrication with quinazoline ionophore in PVC.
In previous studies, a method was developed by R. Selva Kumar and his co-workers in 2019 for the selective measurement of Th4+, which was devised using dibutyl(8-hydroxyquinolin-2-yl)methylphosphonate as an ionophore, sodium tetraphenylborate (NaTPB) as an excluder, and dioctyl phthalate (DOP) as a plasticizer. The PVC membrane composition produces the optimum electrode response for Th4+: DOP: Ionophore: NaTPhB (33%: 59%: 4%: 4%, respectively). The electrode works well in the pH range of 4 to 6.5, has a response time of 5 s, a detection limit of 1 × 108 M, and a service life of approximately three months [4].
In another study in 2007, Ganjali and co-workers developed a Th4+ ion-selective membrane sensor by using a poly (vinyl chloride) (PVC) matrix membrane that contained 2-(diphenylphosphorothioyl)-N′,N′-diphenyl acetamide (DPTD) as a carrier and potassium tetrakis (p-chlorophenyl) borate (KTpClPB) as anion excluder. The response time of the electrode was 30 s. The sensor worked in the pH range of 3.0–9.0 for about 6 weeks [5].
Various other quinazoline compounds, for example, Erlotinib, Gefitinib and different derivatives, have been studied for their potential as kinase inhibitors, antitumor agents, antibacterial agents and anti-inflammatory agents [3]. Other researchers have explored the synthesis of diverse quinazoline compounds with varying substituents to modulate their properties for specific potential use as ionophores in ion-selective electrodes [6].
Here are some potential uses for ISEs built on ionophores based on quinazoline compounds.
2.2. Clinical Diagnostics
The measurement of blood electrolytes: Quinazoline-based ISEs can be used to measure ions, like sodium, potassium and calcium, in blood samples, aiding in diagnosing and monitoring medical conditions, such as electrolyte imbalances and kidney disorders.
2.3. Environmental Monitoring
Water quality assessment: Quinazoline-based ISEs can detect ions like heavy metals (e.g., lead and cadmium) and anions (e.g., nitrate and chloride) in water sources, contributing to environmental monitoring and pollution control efforts.
2.4. Pharmaceutical Analysis
Drug analysis: ISEs can be employed to determine the concentration of specific ions in pharmaceutical formulations, helping to ensure the high quality and effectiveness of drugs.
2.5. Agriculture and Soil Analysis
Soil nutrient monitoring: Quinazoline-based ISEs can measure ions, like potassium and ammonium, in soil samples, aiding in optimizing agricultural practices and managing nutrient levels.
2.6. Food and Beverage Industry
Quality control: ISEs can be used to measure ions, such as sodium, calcium and chloride, in food and beverage products, contributing to quality control and compliance with regulatory standards.
2.7. Biotechnology
Cell culture monitoring: Quinazoline-based ISEs can be integrated with bioreactors to monitor ion concentrations in cell culture media, helping to optimize the conditions for cell growth and production.
2.8. Industrial Process Monitoring
Industrial process optimization: ISEs can be employed to monitor and control ion concentrations in industrial processes, such as wastewater treatment and chemical production.
The success of quinazoline-based ISEs in these applications relies on factors such as the selectivity of the chosen ionophore, the stability of the membrane and the proper calibration and maintenance procedures.
Author Contributions
Conceptualization, C.M. and J.R.; methodology, C.M.; validation, C.M., J.R. and A.N.; investigation, C.M.; resources, A.N.; writing—original draft preparation, C.M.; writing—review and editing, A.N.; supervision, C.M.; project administration, C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Monteiro, M.C.; Winiarski, J.P.; Santana, E.R.; Szpoganicz, B.; Vieira, I.C. Ratiometric Electrochemical Sensor for Butralin Determination Using a Quinazoline-Engineered Prussian Blue Analogue. Materials 2023, 16, 1024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yuan, A.; Xu, H.; Wang, H.; Zheng, C.; Weng, Y.; Bai, Y.; Zhu, Q.; Zhong, B. A fluorescent sensor based on quinazoline ketone derivatives for selectivity of Fe3+. Int. J. Environ. Anal. Chem. 2015, 95, 650–656. [Google Scholar] [CrossRef]
- Ovádeková, R.; Jantová, S.; Labuda, J. Detection of the Effective DNA Protection by Quinazolines Using a DNA-Based Electrochemical Biosensor. Anal. Lett. 2005, 38, 2625–2638. [Google Scholar] [CrossRef]
- Selva Kumar, R.; Ashok Kumar, S.K.; Vijayakrishna, K.; Krishna, A.; Rao, C.B.; Sivaraman, N.; Sahoo, S. Development of highly selective potentiometric thorium(IV) ion-selective electrode: Exploration supported with optical and DFT analysis. Anal. Methods 2019, 11, 1338–1345. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Norouzi, P.; Faridbod, F.; Riahi, S.; Yaftian, M.R.; Zamani, A.; Matt, D. Highly selective and sensitive Th4+-PVC-based membrane sensor based on 2-(diphenylphosphorothioyl)-N′,N′-diphenylacetamide. J. Appl. Electrochem. 2007, 37, 827–833. [Google Scholar] [CrossRef]
- Aghayizadeh, M.M.; Nasirizadeh, N.; Bidoki, S.M.; Yazdanshenas, M.E. Electrochemical Behavior of a Thio-Quinazoline Derivative Electrodeposited on a Glassy Carbon Electrode Modified with Multi-Wall Carbon Nanotubes: Application for Simultaneous Determination of Hydroxylamine and Nitrite. Int. J. Electrochem. Sci. 2013, 8, 8848–8862. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).