Abstract
Here, novel melamine functionalized gum (gum@Melamine@Cu(II)) is synthesized and its catalytic performance is investigated in the oxidation of benzyl alcohols. The gum@Melamine was prepared through the grafting of 3-chloropropyltrimethoxysilane onto gum, followed by treatment with melamine. The synthesized gum@Melamine@Cu(II) was used to achieve an effective and selective method for the oxidation of different alcohols. All substituted benzyl alcohols were oxidized in good yields under mild conditions at room temperature. This gum@Melamine@Cu(II) has great stability and could be recycled five times without noteworthy loss of activity.
1. Introduction
In recent decades, the use of suitable catalytic support has become more important and interesting for chemistry researchers [1]. Therefore, the preparation and use of catalysts with suitable support have been considered. Cherry gum is widely used as a heterogeneous catalytic support due to its advantages such as excellent physical, chemical, and thermal stability; abrasion resistance; high hardness and low density; versatile performance; low cost for synthesis; and recyclability [2,3].
On the other hand, the preparation of catalysts with use of as an essential factor, using agents that are nontoxic, low cost, and economical. Without a doubt, melamine is one of these agents with the aforementioned properties.
In the last decade, with the development of oxidation processes, the use of these reactions has attracted much attention. In addition, the synthesis of their product with high yield is very important and considerable. Oxidation of benzyl alcohol to aldehydes and acid carboxylic is one of these oxidation reactions [4,5,6].
Some of these reactions are carried out under conditions such as high temperature, the use of unfavorable solvents for the environment, and the use of oxidants that produce undesirable by-products. Due to these limitations, using efficient catalysts, suitable oxidants, and environmentally friendly solvents is a priority target.
In this paper, we prepared gum-melamine as a heterogeneous recyclable catalyst and used it to obtain oxidize benzyl alcohol and its derivatives (Scheme 1).

Scheme 1.
Synthesized derivatives in an oxidation reaction.
2. Experimental
2.1. General
All reagents were purchased from Fluka and Merck companies and used without further purification. Thin layer chromatography (TLC) was used for the purity determination of substrates, products, and reaction monitoring over a silica gel 60 F254 aluminum sheet. Melting points were measured in open capillary tubes with Electro thermal 9100 melting point apparatus.
2.2. Synthesis of gum@Melamine@Cu(II)
The gum (1 g) was disperesed in toluene (40) mL. After complete dispersion, melamine (1 g) and Triethylamine (3 mmol) were added to them and was refluxed for 24 h. It was then washed with ethanol and dried at 80 °C for 24 h. gum@Melamine (0.5 g) was dispersed in 10 mL distilled water, then Cu(OAc)2 (0.5 g) was added slowly and stirred at room temperature for 24 h. Then, the mixture was filtered, washed with H2O and EtOH, and dried at 60 °C for 5 h.
2.3. General Procedure for Derivatives Synthesis
The oxidation of alcohols to aldehydes was conducted in a 50 mL flask in an oil bath. 1mmol of aldehyde, 5 mL CH3CN, and 50 mg of gum@Melamine@Cu(II) were added to flask and stirred. After the completion the reaction, which was followed by TLC, the mixture was filtered and the products were purified.
3. Results and Discussion
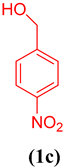
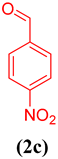
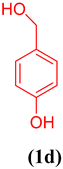
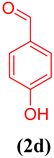
In order to extend the application of gum@Melamine@Cu(II), the effect of substrate was also studied under optimal conditions (Table 1). As can be seen, the reaction conditions were compatible with both an electron acceptor and electron donor substituents aromatic ring.

Table 1.
Oxidation of different benzyl alcohols catalyzed by gum@Melamine@Cu(II) under optimized conditions.
4. Conclusions
In summary, a heterogeneous gum-melamine catalyst was prepared and used for the oxidation of benzyl alcohol and its derivatives with a high yield. The main advantages of this catalyst are its reusability, simple separation from the reaction mixture, and short reaction time. In addition, the use of an easy and convenient method for the preparation of the catalyst is another advantage of this catalyst over other reported catalysts.
Author Contributions
Methodology, H.G.; software, B.A.; investigation, Y.R. and N.G.; resources, Y.R. and N.G.; writing—review and editing, Z.T. and N.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parida, K.N.; Jhulki, S.; Mandal, S.; Moorthy, J.N. Oxidation of benzyl alcohols, benzyl halides, and alkylbenzenes with oxone. Tetrahedron 2012, 68, 9763–9768. [Google Scholar] [CrossRef]
- Al-idee, T.; Habbal, H.; Karabt, F.; Alzubi, H. Study of some functional properties and antioxidant activity of two types of cherry trees (Prunus avium) gum exudates grown in Syria. Iraqi J. Sci. 2020, 61, 13–22. [Google Scholar] [CrossRef]
- Li, T.; Liu, R.; Zhang, C.; Meng, F.; Wang, L. Developing a green film from locust bean gum/carboxycellulose nanocrystal for fruit preservation. Future Foods 2021, 4, 100072. [Google Scholar] [CrossRef]
- Feng, D.; Dong, Y.; Zhang, L.; Ge, X.; Zhang, W.; Dai, S.; Qiao, Z.A. Holey lamellar high-entropy oxide as an ultra-high-activity heterogeneous catalyst for solvent-free aerobic oxidation of benzyl alcohol. Angew. Chem. Int. Ed. 2020, 59, 19503–19509. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, N.; Ghafuri, H.; Zand, H.R.E. Graphene Oxide-Supported Hypervalent Organoiodine (III): Recyclable Reagent for Selective and Metal-Free Oxidation of Alcohols. ChemistrySelect 2018, 3, 3394–3399. [Google Scholar] [CrossRef]
- Göksu, H.; Burhan, H.; Mustafov, S.D.; Şen, F. Oxidation of benzyl alcohol compounds in the presence of carbon hybrid supported platinum nanoparticles (Pt@ CHs) in oxygen atmosphere. Sci. Rep. 2020, 10, 5439. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).