Abstract
In this work, an optical fiber sensor based on the localized surface plasmon resonance (LSPR) phenomenon is presented as a powerful tool for the detection of heavy metals (). The resultant sensing film was fabricated using a nanofabrication process, known as layer-by-layer embedding (LbL-E) deposition technique. In this sense, both silver nanoparticles (AgNPs) and gold nanoparticles (AuNPs) were synthesized using a synthetic chemical protocol as a function of a strict control of three main parameters: polyelectrolyte concentration, loading agent, and reducing agent. The use of metallic nanostructures as sensing materials is of great interest because well-located absorption peaks associated with their LSPR are obtained at 420 nm (AgNPs) and 530 nm (AuNPs). Both plasmonic peaks provide a stable real-time reference that can be extracted from the spectral response of the optical fiber sensor, giving a reliable monitoring of the Hg2+ concentration.
1. Introduction
The presence of heavy metals in a human’s daily life has become a concern due to their adverse health effects. Among all of them, mercury is receiving major attention because its presence is associated with serious problems, such as lung or nervous system damage, heart diseases, and even neurological and psychological symptoms [1]. Due to this, a wide variety of detection methods can be found in the bibliography, ranging from electrochemical sensors [2,3,4] to colorimetric sensors [5,6,7] and optical sensors [8,9,10]. This work focuses on optical fiber sensors based on the localized surface plasmon resonance (LSPR) phenomenon. It is well known that LSPR is an optical phenomenon that is generated, thanks to the interaction between the incident light and the electrons in the conduction band of the metal surface [11]. It has been demonstrated that the resultant amplitude and the plasmonic resonance energy can vary as a function of the geometry and the distance between the nanoparticles. Until now, LSPR optical fiber sensors for mercury ion detection mostly contain gold nanoparticles (AuNPs) as the main plasmonic sensing material, showing the interaction between gold and mercury as a change in the physical and chemical properties of the metallic nanoparticles [12,13]. The novelty of this work is the possibility of introducing two different metallic nanoparticles, AgNPs and AuNPs, into LbL films with the aim of obtaining two different LSPR sensing signals for the detection of mercury ions. This deposition technique makes it possible to obtain thin films with a good control in the resultant thickness in the nanometric range as a function of operational parameters, such as pH, ionic strength, and number of bilayers deposited [14,15,16]. An initial study is performed on glass slides in order to optimize the nanofabrication technique, and then sensing coating is implemented on an optical fiber. Finally, a change in the wavelength position of the LSPR band can be observed as a function of the concentration of the analyte. To sum up, this is the first time that an optical fiber sensor with a dual reference state is presented for mercury ion detection.
2. Methods
2.1. Materials
The polymeric matrix is composed of poly(allylamine hydrochloride) (PAH) (Mw ~15.000), which acts as a polycation, and poly(acrylic acid) (PAA) 35 wt% solution in water, which acts as a polyanion. In order to obtain AuNPs and AgNPs, gold(III) chloride trihydrate (HAuCl4·3H2O) and silver nitrate (AgNO3) were used as loading agents for the synthesis of metallic nanoparticles. Finally, dimethylamine borane complex (DMAB) was used as a reducing agent.
2.2. Chemical Process for the Synthesis of Metallic Nanoparticles
2.2.1. Gold Nanoparticle (AuNP) Synthesis
First, aqueous solutions of HAuCl4·3H2O (20 mL, 5 mM) and PAA (120 mL, 10 mM), which acts as a stabilizing agent, were mixed and stirred for a period of 2 h. After that, an aqueous solution of the reducing agent, DMAB (5 mL, 100 mM), was added to the previous solution, and the mixture was stirred for 24 h at room temperature. Finally, a color change from yellow to violet was obtained, indicating the synthesis of AuNPs. The combination of PAA and AuNPs is denoted as PAA-AuNPs. This colloidal dispersion solution (PAA-AuNPs) showed a spherical shape and nanometric range (10–20 nm) corroborated by transmission electron microscopy (TEM) [17].
2.2.2. Silver Nanoparticle (AgNPs) Synthesis
For the synthesis of AgNPs, first, aqueous solutions of AgNO3 (20 mL, 10 mM) and PAH (120 mL, 10 mM), which acts as a stabilizing agent, were mixed and stirred for a period of 2 h. After that, an aqueous solution of the reducing agent, DMAB (5 mL, 100 mM), was added to the initial solution, and the mixture was stirred for 24 h at room temperature. Finally, a color change from transparent to orange was obtained, indicating the synthesis of AgNPs. The combination of PAH and AgNPs is denoted as PAH-AgNPs. The location of the LSPR absorption band clearly indicates the synthesis of AgNPs with a spherical shape and nanometric size [18].
2.3. Optical Characterization
The optical properties of the synthesized metallic nanoparticles were determined by using a Jasco V-630 spectrophotometer (Agilent, Santa Clara, CA, USA). Two different and well-separated absorption bands were obtained.
2.4. Layer-by-Layer Nanoassembly
The layer-by-layer nanoassembly technique was used for the fabrication of the thin films. In this work, the presence of PAH and PAA was used as the positively and negatively charged polyelectrolytes for the buildup of the polyelectrolyte structure film. In addition, as demonstrated in the previous section, these charged structures also play a key role in stabilizing the synthesized nanoparticles. More specifically, the polycationic solution PAH-capped AgNPs (PAH-AgNPs) and the polyanionic PAA-capped AuNPs (PAA-AuNPs) were used for the fabrication of the thin films.
2.5. Optical Fiber Detection Setup
A multimode optical fiber with a 200 μm core diameter with polymeric cladding, 0.39 NA (Thorlabs FT-200-EMT), was used for the fabrication of the optical fiber sensor. First, it was necessary to remove the acrylate cladding of a segment of approximately 2 cm of the optical fiber, and for this, a few drops of dry acetone and a blade were used, exposing the bare optical fiber core in its entire cylindrical section. This optical fiber segment was immersed for 5 min in piranha solution to eliminate the acetone that could remain. Temporary SMA connectors were used at the end of the optical fiber, exciting the sensor from one of the connectors with a halogen white source, and the other end collected the optical response with a CCD spectrometer (HR4000-UV Ocean Optics, Ocean Insight, FL, USA). A scheme of the deposition process and the optical setup is presented in Figure 1.
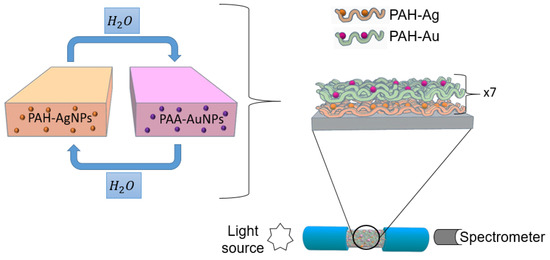
Figure 1.
Schematic representation for the fabrication of the LbL films by using PAH-AgNPs as a polycation and PAA-AuNPs as a polyanion.
2.6. Mercury Sample Preparation
The mercury samples were prepared using mercury(II) chloride (. Every concentration of mercury was prepared with phosphate buffer (PB) solution for achieving a constant pH = 7.6. The Hg concentrations were varied from 50 to 1 and 0.1 ppm. An important aspect is that for each measurement, the fiber optical sensor was immersed in PB + DMAB buffer solution with the aim of obtaining a stable baseline for further mercury detection.
3. Results and Discussion
As an initial step, the nanofabrication process was performed on glass substrates, and then, the same procedure was extrapolated to the optical fiber for further chemical sensing. The selected pH for the fabrication of the whole process was 7.0 in the dipping polyelectrolytes. As can be observed in Figure 2, the sample for thickness coating with 10 bilayers showed a clear predominance of the LSPR in relation to AgNPs (plasmonic peak centered at 450 nm), without being able to identify the peak in relation to AuNPs. However, when the thickness coating was gradually increased up to a total thickness of 30 bilayers, both LSPR peaks can be clearly observed, which were centered at 420 nm (AgNPs) and 540 nm (AuNPs), although transparent films were still obtained, which were observed by the naked eye.
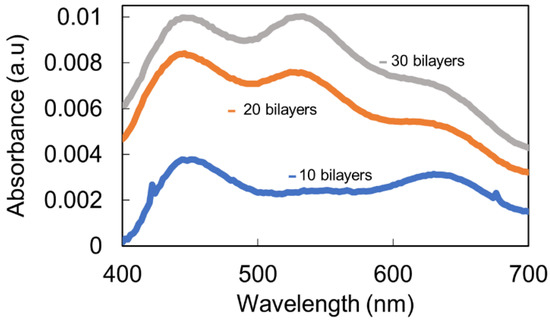
Figure 2.
UV–VIS spectra of the LbL coatings based on PAH/AgNPs and PAA/AuNPs deposited on glass slides as a function of the thickness coating (10, 20, and 30 bilayers) for pH 7.0.
Once the presence of both LSPR peaks was demonstrated on glass slides, the next step was based on the deposition of this same thin film onto an optical fiber at the same pH value (87.0) in order to appreciate both absorption bands in the UV–VIS spectra. In Figure 3, it is demonstrated that by only a final thickness of 7 bilayers, it is possible to appreciate the LSPR of the AgNPs (centered at 420 nm) and AuNPs (centered at 540 nm), this sensing thin film being for the mercury ion detection.

Figure 3.
UV–VIS spectrum of the LbL coatings based on PAH/AgNPs and PAA/AuNPs deposited on an optical fiber for a thickness coating of 7 bilayers.
Detection of Mercury Ions with Fiber Optic Sensor
Once the thin film was fabricated, the optical fiber was immersed in the Buffer PB + DMAB solution for 1 h in order to have a stable baseline for the mercury detection stage. After that, the sensing film was immersed at a fixed mercury concentration of 50 ppm, and a very interesting result was that a clear wavelength shift of 23 nm was observed for LSPR (AuNPs), which remained stable in wavelength, compared with the wavelength shift observed in the AuNP LSPR band. According to this, LSPR-AuNPs are much more sensitive to the presence of Hg than LSPR-AgNPs (Figure 4). The UV–VIS spectra for the minimum (0.1 ppm) and maximum (50 ppm) H2O2 concentrations are presented in Figure 5 in order to have a better appreciation of the wavelength shift related to the LSPR-AuNPs, with the detection range being in the order of 100 ppb.
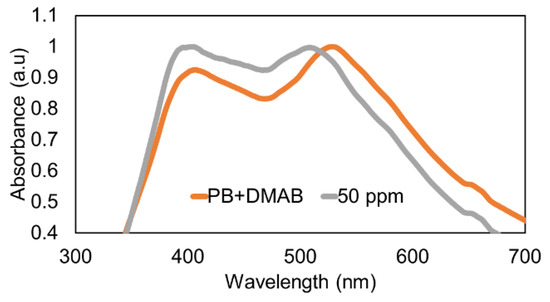
Figure 4.
Wavelength shift of the LSPR absorption bands at 50 ppm of mercury concentration.
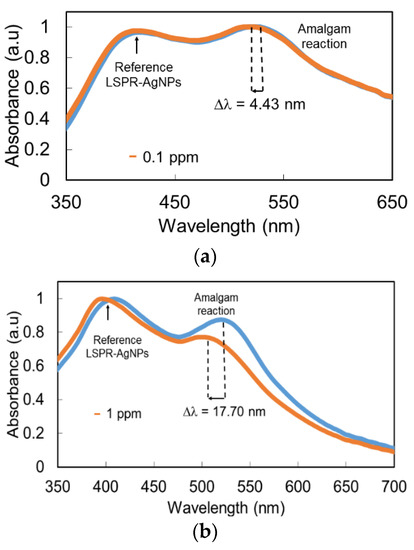
Figure 5.
UV–VIS spectra for (a) the minimum concentration (0.1 ppm) and (b) the maximum Hg concentration (50 ppm).
The wavelength shift observed in the AuNP LSPR absorption band can be explained by the chemical reaction of the mercury present in the sample with the AuNPs. As previously reported [8], mercury ions, in the presence of DMAB of the stock buffered solution, are reduced to metallic mercury, which is known to show a high affinity for gold to form an amalgam [13]. Mercury reacts with AuNPs, changing their surface chemistry, so it is possible that their effective diameter in terms of LSPR resonances is reduced, which explains the blue shift in the LSPR band maximum. The higher reaction affinity of mercury towards gold compared with silver makes the stability of the AgNP absorption band possible, which can be used as a quite stable wavelength reference.
Different sensors were fabricated with the same sensing materials in order to detect a particular mercury concentration. Although both LSPR bands experimented changes in the presence of mercury ions, it is clearly visible that the LSPR band corresponding to AuNPs showed a greater blue shift in comparison with the LSPR of AgNPs. Finally, the dynamic response of the LSPR band inherent in AuNPs is presented in Figure 6 for different mercury concentrations (0.1, 1, and 50 ppm).

Figure 6.
Dynamic response of the optical fiber sensors for the LSPR (AuNPs) to different Hg concentrations, ranging from 50 to 0.1 ppm.
4. Conclusions
In this work, a fiber optic sensor based on two different LSPR sensing signals for the detection of Hg2+ was presented. The metallic nanoparticles were incorporated into the sensing films by using the layer-by-layer nanoassembly technique. The sensors were exposed to different Hg2+ concentrations, with the wavelength response of the AuNP LSPR greater than that of the AgNP LSPR. Finally, this resultant sensing material can be extrapolated for the detection of different heavy metals in environmental applications.
Author Contributions
Conceptualization and methodology, M.E.M.-H., X.S., P.J.R., J.G. and F.J.A.; investigation and validation, M.E.M.-H. and X.S.; writing—original draft preparation, M.E.M.-H., X.S. and P.J.R.; writing—review and editing, P.J.R., J.G. and F.J.A.; supervision, P.J.R., J.G. and F.J.A.; project administration and funding acquisition, F.J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the Spanish State Research Agency (AEI) through the project PID2019-106070RB-I00 and the Public University of Navarre with a PhD research grant.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.; Yu, L.; Li, F.; Xie, J. A dual functional electrochemical “on-off” switch sensor for the detection of mercury(II) and melamine. Sens. Actuators B Chem. 2015, 212, 446–450. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, S.; Zhang, Q.; Grigholm, B.; Kaspari, S.; You, Q.; Qin, D.; Mayewski, P.A.; Cong, Z.; Huang, J.; et al. A 500year atmospheric dust deposition retrieved from a Mt. Geladaindong ice core in the central Tibetan Plateau. Atmos. Res. 2015, 166, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, H.; Wu, D.; Li, Y.; Du, B.; Wei, Q. Label-free immunosensor based on Au@Ag2S nanoparticles/magnetic chitosan matrix for sensitive determination of ractopamine. J. Electroanal. Chem. 2015, 741, 14–19. [Google Scholar] [CrossRef]
- Lee, J.S.; Han, M.S.; Mirkin, C.A. Colorimetric detection of mercuric ion (Hg2+) in aqueous media using DNA-functionalized gold nanoparticles. Angew. Chem.-Int. Ed. 2007, 46, 4093–4096. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Galán-Mascarós, J.R.; Martí-Gastaldo, C.; Palomares, E.; Durrant, J.R.; Villar, R.; Gratzel, M.; Nazeeruddin, M.K. Reversible colorimetric probes for mercury sensing. J. Am. Chem. Soc. 2005, 127, 12351–12356. [Google Scholar] [CrossRef]
- Chen, X.; Nam, S.W.; Jou, M.J.; Kim, Y.; Kim, S.J.; Park, S.; Yoon, J. Hg2+ selective fluorescent and colorimetric sensor: Its crystal structure and application to bioimaging. Org. Lett. 2008, 10, 5235–5328. [Google Scholar] [CrossRef]
- Martínez-Hernández, M.E.; Goicoechea, J.; Arregui, F.J. Hg2+ optical fiber sensor based on LSPR generated by gold nanoparticles embedded in LBL nano-assembled coatings. Sensors 2019, 19, 4906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Sun, T.; Grattan, K.T.V. Gold nanorod-based localized surface plasmon resonance biosensors: A review. Sens. Actuators B Chem. 2014, 195, 332–351. [Google Scholar] [CrossRef]
- James, J.Z.; Lucas, D.; Koshland, C.P. Gold nanoparticle films as sensitive and reusable elemental mercury sensors. Environ. Sci. Technol. 2012, 46, 9557–9562. [Google Scholar] [CrossRef] [PubMed]
- Caucheteur, C.; Guo, T.; Albert, J. Review of plasmonic fiber optic biochemical sensors: Improving the limit of detection. Anal. Bioanal. Chem. 2015, 407, 3883–3897. [Google Scholar] [CrossRef] [PubMed]
- Sadani, K.; Nag, P.; Mukherji, S. LSPR based optical fiber sensor with chitosan capped gold nanoparticles on BSA for trace detection of Hg (II) in water, soil and food samples. Biosens. Bioelectron. 2019, 134, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Schopf, C.; Martín, A.; Schmidt, M.; Iacopino, D. Investigation of Au-Hg amalgam formation on substrate-immobilized individual Au nanorods. J. Mater. Chem. C 2015, 3, 8865–8872. [Google Scholar] [CrossRef]
- Shiratori, S.S.; Rubner, M.F. pH-dependent thickness behavior of sequentially adsorbed layers of weak polyelectrolytes. Macromolecules 2000, 33, 4213–4219. [Google Scholar] [CrossRef]
- Decher, G.; Eckle, M.; Schmitt, J.; Struth, B. Layer-by-layer assembled multicomposite films. Curr. Opin. Colloid Interface Sci. 1998, 3, 32–39. [Google Scholar] [CrossRef]
- Rivero, P.J.; Goicoechea, J.; Matias, I.R.; Arregui, F.J. A comparative study of two different approaches for the incorporation of silver nanoparticles into layer-by-layer films. Nanoscale Res. Lett. 2014, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goicoechea, J.; Rivero, P.J.; Sada, S.; Arregui, F.J. Self-Referenced Optical Fiber Sensor for Hydrogen Peroxide Detection based on LSPR of Metallic Nanoparticles in Layer-by-Layer Films. Sensors 2019, 19, 3872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivero, P.J.; Ibañez, E.; Goicoechea, J.; Urrutia, A.; Matias, I.R.; Arregui, F.J. A self-referenced optical colorimetric sensor based on silver and gold nanoparticles for quantitative determination of hydrogen peroxide. Sens. Actuators B Chem. 2017, 251, 624–631. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).