Abstract
In this work, we explore the possibility of using anti-crown ether (C6HgF4)3 as a membrane-active component for potentiometric cross-sensitive sensors. Anti-crown ligands have already been employed as ionophores in plasticized polymeric membranes; however, the results of these studies are contradictory. In order to clarify the electrochemical sensitivity patterns of anti-crown-based sensors, we have studied plasticized polymeric membranes containing cation and anion-exchanging additives and various solvent-plasticizers. We explored the electrochemical sensitivity of these membranes in a wide variety of aqueous solutions of inorganic salts. Alkaline, alkaline-earth, and d-element salts with different anions were studied. It was found that the sensors based on anti-crown (C6HgF4)3 exhibit cationic sensitivity, and no considerable anionic responses were observed.
1. Introduction
In this work, we explore the possibility of using the so-called anti-crown compound (C6HgF4)3 as a membrane-active component for potentiometric cross-sensitive sensors. Anti-crown ethers are macrocyclic organometallic compounds consisting of mercury atoms or tin atoms separated by carbon atoms, including fluorinated macrocycles and mercury-carborands.
The chemistry of anti-crowns is quite extensive and it is a rapidly grown field of research. Recently, it was reported that (o-C6F6Hg)3 is readily coordinated with bromide and iodide anions to yield the following complexes: {o-[(C6F6Hg)3Br]−} and {o-[(C6F6Hg)3I]−}. When anti-crowns interact with halogens, they form polydecker wedge-shaped sandwiches: [(∙∙∙(C6F6Hg)3∙∙∙X∙∙∙)n]n−, where X = chloride and bromide anions [1]. The ability of anti-crown ethers to coordinate with single-charge anions has attracted the attention of analytical chemists. Several attempts have been made to create sensors based on anti-crown ethers to detect anions. For example, mercury-carborand was used as an ionophore for the determination of chloride ions [2]. The authors made a PVC-plasticized membrane consisting of NPOE as a plasticizer, TDDMA-Cl as an anion exchange additive, and mercury-carborand-3 as an ionophore. It was experimentally shown that the sensors exhibit a near-Nernstian response when interacting with Cl− in a wide range of concentrations, and detection limits were in the micromole range. It was also found that the presence of TDDMA-Cl in the membrane causes an increase in sensor sensitivity. Another study explored three mercury anti-crown ethers to develop sensors for the Me4N+ cation [3]. The authors of the article synthesized three different PVC-plasticized membranes with various plasticizers: bis(2-ethylhexyl) sebacate (DOS), dioctyl phthalate (DOP), and dibutyl phthalate (DBP). Potassium tetrakis(4-chlorophenyl)-borate (KTpClPB) was used as a cation exchanger. A near-Nernstian response of 54.2 mV/dec towards the Me4N+ cation was observed when DOS was employed as a plasticizer. With other plasticizers, the responses were smaller. The electrode with the ionophore was more sensitive, with a response of 54.9 mV/dec. Figure 1 shows several typical anti-crown ether structures.
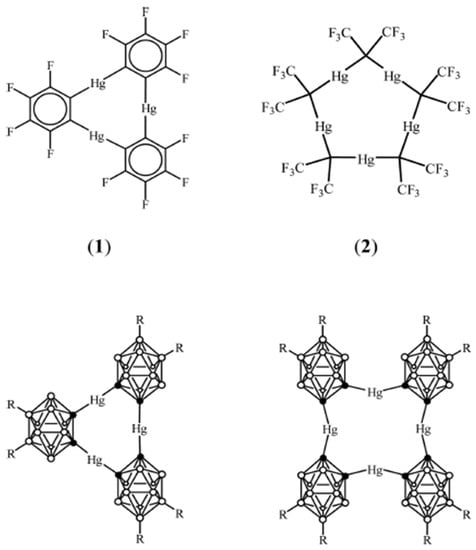
Figure 1.
Structural formulas of typical anti-crown ethers based on fluorinated macrocycles and mercury-carborand: (1) Containing 3 mercury atoms; (2) containing 4 and 5 mercury atoms.
2. Experimental Part
2.1. Reagents
Three-mercury anti-crown ether was used as an ionophore in all membranes. The anti-crown was synthesized at A.N. Nesmeyanov Institute of Organoelement Compounds. The polymer matrix of the membranes was made of PVC (poly(vinylchloride)). O-nitrophenyloctyl ether (NPOE) and dioctyl sebacate (DOS) were used as a solvent-plasticizers. NaTpClPB was used as an anionic additive, and TDDMA-NO3 was used as a cationic additive. All these substances were from SigmaAldrich, at Selectophore grade. We made three membranes of each type to assess repeatability of the results.
2.2. ISE Preparation and Potentiometric Measurements
The composition of the sensor membranes is shown in Table 1. The membranes were made by mixing the following components: 200 mg plasticizer, 109 mg PVC, 17 mg anti-crown ether, 30 mg NaTpClPB (membranes 2 and 5), and 20 mg TDDMA-NO3 (membranes 3 and 6). The components where mixed with 5 mL THF using a magnetic stirrer until completely dissolved. After that, the mixture was transferred to a flat-bottom teflon beaker and left overnight for solvent evaporation. The disks (7 mm in diameter) were cut from the parent membrane and glued into electrode bodies. After the glue dried, the housing was connected to the electrodes and filled with 0.01 M NaCl solution. Finally, the electrodes were soaked for a day in a solution of sodium chloride of the same concentration. Between subsequent measurements, the sensors were stored in air. In total, we made 6 membranes and 18 sensors.

Table 1.
Membrane compositions.
Solutions with a concentration of 1 M were prepared by weight, and less concentrated solutions were prepared by sequential volume dilution of the parent solution. All solutions were prepared with doubly distilled water.
The galvanic cell for the potentiometric measurements was the following:
Ag|AgCl, KCl(sat.)|analyzed solution|PVC membrane|NaCl, 0.01 M, AgCl|Ag
The reference electrode was a silver chloride electrode filled with a saturated solution of potassium chloride. A glass electrode was used to control the pH during the experiment. All measurements were carried out at room temperature.
3. Results and Discussion
3.1. Anion Sensitivity
To study the anion sensitivity of the sensors, a series of measurements was carried out in aqueous solutions of Li2SO4, NaCl, NaOAc, NaF, and Ca(NO3)2 in the concentration range of 10−6–10−2 M. Based on the results of three parallel measurements, the slopes of the linear parts (10−5–10−2 M) of the calibration curves were calculated. The sensor compositions 2 and 5 (Table 2) contain cation-exchange NaTpClPB, compositions 3 and 6-TDDMA-NO3 contain an anion-exchange additive.

Table 2.
Response characteristics of the electrodes to anions (±2 mV/dec).
As can be observed, the developed sensors in most cases do not demonstrate pronounced sensitivity to anions. The sensitivity values obtained for chloride for the sensors of composition 6 (−40.85 mV/dec with NPOE plasticizer) are the closest to the theoretical values. The sensors without any ion-exchanging additive showed cation sensitivity, thus suggesting that three mercury anti-crown ether promotes cation sensitivity.
3.2. Cation Sensitivity
We also studied the sensitivity of the electrodes to cations. A broad variety of inorganic cations was studied: Li+, Na+, Mg2+, K+, Ca2+, Co2+, Ni2+, Cu2+, Zn2+, Sr2+, Cd2+, Cs+, Pb2+, La3+, Eu3+, and Lu3+. The measurements were performed in the concentration range of 10−7–10−3 M for lanthanides solutions; other salts were in the concentration range of 10−6–10−2 M. The slopes were calculated for the concentration range of 10−5–10−2 M (10−5–10−3 M for La3+, Eu3+, and Lu3+). The results are provided in Table 3.

Table 3.
Cation sensitivities of the electrodes (±2 mV/dec).
It can be seen that the sensors have pronounced cross-sensitivity to cations. The sensors of compositions 3 and 6 in most cases showed anion sensitivity, apparently due to the presence of the anion exchanger. The sensor with a cation-exchange additive based on the plasticizer DOS (composition 2) was more sensitive to all the cations than the sensor without an additive with the same plasticizer (composition 1). For example, for Cs+ and Pb2+, the slopes for sensor 1 were 35.8 mV/dec and 2.1 mV/dec, and the slopes for sensor 2 were 51.2 mV/dec and 18.7 mV/dec, respectively. Comparing sensors based on different plasticizers (sensors 2 and 5; plasticizers DOS and NPOE), we can say that for most of the cations (Li+, Mg2+, Ni2+, Cu2+, Sr2+, and Cd2+), the values of the slope of the electrode function are higher for the sensor based on o-nitrophenyloctyl ether (composition 5). For example, for Cd2+, the slope for sensor 2 was 8.4 mV/dec, and the slope for sensor 5 was 12.9 mV/dec. At the same time, it is worth noting that the observed sensitivity values in most cases significantly differ from the theoretical values. The developed sensors did not show sensitivity to the La3+, Eu3+, and Lu3+ cations.
4. Conclusions
We studied the possibility of using a three-mercury anti-crown as an ionophore. Also, the electrochemical sensitivity of developed sensor membranes in solutions of inorganic anions and cations was studied. It was found that the presence of three-mercury anti-crown ether in the polymer plasticized membrane promoted the cation sensitivity of the sensors.
In general, the developed sensors may have potential in the development of potentiometric multi-sensor systems; however, further studies are needed to confirm that there is some benefit to using anti-crown compounds as ionophores, since the observed sensitivities followed the lipophilicity of the cations (the highest values were observed for cesium and lead).
Author Contributions
Conceptualization, D.K. and V.B.; methodology, E.Y., K.T., V.B.S. and I.A.T.; formal analysis, E.Y.; investigation, E.Y., K.T., V.B.S. and I.A.T.; resources, D.K.; writing—original draft preparation, E.Y.; writing—review and editing, D.K. and V.B.; visualization, E.Y.; supervision, D.K. and V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tugashov, K.I.; Gribanyov, D.A.; Dolgushin, F.M.; Smol’yakov, A.F.; Peregudov, A.S.; Minacheva, M.K.; Tikhonova, I.A.; Shur, V.B. Coordination Chemistry of Anticrowns. Synthesis and Structures of Double-Decker Sandwich Complexes of the Three-Mercury Anticrown (o-C6F4Hg)3 with Halide Anions Containinf and Not Containing Coordinated Dibromomethane Molecules. Organometallic 2016, 3, 2197–2206. [Google Scholar] [CrossRef] [Green Version]
- Badr, I.H.; Martin Diaz, M.; Hawthorne, F.; Bachas, L.G. Mercuracarborand “Anti-crown Ether”-Based Chloride-Sensitive Liquid/Polymeric Membrane Electrodes. Anal. Chem. 1999, 71, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhou, Z.; Wu, Y.; Shur, V.B.; Tikhonova, I.A.; Shur, V.B. Cyclic Trimeric Perfluoro-o-Phenylenemercury as a New Ionophore for Quaternary Ammonium Cation-Selective Membrane Electrode. Anal. Lett. 2005, 38, 377–388. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).