Abstract
This work introduces a method specially developed to produce a biorecognition element based on modified Stöber silica nanoparticles by the covalent immobilization of the human IgG. The sensing structure is based on long period fiber gratings (LPFG), specially developed to allow the interaction of the electromagnetic wave with the target analytes through its evanescent field. The surface was modified by the immobilization of the IgG-modified nanoparticles serving has recognition elements for specific target molecules. The resulting configuration was tested in the presence of anti-human IgG, recording the refractometric response of the modified LPFG in contact with different amounts of analyte. The selectivity of the sensor was also assessed.
1. Introduction
Biosensors are powerful allies for food safety, drug discovery, environmental monitoring and clinical diagnosis [1,2,3]. The sensing methodology comprises a bioreceptor, a recognition element and a transducer whose properties changes upon analyte binding [4]. Typically, the bioreceptor is immobilized on the surface of the transducer and the binding event can be, e.g., mechanically, electrically or optically transduced [5,6] producing an increase in mass, a change in electrical resistivity or changes in the refractive index at the surface of the used material allowing to be measured. In recent years, optical biosensors are an active field of research worldwide, presenting rapid progress [7,8,9]. In this perspective, optical biosensors based on refractometric sensing schemes have been developed with great successes in the last few decades [7]. Moreover, optical fibers (OF) based on evanescent wave sensing are an excellent platform to develop high-stability and high-sensitive optical biosensors [10]. The quantitative and/or qualitative measurements result from the interaction of the biorecognition element with the evanescent field of light at the fiber surface. Its good biocompatibility makes them appropriate for biochemical functionalization, creating very sensitive structures targeting viruses, drugs and proteins [11]. In recent years, several authors have reported transduction scheme’s using optical fibers for optical biosensing. Lobry et al. demonstrated a plasmon-assisted tilted fiber Bragg gratings (TFBGs) based biosensor for non-enzymatic D-glucose using polydopamine-immobilized concanavalin A [12]. More recently, Liyanage et al. developed a label-free sensitive tapered optical fiber plasmonic biosensor targeting microRNAs. The sensing platform comprises different types of gold nanoparticles immobilized on the surface of the fiber to enhance the evanescent mode, followed by self-assembled ssDNA probes [13]. Using a long-period fiber grating (LPFG) platform, Liu et al. demonstrated the use of a LPFG coated with graphene oxide (GO) nanosheets using the changes in resonant intensity to measure different concentrations of hemoglobin adsorbed by the GO layer [14]; Chiavaioli et al. reported a D-shaped single mode optical fiber (SMF) nanocoated with different metals for IgG/anti-IgG assays, reaching limit of detections (LOD) around to the femtomolar values [15]; and Dey et al. presented a sensitivity-enhanced LPFG near the turning point by etching the fiber with hydrofluoric acid to detect anti-mouse IgG [16]. In a similar approach to this work, Liu et al. demonstrated an LPFG-coated with silica nanoparticles modified with gold nanoparticles (AuNPs). The gold surface was modified with anti-IgM receptors and the sensing platform was tested to understand its suitability for the detection of human IgM antibodies [17].
In this work, a method based on silica nanoparticles (prepared based on Stöber [18] method), immobilized in the surface of commercial SMF28 OF, serving as recognition elements for specific target molecules is presented. The nanoparticles surface was functionalized by the introduction of an aminosilane (APTMS) followed by the covalent immobilization of the immunoglobulin G from human serum (human-IgG). The antibody was activated by the EDC/NHS protocol to allow the interaction of the amine exposed groups, located on the surface of the silica nanoparticles, with the activated carboxyl acid groups of the human-IgG molecules. The resulting template was immobilized onto the surface of an OF by electrostatic interactions between the negative charges of the fiber surface and the positively charged amine groups located in the IgG molecules. The sensing structure is based on LPFGs, specially developed to allow the interaction of the electromagnetic wave with the target analytes through its evanescent field. The refractometric system comprises a Braggmetter unit (HBK, FiberSensing, Darmstadt, Germany) working in a wavelength range from 1500 to 1600 nm and a reference LPFG to correct possible false interactions. The resulting configuration was tested in the presence of anti-human IgG, recording the refractometric response of the modified LPFG in contact with different amounts of analyte.
2. Materials and Methods
2.1. Chemical Reagents
Silica nanoparticles were prepared following the Stöber method [19], using tetraethyl orthosilicate (TEOS; Sigma-Aldrich, St. Louis, MO, USA; ≥98%) and ammonium hydroxide solution (NH4OH; Sigma-Aldrich, 28% m/m) as reagents. The functionalization of the nanoparticles surface was attained using the following reagents: (3-aminopropyl)trimethoxysilane solution (Sigma-Aldrich, St. Louis, MO, USA; 97%), anhydrous toluene (Sigma-Aldrich, St. Louis, MO, USA; 99.8%), phosphate buffered saline (PBS; pH 7.4, tablets, Sigma-Aldrich, St. Louis, MO, USA), 2-(N-morpholino)ethanesulfonic acid (MES; Sigma-Aldrich, St. Louis, MO, USA), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC; Sigma-Aldrich, St. Louis, MO, USA; ≥99%), N-hydroxysuccinimide (NHS; Sigma-Aldrich, St. Louis, MI, USA; ≥98%) and immunoglobulin G from human serum (human-IgG; Sigma-Aldrich, St. Louis, MO, USA; ≥95%). For the LPFG surface cleaning were used sodium hydroxide anhydrous (NaOH; Sigma-Aldrich, St. Louis, MO, USA; ≥98%) and hydrochloric acid (HCl; Sigma-Aldrich, St. Louis, MO, USA; 37%). For surface activation were used sulfuric acid (H2SO4; Sigma-Aldrich, St. Louis, MO, USA; 95%–98%) and hydrogen peroxide solution (H2O2; Sigma-Aldrich, St. Louis, MO, USA; 30%) to perform piranha solution. For the affinity and selectivity assays were used the human IgG, the human serum albumin (HAS; Sigma-Aldrich, St. Louis, MO, USA; ≥98%) and the anti-human IgG (Fab specific; antibody produced in goat, Sigma-Aldrich). Ultra-pure water (type II-analytical grade, <1 µS·cm−1) and ethanol (Labchem, Zelienople, PA, USA; 96%) were also used.
2.2. Synthesis of the SiO2 Nanoparticles
The SiO2 nanoparticles were synthesized according with the Stöber method. Briefly, in a proper container the ethanol, the ultra-pure water and the TEOS, were mixed in that order. The mixture was sonicated for 20 min and was added, under stir, the ammonia hydroxide and the final mixture was stirred for 24 h at room temperature. The resulted solution was centrifuged at 6000 rpm for 10 min and the beads were redispersed/centrifuged (five times) in deionized water and acetone. The resulted beads were dried at 40 °C for 12 h in the oven. The average size of the nanoparticles was determined by W130i Dynamic Light Scattering (DLS, AvidNano, Wycombe, UK), showing an average diameter ranging from 300–400 nm and were evaluated by attenuated total reflectance (FTIR-ATR, Bruker, Billerica, MA, USA).
2.3. Immobilization of the Biorecognition Molecule onto the SiO2 Surface
The dry beads were incubated in freshly prepared APTMS solution 2% (v/v) in anhydrous Toluene for 24 h at room temperature in a closed container, using 10 mg/mL of beads concentration. After incubation, the nanoparticles were centrifuged at 6000 rpm for 10 min and redispersed/centrifuged for five times in acetone and ethanol. From this step resulted amino-functionalized silica nanoparticles that were verified by ATR-IR. The beads were redispersed in the PBS solution in a concentration of 5 mg/mL. A solution of the biorecognition element in MES buffer (pH 5.5) and the activation of the template was prepared by adding EDC (10× molar excess) to NHS (10× molar excess) and incubating for 30 min at room temperature. The previous prepared beads solution was added to the template solution and the pH was adjusted to 7.4–8.0. The incubation carried out for 4 h at room temperature without stirring (just swirled the mixture every half-hour). The resulted modified nanoparticles were centrifuged at 6000 rpm for 10 min and redispersed/centrifuged in deionized water. The nanoparticles were assessed by FTIR-ATR.
2.4. Working Principle of the Evanescent Wave Based Sensors, Long-Period Fiber Grating Fabrication and Surface Modification
In this work, a long-period fiber grating was microfabricated on the optical fiber surface. This grating works as a wavelength selective filter, displaying a spectrum with several resonances resulting from the combination of the mode of the core and the different cladding modes [20]. Figure 1 shows a description of a LPFG on an optical fiber and the resultant transduced optical signal from the interaction between the recognition molecule and the target.
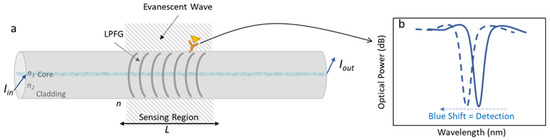
Figure 1.
(a) Schematic figure of a long period fiber grating; and (b) the optical signal resultant from the biorecognition.
Moreover, the LPFGs were fabricated by the induced electric-arc technique following the protocol published by Rego (2016) [20] by creating a modulation in the propagating mode refractive index which, in this case, is achieved point-by-point through electric arc discharges with a current of 9 mA and a duration of 1 s along 30 to 50 mm with a period of 415 µm. Afterwards, the LPFG was chemically modified by the immobilization of the biorecognition molecule on the fiber surface. The sensitive section of the optical fiber was cleaned with a 2 M NaOH solution for 10 min followed by immersion in a 0.5 M HCl solution for 2 h. After washing with deionized water, the sensitive surface was activated with piranha solution (3:1 v/v) for 1 h at 60 °C. Finally, the LPFG was washed with deionized water and kept in the oven for 10 min to completely dry and was cooled with pure nitrogen. The process is schematically presented in Figure 2.
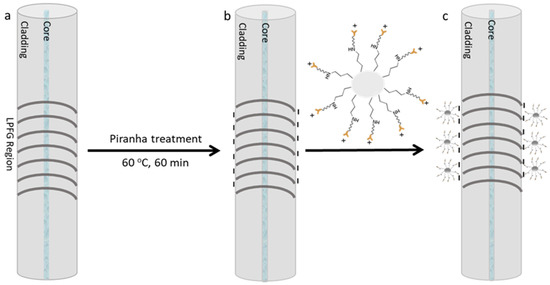
Figure 2.
Schematic figure of the LPFG surface chemical modification: (a) Bare LPFG; (b) activated surface with negative electrical charges; and (c) modified surface by the template immobilization.
2.5. Affinity and Selectivity Assays
The modified LPFGs were tested in the presence of the anti-human IgG in different concentrations ranging from 1.5 × 10−2 to 9 µg/mL to attest the affinity of the sensing platform. To verify the selectivity of the modified optical fiber, the sensing scheme was exposed to a 9 µg/mL of human IgG, HSA and anti-human IgG solutions, in the same experimental conditions. In order to obtain the most trustable values, was used a bare LPFG as a reference signal. All data will be presented as the differential between sensing LPFG and reference LPFG.
3. Results and Discussion
3.1. SiO2 Nanoparticles Bare, SiO2-NH2 and SiO2-NH2-IgG FTIR-ATR Spectra
To confirm the introduction of the new functional groups after each step of the SiO2 nanoparticles surface (nanoSiO2) modification, were made FTIR-ATR analysis. Figure 3 shows the spectra of the bare SiO2 (nanoSiO2), the aminated SiO2 (nanoSiO2-NH2) and the obtained nanospheres after the incubation of the IgG (nanoSiO2-NH2-IgG). In the main figure, the absorption peaks at 3000–3500 cm−1 are related to the stretching -OH bands, the absorption peaks at 1000–1150 cm−1 are assigned to the Si-O-Si asymmetric stretching bands and the peaks at 800–950 cm−1 are appointed to the asymmetric bending of Si-OH. In the Figure 3a, the asymmetric deformation vibration of the -NH2 at around 1550 cm−1 is displayed, suggesting that the amino groups were successfully fixed in the silica nanoparticle surface. Figure 3b show a peak at around 2900 cm−1 that are attributed to the presence of methyl groups of the APTMS structure. Finally, Figure 3c shows the carboxylate peak at 1650 cm−1, assigned to the presence of the IgG molecule. This evaluation is similar to the evaluation made by Feifel and his co-worker when the authors proved the possibility to create electro-active cytochrome C multilayers by using carboxyl-modified SiO2 nanoparticles [21]. Moreover, Hernandez-Leon et al. also showed parallel spectra when the authors modified a core-shell SiO2 nanobeads for capture low molecular weight proteins and peptides [22].
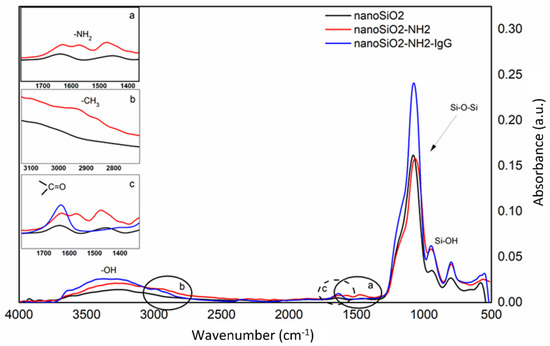
Figure 3.
Obtained absorbance spectra from FTIR-ATR analysis of bare SiO2 nanoparticles (black line), after amino-functionalized SiO2 surface (inset graphs a and b; red line), and after IgG immobilization (inset graph c; blue line).
3.2. Affinity and Slectivity Assays
The SiO2/IgG-modified LPFG probe was tested in the presence of different concentrations of anti-human IgG to attest the affinity of the sensing platform. The sensing LPFG (sensLPFG) was placed in an experimental chamber as well as the reference LPFG (refLPFG). Both gratings were exposed to a freshly prepared standard target solutions ranging from 1.5 × 10−2 to 9 µg/mL in PBS. After 10 min of exposure time, the LPFGs were washed three times with fresh PBS and three times with deionized water. All data were obtained measuring the LPFGs in deionized water at 22 °C. Figure 4a show the experimental data of the wavelength shift (sensLPFG–refLPFG) versus the anti-human IgG. Data are reported as a mean value with standard deviation (n = 3). Other similar works were described recently, such as the interferometric optical fiber biosensor for IgG/anti-IgG immunosensing presented by Wang et al., reporting a limit of detection around of 50 ng/mL [23]. Another approach was demonstrated by Han et al. that combined a Bragg acoustic reflector with an Au electrode and an aluminum nitride piezoelectric thin film, to develop a biosensor for anti-human IgG detection by immobilization of the human IgG antibody onto the modified Au electrode. The sensing platform was able to detect anti-human IgG concentrations smaller than 0.4 mg/mL [24]. The sensing platform presented in this work is able to detect the referenced target below to 0.1 µg/mL. Additionally, the data resulting from the linear fitting of Figure 4a, displaying a sensibility (S) (i.e., the slope) about |S| = 59 pm/(µg/mL).
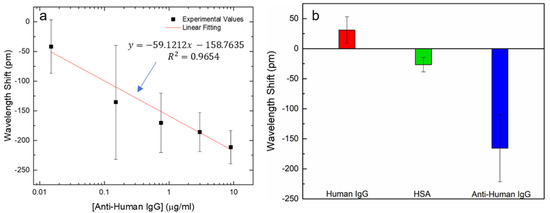
Figure 4.
(a) Resonance shift vs. the anti-human IgG concentration (1.5 × 10−2 to 9 µg/mL). Data is reported as a mean value (n = 3) with standard deviation; (b) Resonance shift obtained by 10 min incubation in 9 µg/mL in human IgG, HSA protein and anti-human IgG. Data is reported as a mean value (n = 3) with standard deviation.
To validate the specificity of the sensing platform, the same protocol was followed in the presence of human IgG antibody, the HSA protein and, finally, the anti-human IgG. Figure 4b show the resulting data after 10 min of incubation time for each target in 9 µg/mL (in PBS). The results are reported as a mean value with standard deviation (n = 3). These results showed the specificity of the built sensing platform to the proposed target, revealing a very relevant wavelength shift when exposed to it. By the other side, the shifts showed by the LPFG in the presence of the other targets are not relevant.
4. Conclusions
In this work, a sensing platform for the detection of the anti-human IgG antigen was developed by chemical modification of long period fiber grating surface. The sensing methodology is based on refractometric changes due to the interactions between the biorecognition molecule and the target. The surface of the optical fiber was changed by immobilization of IgG- modified silica nanoparticles. The FTIR-ATR spectra proved that the biorecognition molecule was successfully attached onto the SiO2 nanoparticles surface and specificity assays demonstrated the selectivity of the method. The use of IgG-modified nanoparticles can bring some advantages, increasing the number of receptors available to interact with the target.
The low-cost and easy-to-use optical sensor reported here can detect anti-human IgG concentrations below 0.1 µg/mL by promoting specific antibody/antigen interactions. In the next step, we aim to imprint molecularly the analogue synthetic molecule of this template. The goal is to produce highly sensitive and selective molecularly imprinted polymers using the template of this work, combining them with highly sensitive optical platforms.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/CSAC2021-10454/s1.
Author Contributions
Conceptualization, J.P.M. and L.C.C.C.; software, L.C.C.C.; formal analysis, J.P.M.; investigation, J.P.M. and V.P.P.; data curation, J.P.M.; writing—original draft preparation, J.P.M.; writing—review and editing, L.C.C.C., P.A.S.J. and C.M.P.; supervision, M.A.A., P.A.S.J. and C.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by National Funds through the Portuguese funding agency, FCT—Fundação para a Ciência e a Tecnologia, within projects “UIDB/50014/2020” and “UIDB/00081/2020 (CIQUP)”and ANI through project “FAMEST”—POCI-01-0247-FEDER-024529 under program P2020|COMPETE.
Acknowledgments
João Mendes would like to thank FCT for the PhD research grant SFRH/BD/130674/2017 and Luís Coelho acknowledges the support from FCT research contract grant CEECIND/00471/2017.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tertis, M.; Hosu, O.; Florea, A.; Cristea, C. Biosensors for Clinical Samples: Consideration and Approaches. In Immunodiagnostic Technologies from Laboratory to Point-Of-Care Testing; Suman, P., Chandra, P., Eds.; Springer: Singapore, 2021. [Google Scholar]
- Chocarro-Ruiz, B.; Fernandez-Gavela, A.; Herranz, S.; Lechuga, L.M. Nanophotonic label-free biosensors for environmental monitoring. Curr. Opin. Biotechnol. 2017, 45, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Balbinot, S.; Srivastav, A.M.; Vidic, J.; Abdulhalim, I.; Manzano, M. Plasmonic biosensors for food control. Trends Food Sci. Technol. 2021, 111, 128–140. [Google Scholar] [CrossRef]
- Peltomaa, R.; Glahn-Martínez, B.; Benito-Peña, E.; Moreno-Bondi, M.C. Optical Biosensors for Label-Free Detection of Small Molecules. Sensors 2018, 18, 4126. [Google Scholar] [CrossRef] [Green Version]
- Wollenberger, U. Chapter 2 Third generation biosensors—Integrating recognition and transduction in electrochemical sensors. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2005; pp. 65–130. [Google Scholar]
- Ong, J.J.; Pollard, T.D.; Goyanes, A.; Gaisford, S.; Elbadawi, M.; Basit, A.W. Optical biosensors—Illuminating the path to personalized drug dosing. Biosens. Bioelectron. 2021, 188, 113331. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Yang, Z.; Wilkinson, J.S.; Zhou, X. Optical biosensors based on refractometric sensing schemes: A review. Biosens. Bioelectron. 2019, 144, 111693. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef]
- Khansili, N.; Rattu, G.; Krishna, P.M. Label-free optical biosensors for food and biological sensor applications. Sens. Actuators B Chem. 2018, 265, 35–49. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhu, Z.-W.; Xiao, W.; Deng, Q.-X. Review of fiber optic sensors in geotechnical health monitoring. Opt. Fiber Technol. 2020, 54, 102127. [Google Scholar] [CrossRef]
- Monfared, Y.E. Overview of Recent Advances in the Design of Plasmonic Fiber-Optic Biosensors. Biosensors 2020, 10, 77. [Google Scholar] [CrossRef]
- Lobry, M.; Lahem, D.; Loyez, M.; Debliquy, M.; Chah, K.; David, M.; Caucheteur, C. Non-enzymatic D-glucose plasmonic optical fiber grating biosensor. Biosens. Bioelectron. 2019, 142, 111506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liyanage, T.; Lai, M.; Slaughter, G. Label-free tapered optical fiber plasmonic biosensor. Anal. Chim. Acta 2021, 1169, 338629. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, B.; Zhou, L.; Sun, Z.; Mao, H.; Zhao, J.; Zhang, L.; Chen, X. Graphene oxide functionalized long period fiber grating for highly sensitive hemoglobin detection. Sens. Actuators B Chem. 2018, 261, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Chiavaioli, F.; Zubiate, P.; Del Villar, I.; Zamarreño, C.R.; Giannetti, A.; Tombelli, S.; Trono, C.; Arregui, F.J.; Matias, I.R.; Baldini, F. Femtomolar Detection by Nanocoated Fiber Label-Free Biosensors. ACS Sens. 2018, 3, 936–943. [Google Scholar] [CrossRef]
- Dey, T.; Tombeli, S.; Biswas, P.; Giannetti, A.; Basumallick, N.; Baldini, F.; Bandyopadhyay, S.; Trono, C. Realization of enhanced evanescent field long period fiber grating near turn around point for label-free immunosensing. In Proceedings of the 1st International Electronic Conference on Biosensors, Basel, Siwtzerland, 2–17 November 2020. [Google Scholar]
- Liu, L.; Marques, L.; Correia, R.; Morgan, S.P.; Lee, S.-W.; Tighe, P.; Fairclough, L.; Korposh, S. Highly sensitive label-free antibody detection using a long period fibre grating sensor. Sens. Actuators B Chem. 2018, 271, 24–32. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Tadanaga, K.; Morita, K.; Mori, K.; Tatsumisago, M. Synthesis of monodispersed silica nanoparticles with high concentration by the Stöber process. J. Sol-Gel Sci. Technol. 2013, 68, 341–345. [Google Scholar] [CrossRef]
- Rego, G. Arc-Induced Long Period Fiber Gratings. J. Sens. 2016, 2016, 3598634. [Google Scholar] [CrossRef] [Green Version]
- Feifel, S.C.; Lisdat, F. Silica nanoparticles for the layer-by-layer assembly of fully electro-active cytochrome c multilayers. J. Nanobiotechnol. 2011, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Leon, S.G.; Sarabia-Sainz, J.A.-I.; Montfort, G.R.-C.; Guzman-Partida, A.M.; Robles-Burgueño, M.D.R.; Vazquez-Moreno, L. Novel Synthesis of Core-Shell Silica Nanoparticles for the Capture of Low Molecular Weight Proteins and Peptides. Molecules 2017, 22, 1712. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.-T.; Wang, Q. An interferometric optical fiber biosensor with high sensitivity for IgG/anti-IgG immunosensing. Opt. Commun. 2018, 426, 388–394. [Google Scholar] [CrossRef]
- Han, C.; Wang, X.; Zhao, Q.; Teng, L.; Zhang, S.; Lv, H.; Liu, J.; Ma, H.; Wang, Y. Solidly mounted resonator sensor for biomolecule detections. RSC Adv. 2019, 9, 21323–21328. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).