Abstract
Natural phenolic antioxidants are extensively studied compounds due to their positive health effect and wide distribution in human diets. The simultaneous occurrence in samples requires selective methods for their determination. Electrochemical sensor based on the polyaminobenzene sulfonic acid functionalized single-walled carbon nanotubes (f-SWCNT) and electropolymerized bromocresol purple has been developed for the simultaneous quantification of ferulic acid and vanillin. The electrode has been characterized by scanning electron microscopy (SEM) and electrochemical methods, and the effectivity of the developed modifier has been confirmed. Thus, the novel sensitive voltammetric sensor is simple to fabricate, reliable, cost-effective, and can be applied for foodstuff screening.
1. Introduction
Natural phenolic antioxidants are extensively studied compounds in modern electroanalysis due to their positive health effect and wide distribution in the human diet [1]. Simultaneous occurrence in samples requires selective methods for their determination. Among a wide range of natural phenolics, vanillin and its biological precursor ferulic acid [2] are of practical interest. High-performance liquid [3,4] and thin-layer chromatography [5] are usually applied for this purpose. Both phenolics under consideration are electrochemically active, which makes it possible to use electrochemical methods for their quantification. Although various types of electrochemical sensors have been developed for the simultaneous quantification of natural phenolics of different classes [6,7,8], ferulic acid and vanillin are not considered as analytes.
Thus, the current work is focused on the development of an electrochemical sensor based on a poly(bromocresol purple)-modified electrode for the simultaneous quantification of ferulic acid and vanillin.
2. Materials and Methods
Bromocresol purple (90% purity), 99% vanillin from Sigma-Aldrich (Steinheim, Germany), and 99% ferulic acid from Aldrich (Steinheim, Germany) were used. Their standard 10 mM solutions were prepared in ethanol (rectificate). The exact dilution was used for the preparation of less concentrated solutions.
Polyaminobenzene sulfonic acid functionalized single-walled carbon nanotubes (f-SWCNT) (d × l is 1.1 nm × 0.5–1.0 μm) were purchased from Sigma-Aldrich (Steinheim, Germany). A homogeneous 1.0 mg mL−1 suspension of f-SWCNT was obtained by ultrasonic dispersion for 30 min in dimethylformamide.
All reagents had chemical-grade purity. Double distilled water was used for the measurements. The experiments were carried out at laboratory temperature (25 ± 2 °C).
Electrochemical measurements were carried out on the potentiostat/galvanostat Autolab PGSTAT 302N with FRA 32M module (Eco Chemie B.V., Utrecht, The Netherlands) and NOVA 1.10.1.9 software. The 10 mL glassy electrochemical cell with a working glassy carbon electrode (GCE) with a 7.07 mm2 geometric surface area (CH Instruments, Inc., Bee Cave, TX, USA) or modified electrode, a silver-silver chloride saturated KCl reference electrode, and a platinum wire as the counter electrode was used.
An “Expert-001” pH meter (Econix-Expert Ltd., Moscow, Russian Federation) equipped with the glassy electrode was applied for pH measurements.
Scanning electron microscopy (SEM) was carried out on the high-resolution field emission scanning electron microscope MerlinTM (Carl Zeiss, Oberkochen, Germany) at the accelerating voltage of 5 kV and emission current of 300 pA.
3. Results and Discussion
3.1. Characterization of the Electrodes
Bromocresol purple forms a nonconducting film which is confirmed by the disappearance of the oxidation peak with an increase of the cycles number and which is typical for the electropolymerization of phenolics [9].
The conditions of bromocresol purple potentiodynamic electropolymerization (monomer concentration, number of scans, supporting electrolyte pH, electrolysis parameters) have been optimized in order to find the best voltammetric response of the co-existed ferulic acid and vanillin. The peak potential separation of 170 mV on the polymer-based electrode is not affected by electropolymerization conditions, while oxidation currents change significantly. The best response has been obtained for the poly(bromocresol purple) obtained by 10-fold potential cycling from 0.1 to 1.2 V with a scan rate of 100 mV s−1 from the 25 µM monomer solution in 0.1 M phosphate buffer pH 7.0.
The electrodes have been characterized by SEM (Figure 1). The data obtained confirm the successful immobilization of the nanomaterial on the electrode surface.
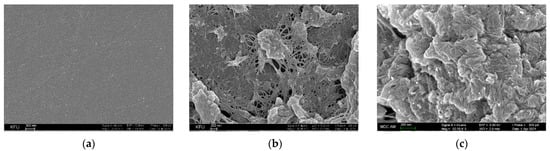
Figure 1.
SEM images for (a) CGE; (b) CGE/f-SWCNT; and (c) CGE/f-SWCNT/Poly(bromocresol purple).
The effective surface area of the electrodes has been evaluated using 1.0 mM [Fe(CN)6]4− as a redox probe under conditions of cyclic voltammetry and chronoamperometry (for GCE). The Cottrell equation for GCE and Randles–Sevcik equation for the cyclic voltammetry data have been applied. A 5.1-fold increase of the effective surface area for the polymer-modified electrode vs. bare GCE has been obtained, which leads to the ferulic acid and vanillin oxidation current increasing. Electrochemical impedance spectroscopy (EIS) has been performed in the presence of [Fe(CN)6]4−/3− as a redox probe at 0.23 V. EIS data show a 7.2-fold lower charge transfer resistance for the poly(bromocresol purple)-based sensor in comparison to GCE, which means an increase of the electron transfer rate.
3.2. Simultaneous Quantification of Natural Phenolic Antioxidants
The well-resolved oxidation peaks of the ferulic acid and vanillin at 0.732 and 0.903 V, respectively, with a potential separation of 170 mV have been obtained on the created sensor (Figure 2).
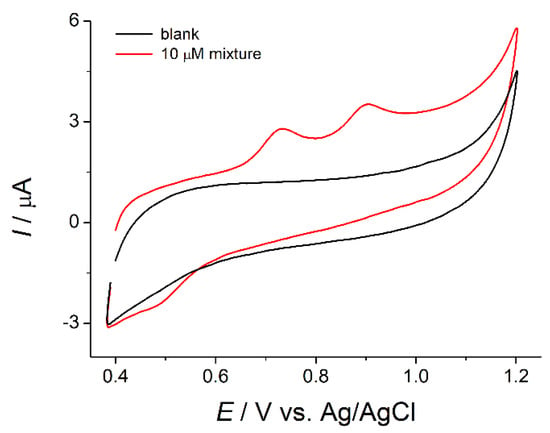
Figure 2.
Cyclic voltammogram of 10 µM mixture of ferulic acid and vanillin on the poly(bromocresol purple)–based electrode in Britton−Robinson buffer pH 2.0.
The analytes’ electrooxidation parameters have been studied. Both phenolics are oxidized under a diffusion control and irreversible with the participation of two electrons and protons (Figure 3).
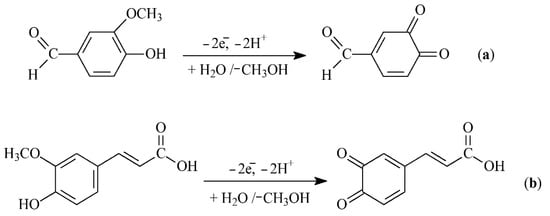
Figure 3.
Electrooxidation scheme of (a) vanillin and (b) ferulic acid.
The developed sensor has been operated under conditions of differential pulse voltammetry. The pulse parameters have been optimized, and it has been found that a modulation amplitude of 75 mV and modulation time of 25 ms provide the best response of the target analytes. The sensor allows a direct simultaneous quantification of ferulic acid and vanillin in the ranges of 0.1–5.0 and 5.0–25 µM for both analytes (Figure 4) with detection limits of 72 and 64 nM, respectively. The accuracy of determination has been tested on the model mixtures of ferulic acid and vanillin. The relative standard deviation and recovery values obtained confirm the absence of a random error and the precision of the developed sensor.
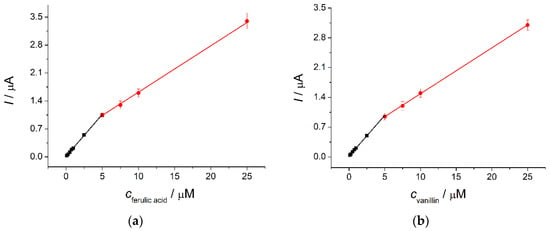
Figure 4.
Calibration plots for (a) ferulic acid and (b) vanillin based on the differential pulse voltammetry data on the poly(bromocresol purple)-based electrode in Britton—Robinson buffer pH 2.0.
Thus, the novel sensitive voltammetric sensor is simple to fabricate, reliable, cost-effective, and can be applied for the foodstuff screening.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhuyan, D.J.; Basu, A. Phenolic Compounds potential health Benefits and toxicity. In Utilisation of Bioactive Compounds from Agricultural and Food Waste, 2nd ed.; Vuong, Q.V., Ed.; CRC Press: Boca Raton, FL, USA, 2017; Chapter 2; pp. 27–59. [Google Scholar]
- Gallage, N.J.; Hansen, E.H.; Kannangara, R.; Olsen, C.E.; Motawia, M.S.; Jørgensen, K.; Holme, I.; Hebelstrup, K.; Grisoni, M.; Møller, B.L. Vanillin formation from ferulic acid in Vanilla planifolia is catalysed by a single enzyme. Nat. Commun. 2014, 5, 4037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranadive, A.S. Vanillin and related flavor compounds in vanilla extracts made from beans of various global origins. J. Agric. Food Chem. 1992, 40, 1922–1924. [Google Scholar] [CrossRef]
- Sinha, A.K.; Verma, S.C.; Sharma, U.K. Development and validation of an RP-HPLC method for quantitative determination of vanillin and related phenolic compounds in Vanilla planifolia. J. Sep. Sci. 2007, 30, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Hingse, S.S.; Digole, S.B.; Annapure, U.S. Method development for simultaneous detection of ferulic acid and vanillin using high-performancethin layer chromatography. J. Anal. Sci. Technol. 2014, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Ziyatdinova, G.K.; Guss, E.V.; Morozova, E.V.; Budnikov, H.C. An electrode based on electropolymerized sunset yellow for the simultaneous voltammetric determination of chlorogenic and ferulic acids. J. Anal. Chem. 2021, 76, 371–380. [Google Scholar] [CrossRef]
- Zhupanova, A.; Guss, E.; Ziyatdinova, G.; Budnikov, H. Simultaneous voltammetric determination of flavanones using an electrode based on functionalized single-walled carbon nanotubes and polyaluminon. J. Anal. Lett. 2020, 53, 2170–2189. [Google Scholar] [CrossRef]
- Guss, E.V.; Ziyatdinova, G.K.; Zhupanova, A.S.; Budnikov, H.C. Voltammetric determination of quercetin and rutin in their simultaneous presence on an electrode modified with polythymolphthalein. J. Anal. Chem. 2020, 75, 526–535. [Google Scholar] [CrossRef]
- Yang, G.-J.; Qu, X.-L.; Zhu, A.-P.; Wang, C.-Y.; Qu, Q.-S.; Hu, X.-Y. Characterization, growth mechanism and application of network poly(bromophenol blue). J. Electroanal. Chem. 2007, 604, 48–56. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).