Abstract
An array of 2,4-disubstituted thiazolines was obtained through Asinger reaction approach from the straightforward treatment of diverse aldehydes/ketones with 1-mercaptopropan-2-one, in the presence of NH3 assisted by microwave irradiation, displaying similar and sometimes higher yields, as well as shorter reaction times that traditional Asinger reaction conditions at room and lower temperatures.
1. Introduction
Thiazolines are five-membered heterocycles that contain both nitrogen and sulfur, belonging to 1,3-azole heterocycles group. Compared with other azole rings, the thiazoline moiety is relatively less studied, in spite of the growing interest raised by this heterocycle, as a component of many natural products and synthetic compounds displaying a wide range of biological activities [1]. In this regard, diverse synthetic protocols for thiazolines were developed from condensations of thioamides and derivatives [2,3,4,5,6], as well as cysteine and other amino acids [7,8,9].
On the other hand, since its discovery in the late fifties [10,11,12], Asinger reaction showed the distinctive advantages provided by multicomponent reactions applied to the synthesis of cyclic compounds [13,14,15,16,17]. However, these apparent benefits were missed probably due to the few starting materials reported in the literature, which are compatible (and sometimes soluble) with aqueous ammonia, in addition to reaction temperatures close to ambient conditions or lower [18,19].
These facts motivated us to explore alternative conditions for the Asinger Reaction, in order to incorporate structurally diverse feedstock, keeping the attractive characteristics of this process and inherent multicomponent reaction approach. In this report, we disclose our first findings in this area.
2. Results and Discussion
Initial experiments were carried out following a four-component reaction approach, according to the literature [18,19], through a simultaneous mixing of a 2-bromoketone, an ammonia source, NaSH, and a ketone under diverse reaction conditions, which are summarized in Scheme 1 and Table 1. Preliminary results represented in entries 1–3 showed poor yields of thiazoline, as a consequence of incompatibility of aqueous ammonia with the other components, especially 2-bromoketone, affording some degradation products related to competition in nucleophilic substitution on 2-bromoketones, which resulted in it being sensitive to ammonia. This behavior was kept with the introduction of other solvents (entries 4–7) at different temperatures (entries 8–10), as well as diverse ammonia sources (entries 11–18).
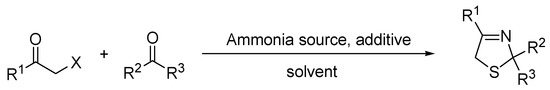
Scheme 1.
Preparation of thiazolines from a four-component reaction.

Table 1.
Synthesis of thiazoline 4 catalyzed by phenylacetylide 1.
The change of a ketone by an aldehyde in Asinger Reaction slightly improved thiazoline yields up to 20%, using the ammonia solution in methanol described by Silvani et al. [14]. According to these authors, anhydrous conditions using MgSO4 also contributed to yield optimization, however, a radical change in reaction yield was observed when 1-mercaptopropan-2-one was used instead of the 2-bromoketone/NaSH system, avoiding secondary nucleophilic substitutions. This reactivity pattern was corroborated through the use of cyclohexanone giving the corresponding thiazoline in 68% yield. Hence, the components reduction in the Asinger reaction was compensated by a substantial yield increase.
From these improvements, a general three-component synthetic protocol was developed via straightforward reaction of 1-mercapto propan-2-one and ammonia/MeOH, and was modulated by an aldehyde (Scheme 2). Results in Table 2 show the procedure scope covering distinct substituents in the aldehyde component, giving yields ranged from 33% to 90%.
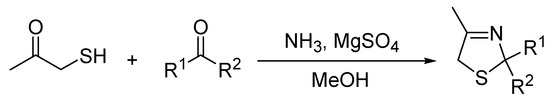
Scheme 2.
Preparation of thiazolines from a three-component reaction at room temperature and under microwave irradiation.

Table 2.
Thiazoline yields obtained at room temperature and under microwave irradiation.
Based on our previous experiences on microwave-assisted reactions [20], we considered that these conditions could be extended to the Asinger reaction. Thus, early investigations revealed thiazoline formation from the last studied three-component reaction treated under microwave irradiation. The best yields were obtained at 40 °C (200 W), which is comparable to, and in some cases higher than those observed at room temperature (see Table 2), but showed shorter reaction times. To the best of our knowledge, this was the first report about the synthesis of thiazolines from Asinger reaction using microwave irradiation as an alternative energy source.
The above examples demonstrate the feasibility of this methodology, and future studies are envisioned to broaden the applications of thiazolines in the synthesis of biologically active molecules, potential drugs, and ligands for catalysis.
3. Experiment
The starting materials were purchased from Aldrich Chemical Co. and were used without further purification. Solvents were distilled before use. Silica plates of 0.20 mm thickness were used for thin layer chromatography. The melting points were determined with a Krüss Optronic melting point apparatus and they were uncorrected. 1H and 13C NMR spectra were recorded using a Bruker Avance 300-MHz; the chemical shifts (δ) are given in ppm relative to TMS as the internal standard (0.00). For analytical purposes, the mass spectra were recorded on a Shimadzu GCMS-QP2010 Plus in the EI mode, 70 eV, 200 °C via direct inlet probe. Only the molecular and parent ions (m/z) were reported. IR spectra were recorded on a Bruker Tensor 27 equipment. The microwave-assisted reactions were performed using a CEM Discover microwave unit (constant factor of the microwave 1.214). The temperature was monitored with an IR temperature sensor. In all experiments, the microwave temperature was held constant. Reactions were carried out in 5-mL glass vessels, which were sealed with a cap septum. The specific reaction time corresponded to the total irradiation time.
3.1. Synthesis of Thiazolines under Conventional Conditions—General Procedure
The appropriate aldehyde or ketone (1 mmol) and anhydrous MgSO4 (0.360 g, 3 mmol) were added to a ~13 M ammonia solution in MeOH (1 mL), and the mixture was stirred for 1 h at room temperature. Mercaptoacetone (0.099 g, 1.1 mmol) was added dropwise, over 5 min. The resulting mixture was stirred for 24 h at room temperature. The mixture was filtered through celite. The solvent was removed under reduced pressure and the final product was purified by column chromatography (SiO2, hexane/AcOEt 8:2).
3.2. Synthesis of Thiazolines under Microwave Irradiation Conditions—General Procedure
A 5-mL microwave vial was charged with the corresponding aldehyde or ketone (1 mmol), MgSO4 (0.360 g, 3 mmol) and a ~13 M ammonia solution in MeOH (1 mL) and a cylindrical magnetic stirring bar. The vessel was sealed with a septum, placed into the microwave cavity of a CEM Discover microwave unit and irradiated to heat the reaction mixture at 40 °C. The total heating time was 10 min at 200 W, 40 °C. After the first cycle, mercaptoacetone (0.099 g, 1.1 mmol) was added and the mixture was irradiated with same parameters for 10 min. When the reaction was completed, the vial was cooled to room temperature. The vial was then opened and the mixture was filtered through celite. The solvent was removed under reduced pressure and the final product was purified by column chromatography (SiO2, hexane/AcOEt 8:2).
3.3. 2-(4-Chlorophenyl)-4-methyl-2,5-dihydrothiazole 1
4-Chlorobenzaldehyde and 1-mercaptopropan-2-one afforded 2-(4-chlorophenyl)-4-methyl-2,5-dihydrothiazole 1 as white solid. Yields: 135.0 mg (64% at room temperature conditions) and 149.8 mg (71% under microwave conditions). IR (ATR) νmax 2922, 1489, 1089, 828 cm−1. 1H NMR (300 MHz, CDCl3) δ 7.3 (d, J = 6 Hz, 2H), 7.2 (d, J = 6 Hz, 2H) 6.56 (s, 1H), 4.09 (dd, J = 5 Hz, J = 16 Hz, 1H), 3.98 (dd, J = 3 Hz, J = 16 Hz,1H), 2.23 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 170.8, 140.5, 133.6, 128.7, 128.2, 83.8, 47.3, 19.5. MS [EI+] m/z (%): 211 [M]+ (10).
3.4. 2-(2-Chlorophenyl)-4-methyl-2,5-dihydrothiazole 2
2-Chlorobenzaldehyde and 1-mercaptopropan-2-one afforded 2-(2-chlorophenyl)-4-methyl-2,5-dihydrothiazole 2 as the white solid. Yields: 162.4 mg (77% at room temperature conditions) and 99.2 mg (47% under microwave conditions). IR (ATR) νmax 2916, 1469, 1036, 740 cm−1. 1H NMR (300 MHz, CDCl3) δ 7.37–7.17 (m, 4H), 6.9 (s, 1H), 3.89 (s, 2H), 2.25 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 172.2, 140.0, 132.3, 129.4, 128.8, 127.4, 127.1, 81.0, 46.6, 19.7. MS (EI+) m/z (%): 211[M]+ (5).
3.5. 4-Methyl-2-phenyl-2,5-dihydrothiazole 3
Benzaldehyde and 1-mercaptopropan-2-one afforded 4-methyl-2-phenyl -2,5-dihydrothiazole 3 as white solid. Yields: 58.4 mg (33% at room temperature conditions) and 88.5 mg (50% under microwave conditions). IR (ATR) νmax 2915, 1662, 1421 cm−1. 1H NMR (300 MHz, CDCl3) δ 7.42–7.25 (m, 5H), 6.62 (bs, 1H), 4.10 (dd, J = 5 Hz J = 15 Hz, 1H), 3.98 (dd, J = 3 Hz, J = 15 Hz, 1H), 2.23 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 170.3, 141.9, 128.6, 128.41, 127.9, 126.8, 84.5, 47.2, 19.5. MS (EI+) m/z (%): 177 [M]+ (15).
3.6. 4-(4-Methyl-2,5-dihydrothiazol-2-yl)phenol 4
4-Hydroxybenzaldehyde and 1-mercaptopropan-2-one afforded 4-(4-methyl-2,5-dihydrothiazol-2-yl)phenol 4 as white solid. Yields: 106.1 mg (55% at room temperature conditions) and 125.4 mg (65% under microwave conditions). IR (ATR) νmax 3065, 2900, 1663, 1514 cm−1. 1H NMR (300 MHz, CDCl3) 8.69 (bs, 1H, -OH), 7.12 (d, J = 9 Hz, 2H), 6.79 (d, J = 9 Hz), 6.53 (bs, 1H), 4.10 (dd, J = 5 Hz, J = 16 Hz, 1H), 3.96 (dd, J = 3 Hz, J = 16 Hz,1H) 2.22 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 169.6, 156.9, 132.6, 127.9, 115.48, 84.3, 47.0, 19.4. MS (EI+) m/z (%): 193 [M]+ (5).
3.7. 4-Methyl-2-(p-tolyl)-2,5-dihydrothiazole 5
4-Methylbenzaldehyde and 1-mercaptopropan-2-one afforded 4-methyl-2-(p-tolyl)-2,5-dihydrothiazole 5 as white solid. Yields: 131.7 mg (69% at room temperature conditions) and 93.5 mg (49% under microwave conditions). IR (ATR) νmax 2916, 1662, 817 cm−1. 1H NMR (300 MHz, CDCl3) δ 7.19 (d, J = 9 Hz, 2H), 7.13 (d, J = 9 Hz, 2H), 6.59 (bs, 1H), 4.11 (dd, J = 6 Hz, J = 15 Hz, 1H) 3.98 (dd, J = 6 Hz, J = 15 Hz, 1H), 2.33 (s, 3H) 2.24 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 169.7, 138.7, 137.3, 128.9, 126.3, 84.1, 46.9, 29.4, 20.8, 19.2. MS (EI+) m/z (%): 191 [M]+ (10).
3.8. 2-(2,3-Dimethoxyphenyl)-4-methyl-2,5-dihydrothiazole 6
2,3-Dimethox-benzaldehyde and 1-mercaptopropan-2-one afforded 2-(2,3-dimethoxyphenyl)-4-methyl-2,5-dihydrothiazole 6 as white solid. Yields: 163.5 mg (69% at room temperature conditions) and 177.7 mg (75% under microwave conditions). IR (ATR) νmax 2935, 2834, 1665, 1478, 1263 cm−1. 1H NMR (300 MHz, CDCl3) δ 7.02 (dd, J = 9 Hz, J = 6 Hz, 1H), 6.94 (bs, 1H), 6.84 (dd, J = 3 Hz, J = 6 Hz, 1H), 6.76 (dd, J = 3 Hz, J = 6 Hz, 1H), 4.05 (dd, J = 6 Hz, J = 15 Hz, 1H) 3.98 (dd, J = 6 Hz, J = 15 Hz, 1H), 3.91 (s, 3H), 3.86 (s, 3H), 2.26 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 169.2, 150.9, 144.3, 134.6, 122.5, 117.0, 110.3, 59.4, 54.2, 45.2, 18.1. MS (EI+) m/z (%): 237 [M]+ (20).
3.9. 2-Methoxy-4-(4-methyl-2,5-dihydrothiazol-2-yl)phenol 7
4-Hydroxy-3-methoxy-benzaldehyde and 1-mercaptopropan-2-one afforded 2-methoxy-4-(4-methyl-2,5-dihydrothiazol-2-yl)phenol 7 as white solid. Yields: 200.7 mg (90% at room temperature conditions) and 156.1 mg (70% under microwave conditions). IR (ATR) νmax 3101, 2920, 1595, 1368, 1270 cm−1. 1H NMR (300 MHz, CDCl3) δ 6.85–6.82 (m, 3H), 6.55 (bs, 1H), 5.73 (bs, 1H), 4.11 (dd, J = 3 Hz, J = 16 Hz, 1H), 3.98 (dd, J = 3 Hz, J = 16 Hz, 1H), 3.88 (s, 3H), 2.24 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 170.0, 146.5, 145.4, 133.7, 119.9, 114.3, 109.4, 84.5, 55.9, 47.2, 19.6. MS (EI+) m/z (%): 223 [M]+.
3.10. 4-Methyl-2-(2-(prop-2-yn-1-yloxy)phenyl)-2,5-dihydrothiazole 8
2-Prop-2-ynyloxy-benzaldehyde and 1-mercaptopropan-2-one afforded 4-methyl-2-(2-(prop-2-yn-1-yloxy)phenyl)-2,5-dihydrothiazole 8 as white solid. Yields: 203.2 mg (88% at room temperature conditions) and 168.3 mg (73% under microwave conditions). IR (ATR) νmax 3285, 2918, 2120, 1663, 1487, 1220, 751 cm−1. 1H NMR (300 MHz, CDCl3) δ 7.25–7.23 (m, 1H), 7.21–7.19 (m, 1H) 6.99–6.95 (m, 1H), 6.93 (bs, 1H), 4.75 (s, 2H) 4.03 (dd, J = 3 Hz, J = 9 Hz, 1H), 3.94 (dd, J = 1.5 Hz, J = 9 Hz, 1H), 2.52 (s, 1H) 2.26 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 154.1, 131.6, 128.6, 126.8, 121.6, 112.0, 78.8, 78.5, 75.5, 56.2, 46.4, 19.7. MS [EI+] m/z (%): 231 [M]+ (5).
3.11. 3-Methyl-1-thia-4-azaspiro[4.5]dec-3-ene 9
Cyclohexanone and 1-mercaptopropan-2-one afforded 3-methyl-1-thia-4-azaspiro[4.5]dec-3-ene 9 as white solid. Yields: 114.9 mg (68% at room temperature conditions) and 126.7 mg (75% under microwave conditions). IR (ATR) νmax 2927, 1691, 1444, 894 cm−1. 1H NMR (300 MHz, CDCl3) δ 3.77 (s, 2H), 2.10 (s, 3H), 2.04–1.16 (m, 10H). 13C NMR (75 MHz, CDCl3) δ 165.7, 95.4, 44.3, 40.9, 24.8, 24.1, 19.8. MS [EI+] m/z (%): 169 [M]+ (10).
4. Conclusions
Thiazolines are readily available from microwave assisted Asinger reaction through a synthetic procedure that takes advantage of joining both, a multicomponent reaction approach and microwave irradiation methods. The simplicity of this methodology suggests that this route to thiazolines can have widespread application.
Author Contributions
Conceptualization, E.C.-Y.; methodology, R.E.G.-C., L.G.-R., M.C.-R., and N.Z.-S.; formal analysis, N.Z.-S.; investigation, R.E.G.-C., N.Z.-S., E.C.-Y., B.A.F.-U., M.A.G.-E., and M.V.B.U.; resources, B.A.F.-U. and E.C.-Y.; data curation, N.Z.-S., M.A.G.-E., and M.V.B.U.; writing—original draft preparation, E.C.-Y.; writing—review and editing, E.C.-Y.; supervision, E.C.-Y., M.A.G.-E., and M.V.B.U.; project administration, E.C.-Y.; funding acquisition, B.A.F.-U. and E.C.-Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CONACYT-Mexico, project No. A1-S-18230 and fellowship for R.E.G.C., CVU: 229559.
Acknowledgments
Financial support from CONACYT is gratefully acknowledged. The authors would like to thank A. Nuñez, L. Triana and M. C. Martínez for the technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gaumont, A.C.; Gulea, M.; Levillain, J. Overview of the Chemistry of 2-Thiazolines. Chem. Rev. 2009, 109, 1371–1401. [Google Scholar] [CrossRef] [PubMed]
- Alom, N.E.; Wu, F.; Li, W. One-Pot Strategy for Thiazoline Synthesis from Alkenes and Thioamides. Org. Lett. 2017, 19, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, Z.A.; Alam, M. Modular synthesis of thiazoline and thiazole derivatives by using a cascade protocol. RSC Adv. 2017, 7, 32647. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ahmad, A.; Sudhakar, R.; Varshney, H.; Subbarao, N.; Ansari, S.; Rauf, A.; Khan, A.U. Designing, synthesis and antimicrobial action of oxazoline and thiazoline derivatives of fatty acid esters. J. Biomol. Struct. Dyn. 2017, 35, 3412–3431. [Google Scholar] [CrossRef] [PubMed]
- Oniga, O.; Ndongo, J.T.; Moldovan, C.; Tiperciuc, B.; Oniga, S.; Pîrnău, A.; Vlase, L.; Verité, P. Synthesis and antimicrobial activity of some new 2-hydrazone-thiazoline-4-ones. Farmacia 2012, 60, 785–797. [Google Scholar]
- You, S.L.; Razavi, H.; Kelly, J.W. A Biomimetic Synthesis of Thiazolines Using Hexaphenyloxodiphosphonium Trifluoromethanesulfonate. Angew. Chem. Int. Ed. 2003, 42, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.M.F.M.; Sales, E.S.; Livotto, P.R.; Schneider, P.H.; Merlo, A.A. Synthesis of New Family of Thiazoline and Thiazole Esters and Investigation of their Thermal Properties. J. Braz. Chem. Soc. 2014, 25, 1493–1503. [Google Scholar] [CrossRef]
- Diness, F.; Nielsen, D.S.; Fairlie, D.P. Synthesis of the Thiazole−Thiazoline Fragment of Largazole Analogues. J. Org. Chem. 2011, 76, 9845–9851. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Forsyth, C.J. Total synthesis of the marine cyanobacterial cyclodepsipeptide apratoxin A. Proc. Natl. Acad. Sci. USA 2004, 101, 12067–12072. [Google Scholar] [CrossRef]
- Asinger, F. Über die gemeinsame Einwirkung von Schwefel und Ammoniak auf Ketone. Angew. Chem. 1956, 68, 413. [Google Scholar] [CrossRef]
- Asinger, F. Chemiker-Treffen Salzburg. Angew. Chem. 1956, 68, 377. [Google Scholar]
- Asinger, F.; Thiel, M.; Pallas, E. Die gemeinsame einwirkung von schwefel und ammoniak auf diathylketon. Liebigs Ann. Chem. 1957, 602, 37–49. [Google Scholar] [CrossRef]
- Schlüter, T.; Frerichs, N.; Schmidtmann, M.; Martens, J. Consecutive Multicomponent Reactions: Synthesis of 3-Acyl-4-alkynyl-Substituted 1,3-Thiazolidines. Synthesis 2018, 50, 1123–1132. [Google Scholar]
- Rainoldi, G.; Begnini, F.; Silvani, A.; Lesma, G. Efficient Synthesis of Spirooxindole-Fused 3-Thiazoline Derivatives by a One-Pot Asinger-Type Reaction. Synlett 2016, 27, 2831–2835. [Google Scholar]
- Brockmeyer, F.; Schoemaker, R.; Schmidtmann, M.; Martens, J. Multicomponent reaction for the first synthesis of 2,2-dialkyl- and 2-alkyl-2-aralkyl-5,6-diaryl-2H-1,3-thiazines as scaffolds for various 3,4-dihydro-2H-1,3-thiazine derivatives. Org. Biomol. Chem. 2014, 12, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Brockmeyer, F.; van Gerven, D.; Saak, W.; Martens, J. Two Sequential Multicomponent Reactions: Synthesis of Thiazolidin-4-yl-1,3,4-oxadiazoles under Mild Conditions. Synthesis 2014, 46, 1603–1612. [Google Scholar] [CrossRef]
- Zeinab Faghihi, Z.; Oskooie, H.A.; Heravi, M.M.; Tajbakhsh, M.; Shiri, M. A novel analogue of Asinger reaction for the synthesis of thiazinoquinoline derivatives. Monat. Chem. 2017, 148, 315–320. [Google Scholar] [CrossRef]
- Schlemminger, I.; Janknecht, H.H.; Maison, W.; Saak, W.; Martens, J. Synthesis of the First enantiomerically pure 3-thiazolines via Asinger reaction. Tetrahedron. Lett. 2000, 41, 7289–7292. [Google Scholar] [CrossRef]
- Asinger, F.; Offermanns, H. Syntheses with Ketones, Sulfur, and Ammonia or Amines at Room Temperature. Angew. Chem. Int. Ed. 1967, 6, 907–919. [Google Scholar] [CrossRef]
- García-Muñoz, A.; Ortega-Arizmendi, A.I.; García-Carrillo, M.A.; Díaz, E.; Gonzalez-Rivas, N.; Cuevas-Yañez, E. Direct, metal-free synthesis of benzyl alcohols and deuterated benzyl alcohols from p-toluenesulfonylhydrazones using water as solvent. Synthesis 2012, 44, 2237–2242. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).