Abstract
N,N′-diphenyldithiomalonamide (dithiomalondianilide) smoothly reacts with various Michael acceptors to give either stable Michael adducts or products of their further heterocyclization. The structure of the products was confirmed by 2D NMR experiments and X-ray data. The mechanisms of the reactions are discussed.
1. Introduction
N-Substituted thioamides play an important role in the synthesis of nitrogen- and sulfur-containing heterocyclic compounds. They are widely used in organic synthesis to prepare compounds with new, previously unknown core structures. N,N′-diphenyl malondithioamide (dithiomalondianilide) 1 is a promising compound to build new heterocyclic systems since it has two thioanilide fragments and CH-acidity due to the methylene bridge (Figure 1).
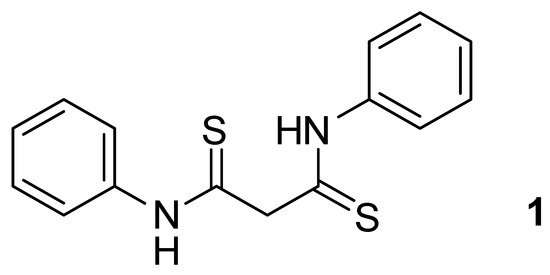
Figure 1.
The structure of dithiomalondianilide.
Earlier, we reported the reaction of N,N′-diphenyldithiomalondiamide 1 with arylmethylenemalononitriles [1]. The products of the reaction were [1,2]dithiolo[3,4-b]pyridines 2, as shown by means of X-ray analysis (Scheme 1).
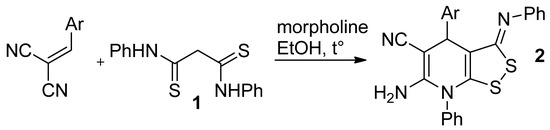
Scheme 1.
The reaction of N,N′-diphenyldithiomalondiamide 1 with arylmethylenemalononitriles.
N,N′-Diphenyldithiomalondiamide 1 is an active methylene compound that is rarely used in heterocyclization reactions. In continuation of our studies in this field, we decided to study the reactions of dithioamide 1 with other Michael acceptors.
2. Results and Discussion
We found that the reaction of dithiomalondianilide 1 with arylmethylidene cyanoacetic ethers proceeds readily in the presence of a basic catalyst and, in the presence of an oxidizing agent (atmospheric oxygen), gives [1,2]dithiolo[3,4-b]pyridines 3 in modest yields (Scheme 2). The structure of the obtained compounds was confirmed by a complex of spectral data including 2D NMR spectroscopy (HSQC, HMBC) as well as X-ray data (Figure 2 and Figure 3).
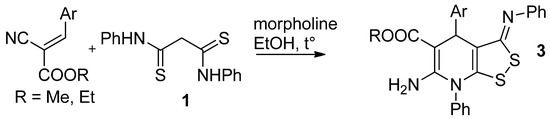
Scheme 2.
The reaction of N,N′-diphenyldithiomalondiamide 1 with arylmethylene cyanoacetates.
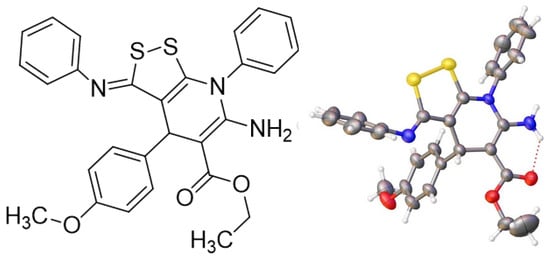
Figure 2.
Structure of the reaction product of (4-methoxyphenyl)methylidenecyanoacetic ester and dithiomalondianilide 1 (according to X-ray diffraction data).
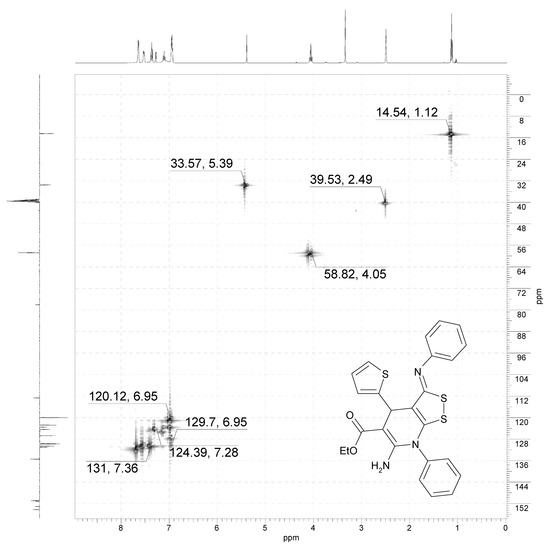
Figure 3.
NMR 1H/13C HSQC spectrum (400/101 MHz, DMSO-d6) of 3b.
Next, we decided to study the reaction of 1 with arylmethylene Meldrum’s acids 4. It was found that with the 4-nitrobenzylidene derivative, the reaction proceeds smoothly to give the Michael adduct 5 (Scheme 3):
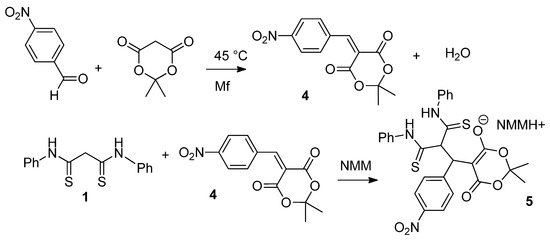
Scheme 3.
The reaction of N,N′-diphenyldithiomalondiamide 1 with 4-nitrobenzylidene Meldrum’s acid.
We decided to expand the base of similar Michael adducts by using various aromatic aldehydes to prepare arylidene Meldrum’s acids. However, we found that, along with the formation of the Michael adducts 5, in most cases, the by-process of further cyclization occurred, giving rise to the formation of tetrohydropyridine-2-thiolates 6. In these cases, the isolated crystalline products are mixtures of adducts 5 and their cyclization products 6 in various ratios. The plausible mechanism consists of two stages. In the first stage, the arylidene Meldrum’s acid 4 is formed. The reaction proceeds as a Knoevenagel-type condensation [2]. Next, the aforementioned activated alkene undergoes the Michael addition with dithiomalondianilide 1 to form Michael adducts 5 and, followed by subsequent by-process, their cyclization products 6 (Scheme 4).
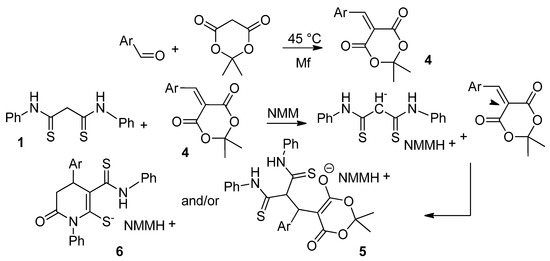
Scheme 4.
The reaction of N,N′-diphenyldithiomalondiamide 1 with arylidene Meldrum’s acids.
The structure of the products was confirmed by a complex of spectral methods (NMR 1H, 13C DEPTQ), IR spectroscopy; the structures of the adducts (5a and 5d) were also proven using X-ray diffraction data (Figure 4):
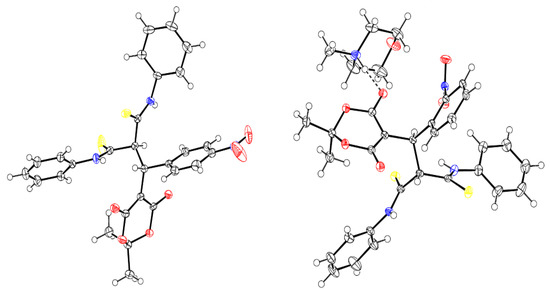
Figure 4.
Structures of the Michael adducts 5 (according to X-ray diffraction data).
3. Experimental
3.1. Method for the Synthesis of Arylidene Derivatives of Meldrum’s Acid 4
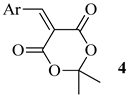
A beaker was charged with 15 mL of ethanol–water mixture (1:2), 0.5 g (0.0035 mol) of Meldrum’s acid, 0.0035 mol of the corresponding aldehyde, and a catalytic amount of morpholine (1–2 drops). The mixture was stirred at 45 °C. Precipitation of the product was observed in the first 10 min. The reaction proceeds within 2 h, then the product is filtered off, and washed with aqueous alcohol.
3.2. Method for the Synthesis of Michael Adducts 5 and Their Cyclization Products 6
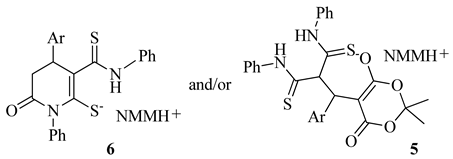
A mixture of 0.25 g of arylidene Meldrum’s acid and an equimolar amount of dithiomalondianilide 1 was refluxed in 15 mL of dry acetone. The resulting solution was filtered through a paper filter to remove insoluble impurities. The filtrate was transferred into a flat-bottom flask and a 1.5-fold excess of base was added. The mixture was refluxed under vigorous stirring. Bright-yellow crystals began to precipitate form the hot solution within 5 min. The crystals obtained were filtered off, and washed with cold acetone.
N-Methylmorpholinium 5-[3-Anilino-2-(anilinothiocarbonyl)-1-(4-chlorophenyl)-3-thioxopropyl]-2,2-dimethyl-4-oxo-4H-1,3-dioxin-6-olate 5a
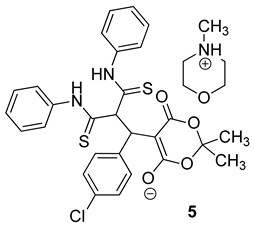
The resulting product was soluble in DMF and DMSO, and insoluble in ethanol, ethyl acetate, acetone. The product contains an admixture (about 15% mol) of the cyclization product. The yield was 52%.
IR spectrum, ν, cm−1: 3190–3128 (N–H), 3024–2862 (C–H), 1654 (C=O), 1595–1494 (C=C). The NMR 1H spectrum is given in Figure 5.
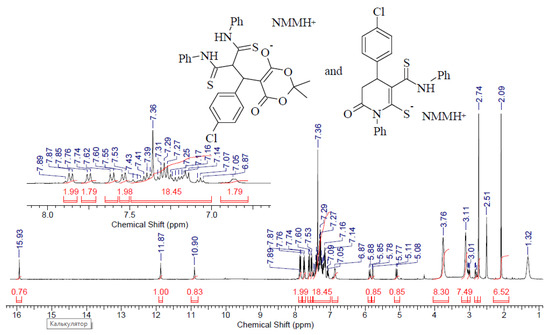
Figure 5.
NMR 1H spectrum (400 MHz, DMSO-d6) of compound 5a.
Author Contributions
Conceptualization, methodology, V.V.D.; synthesis, A.E.S., V.V.D.; analysis, A.E.S., V.V.D., N.A.A.; writing—original draft preparation, V.V.D.; writing—review and editing, V.V.D.; supervision, V.V.D.; funding acquisition, V.V.D. All authors have read and agreed to the published version of the manuscript.
Funding
The research was carried out with the financial support of the Kuban Science Foundation, scientific project No. MFI-20.1-26/20.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dotsenko, V.V.; Krivokolysko, S.G.; Frolov, K.A.; Chigorina, E.A.; Polovinko, V.V.; Dmitrienko, A.O.; Bushmarinov, I.S. Synthesis of [1,2]dithiolo[3,4-b]pyridines via the reaction of dithiomalondianilide with arylmethylidenemalononitriles. Chem. Heterocycl. Compd. 2015, 51, 389–392. [Google Scholar] [CrossRef]
- Kaupp, G. Solvent-free Knoevenagel condensations and Michael additions in the solid state and in the melt with quantitative yield. Tetrahedron 2003, 59, 3753–3760. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).