Abstract
The design and synthesis of chemosensors that allows the selectively detection of heavy metal cations by complexation, is an area of growing interest. The hydrazone functional group has been widely used in supramolecular chemistry due to its complexing capacity, its conjugated electrons, and its simple methods of synthesis. In this way, a nitrogen system derived from the condensation of vanillin (aromatic aldehyde) and 2-hydrazinobenzothiazole to give the corresponding hydrazone for the detection of Cu2+ was developed. Spectroscopic determinations demonstrate whether the complexing took place. The hidrazone showed a response in UV–vis spectroscopy and a color shift is observed with the naked eye. In addition, theoretical analysis based on the Density Functional Theory (DFT) was realized to understand the chemical changes that the ligand suffers in the complexation process with the Cu2+ ion through the study of the structural, electronics, and optical properties.
1. Introduction
Chemical sensors or chemosensors are those systems that transform a chemical phenomenon into a useful analytical signal. This is achieved by generating a change in the electronic, conformational, or optical properties of a supramolecular structure, resulting in a chemical response translated into a signal. Colorimetric and fluorometric sensors use changes in optical properties, absorption or emission, as an output signal [1,2,3,4,5,6]. The great development that spectroscopic methods have undergone in the 20th century made it possible to have adequate analytical techniques for the translation of the signal produced in the coordination process and its subsequent interpretation. Chromogenic systems are characterized by a quick response that is visible to the naked eye, changing their color when they interact with certain analytes. While fluorogenic systems are characterized by their great sensitivity and specificity, chromogenic ones offer the advantages of simplicity, fast response, non-destructive, and real-time monitoring [7]. The combination of spectroscopic methods with theoretical calculations based on the Density Functional Theory (DFT) are powerful tools to understand the electronic structure and formation of these complexes.
Hydrazones can be used as intermediate compounds to synthesize products using the active hydrogen of the azomethine group -CONHN=CH-. The classical synthesis method of these compounds involves the condensation of aldehydes or ketones with hydrazines.
Among the applications that hydrazones have, their use as molecular sensors stands out and is rising due to the wide range of environmental applications that involves detecting trace amounts of potentially harmful chemicals [8].
Taking the above into account, a study will be developed to synthesize a hydrazone molecule from vanillin aldehyde and evaluate its feasibility in metal recognition, analyzing the different spectroscopic determinations that allow to elucidate if the molecular recognition event occurred.
2. Materials and Methods
2.1. Experimental
To synthesize the hydrazone (E)-4-((2-(benzo[d]thiazol-2-yl)hydrazono)methyl)-2-methoxyphenol (VBTH), a condensation reaction was performed (Figure 1). In a balloon containing 50 mL of absolute ethanol, 20 mmol of the corresponding hydrazine were added. Subsequently, 20 mL of an ethanolic solution containing 20 mmol of vanillin was added dropwise. Then, 4 drops of glacial acetic acid were added, and the reaction was kept at 50 °C for 2 h. The colored precipitate was vacuum filtered and purified by recrystallization from ethanol. The solid obtained was placed in a vacuum oven at 40 °C for 48 h.
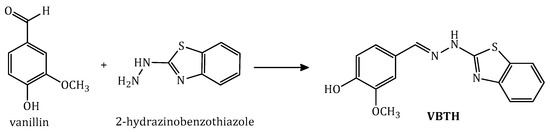
Figure 1.
Reaction scheme of the synthesis of the hydrazone (E)-4-((2-(benzo[d]thiazol-2-yl)hydrazono)methyl)-2-methoxyphenol.
Monitoring of reactions was carried out by thin layer chromatography (TLC). TLC determinations were carried out with 254 nm UV silica gel plates.
To study UV–vis behavior of VBTH against metal cations, 1 x 10-4 M solutions in water of Al3+, Sb3+, Ba2+, Ca2+, Cr3+, Sn2+, Li +, Zn2+, Mg2+, Ni2+, Co2+, Cu +, Cu2+, Fe2+, and Fe3+ in the form of salts of the chloride, nitrate, or sulfate type were prepared. Then, the saline solutions were added to a 1 × 10−4 M solution of VBTH in DMSO. Subsequently, the corresponding absorption spectra were recorded in order to elucidate whether there was a change in the representative bands respect to those of the ligands in the absence of metals.
The limit of detection (LOD) is determined as the sum of the mean of the blank signal and a multiple k of the blank standard deviation. That is to say,
Now, the LOD is given by
There are several ways to determine LOD, but the simplest way is using a calibration curve. m is the sensitivity of the method and by definition is the slope of the calibration curve. In addition, a value of k is usually adopted as the optimum of 3 [9].
2.2. Computational
On the other hand, theoretical calculations were carried out for the ground state and gas phase using the DFT method with the LANDL2DZ set of bases through the Gaussian09 software package. The structural visualization of the ligand and the complex as well as their electronic properties were carried out with the GaussView software. A structural analysis was carried out, and energies of the Frontier Molecular Orbitals (FMO) were calculated with and without the Cu2+ cation.
3. Results and Discussion
Hydrazone was prepared with high yields (75%) and is a white solid directly from vanillin aldehyde and 2-hidrazine benzothiazole. Mechanistically, the oxygen atom of the carbonyl group protonates forming its conjugated acid. The important polarity of the carbonylic carbon favors nucleophilic attack by the corresponding hydrazine or semicarbazide to form an intermediate. This species exchanges a proton between nitrogen and oxygen, transforming the hydroxyl group into water, which is a good leaving group. In this way, the intermediate loses a water molecule, the deprotonation of which finally gives the corresponding hydrazone.
Spectral Data
IR (KBr) [cm−1] 3417 (ν O-H); 3182 (ν N-H); 1623 (ν C=N); 1571 (δ N-H); 1452 (δ CAr=CAr); 1275 (ν C-O); 1242 (ν C-S); 1106 (δ C-H); 1049 (δ N-H); 1040 (δ C-H); 936 (scis. -CNN)
1H RMN (CDCl3, 300 MHz) δ [ppm]: 12,10 (s, 1H, NH); 8,23 (s, 1H, CH=N); 7,95 (d, 1H); 7,82 (d, j = 7.65 Hz, 1H); 7,48 (m, 1H); 7,36 (m, 1H); 7,11 (d, 1H); 7,04 (d 1H); 6,92 (m, 1H); 3,91 (s, 3H, OCH3)
The hydrazone act as ligand in metal complexes by binding to the metal center through imine nitrogen. Likewise, the presence of substituents with donor atoms, both in the carbonyl compound and in the hydrazine, can increase the number of attachment points.
The UV–visible absorption spectrum of VBTH in DMSO showed absorbance in the UV region, centered at 347 nm. The characteristic absorption band showed modifications compared to the addition of salts, producing a significant decrease in absorbance for the Cu2+ salt. In turn, the study of this ligand showed two new absorption bands at 405 nm and 500 nm after Cu2+ solution was added. The absorption spectra of VBTH before and after the addition of different salts are shown in Figure 2.
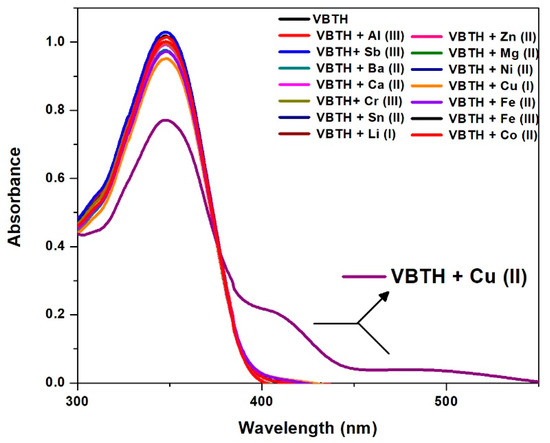
Figure 2.
Absorption spectra of (E)-4-((2-(benzo[d]thiazol-2-yl)hydrazono)methyl)-2-methoxyphenol (VBTH) with the addition of different salts.
Once the addition of one equivalent of the cupric salt was made, the ligand solution which was clear and turned reddish in color. The change was instantly observed and this phenomenon only occurred with Cu2+, which would suggest that VBTH would be efficiently selective for this metal. The color change is shown in Figure 3, where the 10−4 M VBTH solution is seen on the left (a) and the solution after the addition of the Cu2+ solution is seen on the right (b).
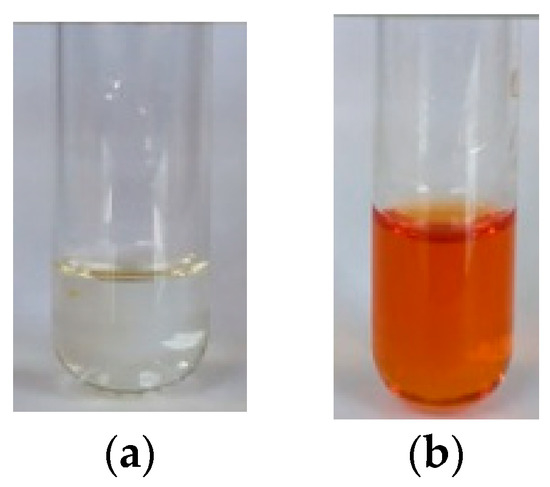
Figure 3.
(a) VBTH solution, (b) VBTH solution after adding the Cu2+ solution.
To calculate the LOD, a calibration curve was made with VBTH and with the Cu2+ cation as it was the one with the highest selectivity. The absorbances of the calibration curve correspond to a 405 nm wavelength (Figure 4).
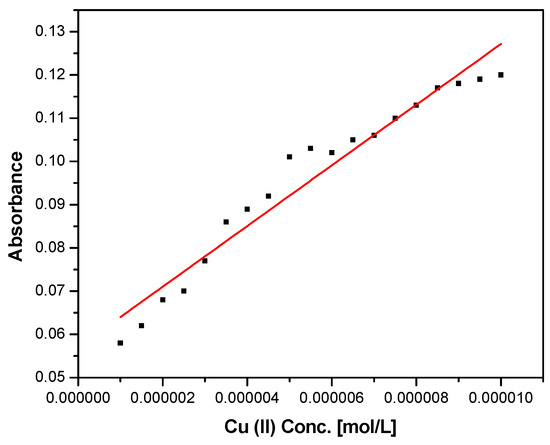
Figure 4.
Absorbance vs. concentration of VBTH, respectively, with Cu2+.
The LOD obtained is 2.43 × 10−6. This LOD was verified experimentally from diluted Cu2+ solutions.
Given the importance of the appearance of new absorption bands for VBTH against Cu2+, theoretical calculation was developed to analyze the complexing capacity of these systems and their stability. The first task for the computational work was to determine the geometric optimization of the ligand (Figure 5). Analytical frequency calculations were performed to confirm that the optimized structure is an energy minimum. Since no imaginary frequency modes were obtained, it is said that a true minimum was obtained at the potential energy surface. Once the ligand was optimized, its structure complexed to Cu2+ was evaluated.
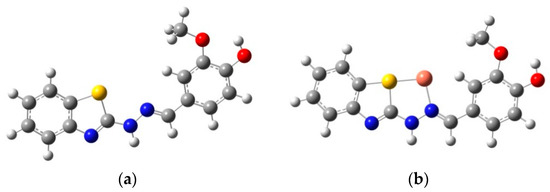
Figure 5.
Optimized geometries of VBTH and VBTH-Cu2+.
Based on this data, the difference in total energy between the VBTH ligand and it complex with the Cu2+ cation (L-Cu2+) and the energy GAP between the FMOs in each case were evaluated. The results are shown in Table 1.

Table 1.
Total energy values and energy gap between the Frontier Molecular Orbitals (FMO) for the VBTH and BBTH ligands with the Cu2+ cation.
In this table it can be observed that the total energy and the GAP energy between the FMOs of the complex are lower than the ones of the ligand on its own, which could increase the stability of the system.
4. Conclusions
Through a condensation reaction in a single stage, the active molecule derived from vanillin aldehyde was synthesized, obtaining high yields.
The sensing properties of the hydrazone system was evaluated. It was thus found that VBTH is capable of acting as a highly selective chromogenic sensor sensitive to Cu2+. The complexation site was determined through computational methods, where sulfur atom and iminic nitrogen are responsible for its ion complexation capacity.
Author Contributions
L.G. and C.O.: methodology, formal analysis, and investigation; M.N.K. and C.A.F.: conceptualization, methodology, resources, writing, visualization, supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was supported by the Agencia Nacional de Ciencia y Tecnología (ANCyT) of Argentina-PICT 2014 No. 1587 and by CAI+D 2017 (PEN 3042150100036 LI) of the Universidad Nacional del Litoral, Santa Fe, Argentina.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fernández-González, A.; Guardia, L. Reconocimiento molecular mediante materiales biomiméticos: Impresión molecular. An. Quím. 2007, 103, 14–22. [Google Scholar]
- Pioquinto-Mendoza, J.; Mendoza-Olvera, D.; Andrade-López, N.; Alvarado-Rodríguez, J.; Moreno-Esparza, R.; Flores-Álamo, M.J. Synthesis and structural characterization of mono- and dinuclear NiII and PdII complexes derived from tetradentate 1,7-bis-(pyridin-2-yl)-2,6-diaza-1,6-heptadiene. Coord. Chem. 2013, 66, 2477–2488. [Google Scholar] [CrossRef]
- Uhlenheuer, D.A.; Petkau, K.; Brunsveld, L. Combining supramolecular chemistry with biology. Chem. Soc. Rev. 2010, 39, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Thakura, A.; Bhatta, S.R.; Mondal, B.; Kakash, D.; Chawla, P. Naphthalene-glycine conjugate: An extremely selective colorimetric chemosensor for iodide ion in aqueous solution. Sens. Actuators B. Chem. 2018, 267, 617–626. [Google Scholar] [CrossRef]
- Lo-Presti, M.; El-Sayed, S.; Martínez-Máñez, R.; Costero, A.M.; Gil, S.; Parra, M.; Sancenón, F. Selective chromo-fluorogenic detection of trivalent cations in aqueous environments using a dehydration reaction. New J. Chem. 2016, 11, 14–17. [Google Scholar] [CrossRef]
- Skoog, D.A.; Holler, F.J.; Nieman, T.A. Principles of Instrumental Analysis, 6th ed.; Cengage: Mexico City, Mexico, 2008; ISBN 0-495-01201-7. [Google Scholar]
- Balamurugan, R.; Liu, J.; Liu, B. A review of recent developments in fluorescent sensors for the selective detection of palladium ions. Coord. Chem. Rev. 2018, 376, 196–224. [Google Scholar] [CrossRef]
- Su, X.; Aprahamian, I. Hydrazone-based switches, metallo-assemblies and sensors. Chem. Soc. Rev. 2014, 43, 1963. [Google Scholar] [CrossRef] [PubMed]
- Gründler, P. Chemical Sensors. An Introduction for Scientists and Engineers; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).