Abstract
A comparison of properties of clips based on diazacrown-n ethers (n = 4, 5, 6) as a central fragment and pendant p-tert-butylcalix[4]arenes was carried out. The clip with diaza-12-crown-4 demonstrates high selectivity towards Rb cations, the clip with diaza-15-crown-5 ether is a selective ligand for Cs cations in the alkali metal series. Clip based on diaza-18-crown-6 is a selective for Ba cations in the alkaline earth metal series. Transition metal cation clips with diaza-12-crown-4 or diaza-18-crown-6 are capable of forming predominantly 1:1 complexes. For diaza-15-crown-5 ether, the formation of 1:2 (L:M) complexes with Ni2+, Mn2+, Fe3+ and Pb2+ is observed.
1. Introduction
The term “molecular tweezers” was first defined by Whitlock, and Klärner later demonstrated the differences between molecular tweezers and molecular clips, which are due to the peculiarities in the structure of the central fragment [1,2].
What does Wikipedia say? According to Wikipedia, “Molecular tweezers, and molecular clips, are host molecules with open cavities capable of binding guest molecules. The open cavity of the molecular tweezers may bind guests using non-covalent bonding, which includes hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, π–π interactions, and/or electrostatic effects. These complexes are a subset of macrocyclic molecular receptors and their structure is that the two “arms” that bind the guest molecule between them are only connected at one end leading to a certain flexibility of these receptor molecules (induced fit model)”.
Previously, we demonstrated the synthesis and comparison of complexing properties of molecular clips with pendant p-tert-butylcalix[4]arenes attached to a central fragment, which were crown ethers that differ in nature—diaza-18-crown ether and diamino-dibenzo-18-crown ether [3]. In order to confirm our assumptions about the possibility of creating a database of supramolecular ligands with such structural features and such a nature that will work on the principle of molecular clips, as well as to compare the properties of these molecular clips, it was expedient to synthesize a series of compounds based on diazacrown-n ethers as the central fragments and study the dependence of the complexing abilities of these substances on the size of the crown ether and the number of heteroatoms in the macrocycle.
2. Materials and Methods
2.1. Chemistry
The 1H and 13C NMR spectra were recorded from 10% solutions in Chloroform-d (Acros Organics BV (Thermo Fisher Scientific), Geel, Belgium) on a Bruker Avance DRX 500 spectrometer (Bruker Analytik GmbH, Rheinstetten, Germany) using tetramethylsilane as internal reference. The mass spectra were recorded on an Agilent 6530 Accurate Mass Q-TOF spectrometer (Agilent Technologies, Inc., Santa Clara, USA) with the LC/MS System. Absorption spectra in the UV region were recorded using a spectrophotometer SPECORD 250 Plus (Analytik Jena GmbH+Co. KG, Jena, Germany). All of the metal chlorides were of analytical grade.
Synthesis of clips: to a solution of 0.7 mmol (0.5 g) of mono(carboxymethoxy)-trihydroxy-p-tert-butylcalix[4]arene [4] and 0.3 mmol crown ether in 15 mL CH3CN or THF (in case of diaza-18-crown-6) at −5 °C a 0.875 mmol (167.7 mg) EDC was added with stirring. The reaction mixture was left at room temperature for 3–5 h. The solvent was evaporated in vacuo and dry residue was dissolved in 50 mL of chloroform, and the solution was washed in succession with water, 10% aqueous HCl, and water again. The solvent was distilled off under reduced pressure, and crude product was purified by recrystallization from CH3CN.
N,N’-bis[5,11,17,23-tetra-tert-butyl-25-mono(carbonylmethoxy)-26,27,28-trihydroxycalix[4]arene]diaza-12-crown-4 (1). White solid; yield 48%. 1H NMR: δ 7.12 (d, 4H, ArH), 7.1 (m, 10H, ArH, OH), 6.91 (d, 2H, ArH), 6.89 (s, 4H, ArH), 4.56 (s, 4H, CH2CO), 4.26 (d, 2H, J = 12.76 Hz, ArCH2Ar), 4.20 (d, 4H, J = 13.45 Hz, ArCH2Ar), 3.72–3.67 (m, 8H, -CH2CH2-N), 3.51–3.46 (m, 10H, O-CH2CH2-O, ArCH2Ar), 3.33 (d, 2H, J = 13.45 Hz, ArCH2Ar), 3.25 (d, 4H, J = 12.76 Hz, ArCH2Ar), 3.17 (d, 4H, J = 13.45 Hz, ArCH2Ar), 1.27 (s, 18H, (CH3)3C), 1.21 (s, 36H, (CH3)3C), 1.18 (s, 18H, (CH3)3C). 13C NMR: δ 177.84, 173.87, 168.58, 168.58, 156.36, 152.19, 151.93, 151.04, 147.17, 146.94, 143.14, 142.74, 142.64, 142.20, 133.05, 137.91, 132.22, 131.97, 131.95, 130.77, 129.54, 129.48, 129.37, 129.04, 128.77, 126.53, 126.43, 126.26, 126.18, 72.29, 69.01, 66.95, 49.50, 44.40, 37.71, 36.21, 34.63, 34.46, 34.34, 31.98, 31.83, 31.78, 31.36, 30.80, 30.30, 27.44. MS ESI(+): m/z 1552.9754 [M+1]+.
N,N’-bis[5,11,17,23-tetra-tert-butyl-25-mono(carbonylmethoxy)-26,27,28-trihydroxycalix[4]arene]diaza-15-crown-5 (2). White solid; yield 65%. 1H NMR: δ 10.20 (s, 2H, OH), 9.31 (br. s, 4H, OH), 7.09 (d, 4H, ArH), 7.04–6.98 (m, 8H, ArH), 6.97 (d, 4H, ArH), 5.23 (s, 4H, CH2CO), 4.42 (d, 4H, J = 13.7 Hz, ArCH2Ar), 4.27 (d, 2H, J = 13.2 Hz, ArCH2Ar), 3.99–3.74 (m, 10H, -CH2CH2-N, ArCH2Ar), 3.65 (d, 4H, J = 14.2 Hz, ArCH2Ar), 3.47–3.36 (m, 16H, O-CH2CH2-O, ArCH2Ar), 1.22 (s, 18H, (CH3)3C), 1.19 (s, 36H, (CH3)3C), 1.18 (s, 18H, (CH3)3C). 13C NMR: δ 171.97, 154.06, 149.70, 148.36, 147.80, 143.62, 143.33, 133.91, 133.53, 128.19, 128.05, 127.95, 127.78, 126.40, 126.33, 125.79, 125.57, 125.45, 125.33, 116.24, 72.28, 67.78, 66.85, 66.84, 50.23, 49.78, 44.72, 44.40, 35.81, 34.10, 33.84, 33.80, 32.73, 32.40, 31.36, 31.32, 31.15, 31.10. MS ESI(+): m/z 1597.1815 [M+1]+, 1663.3287 [M+Na]+.
2.2. Stability Constant Determination
UV–vis titration experiments. A solution of molecular clip (concentration about 2 × 10−5–3.5 × 10−5 M) in methanol was treated with increasing amounts of metal chloride solution (concentration about 1–4 × 10−4 M) containing proper ligand of the same concentration at 20 °C. The host concentration was maintained constant and the molar ratio of guest increased with respect to the host over the range 0.1:1 to 10(20):1 during the titration. The absorbance measurements were carried out at six–ten wavelengths, at which spectral changes were the most notable (210–320 nm) simultaneously, and sets of the obtained experimental values (4 × 21 points) were used for joint computer processing. The data were processed with the nonlinear least squares fitting MatLab Online (basic) [5] software.
In the case of studies involving transition metal and lead salts, a solution of a ligand of a certain concentration was prepared with the addition of 0.01 M tris(hydroxymethyl)aminomethane (TRIS) in methanol.
3. Results and Discussion
Based on diaza-12-crown-4, diaza-15-crown-5, and diaza-18-crown-6, the corresponding molecular clips 1–3 were obtained (Scheme 1). As indicated by the analysis of the NMR spectra of the synthesized compounds, the calixarene skeletons are in the cone conformation. With the exception of 3, the obtained compounds are partially soluble in water, especially at pH < 7.
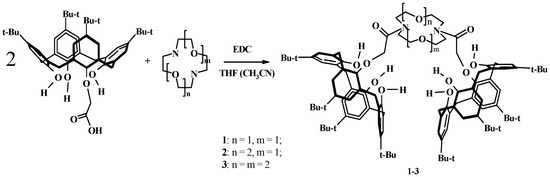
Scheme 1.
Synthesis of clips: EDC, THF (CH3CN), r.t., 3–5 h.
The complexing properties of clips 1–3 towards alkali, alkaline earth, and some transition metal cations were studied using the spectrophotometric titration method. Unfortunately, the partial water solubility of the obtained compounds did not allow obtaining reliable data on their extraction ability.
It was found that the molecular clip based on diaza-12-crown-4 1 demonstrates high selectivity towards rubidium cations among alkali metals and forms 1:1 complexes with this cation with lgK 6.2. The results of spectrophotometric titration of clip 1 with alkaline earth metal salts indicate that this compound is capable of forming binuclear complexes with magnesium and calcium cations; however, the selectivity towards magnesium is 4 logarithmic units higher than that for complexes with calcium (Table 1). In the case of interaction with the strontium cations, the possibility of forming mononuclear biligand complexes along with complexes of the 1:1 composition is observed (lgK11 1.2 and lgK21 5.68).

Table 1.
Stability constants (lgKn) of the complexes of clips 1–3 with alkali and alkaline earth metal cations in MeOH.
The clip based on the unsymmetrical diaza-15-crown-5-ether 2 is an exceptionally selective and effective ligand for cesium cations in the alkali metal series, and is capable of forming binuclear complexes with cations of this metal. Also, the formation of binuclear complexes with calcium cations among alkaline earth metals with a high stability constant lgK > 7 is observed for 2. With barium cations, the ligand 2 is capable of forming biligand complexes, while the clip 3 based on diaza-18-crown-6 demonstrates exceptional selectivity for barium cations in the alkaline earth metal series with the formation of 1:1 complexes [3].
We examined the complexing properties of the obtained compounds toward cations of transition metals and lead also.
In the case of the clip 1, the formation of monoligand mononuclear complexes with manganese, cadmium, copper, and cobalt cations and biligand mononuclear complexes with nickel, lead, and iron cations is observed. It should be noted that the biligand complexes of clip 1, as well as complexes of clip 3, are characterized by too-small stability constants lgK11~0.5–1.82, and high values of lgK21 > 6.5.
A different behavior is characteristic of the ligand 2: interaction with nickel, lead, manganese, and iron cations leads to the formation of complexes of composition 1:2 (L:M) with lgK11 > 4.0, lgK12 < 3.2, but with cadmium, copper, and cobalt cations, the formation of stable biligand mononuclear complexes with lgK21 > 5 is observed (Table 2).

Table 2.
Stability constants (lgKn) of the complexes of clips 1–3 with cations of d- and p-elements in MeOH.
4. Conclusions
Thus, comparing the complexing abilities of the obtained compounds, it can be assumed that, despite the fact that the substance based on diaza-15-crown ether as a central fragment demonstrates high efficiency as a ligand, especially toward to transition metal cations, it would be a mistake to call it a “molecular clip”. The closest in properties to classical clips is a compound based on diaza-18-crown ether.
Author Contributions
Conceptualization, E.A., T.K.; methodology, E.A.; investigation, E.A.; writing—original draft, E.A.; writing—review and editing, T.K.; project administration, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, C.W.; Whitlock, H.W. Molecular tweezers: A simple model of bifunctional intercalation. J. Am. Chem. Soc. 1978, 100, 4921–4922. [Google Scholar] [CrossRef]
- Klärner, F.-G.; Schrader, T. Aromatic Interactions by Molecular Tweezers and Clips in Chemical and Biological Systems. Acc. Chem. Res. 2013, 46, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Kulygina, E.; Alekseeva, E.; Rakipov, I.; Kirichenko, T. Synthesis and Complexing Ability of a New Type of Molecular Clips Based on Diaza-18-crown-6 or Diamino-Dibenzo-18-crown-6 with Pendant p-tert-butylcalix[4]arenes. Chem. Proc. 2023, 14, 72. [Google Scholar] [CrossRef]
- Amaud-Neu, F.; Barrett, G.; Harris, S.J.; Owens, M.; McKervey, M.A.; Schwing-Weill, M.J.; Schwinte, P. Cation Complexation by Chemically Modified Calixarenes. 5. Protonation Constants for Calixarene Carboxylates and Stability Constants of Their Alkali and Alkaline-Earth Complexes. Inorg. Chem. 1993, 32, 2644–2650. [Google Scholar] [CrossRef]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).