Bioactive Potential of Ethyl Acetate Extract from Prosopis laevigata: Antimicrobial and Anti-Inflammatory Effects †
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Extracts
2.3. HPLC-Photo Diode Array (PDA) Analysis of the Ethyl Acetate Extract
2.4. Chromatographic Fractionation of the Ethyl Acetate Extract of P. laevigata
2.5. Microorganisms
2.6. Minimum Inhibitory Concentration (MIC)
2.7. 12-O-tetradecanoylphorbol-13-acetate (TPA)-Induced Mouse Ear Edema
2.8. Statistical Analysis
3. Results
3.1. High-Performance Liquid Chromatography (HPLC) Analysis of the Ethyl Acetate Extract
3.2. Antimicrobial Analysis
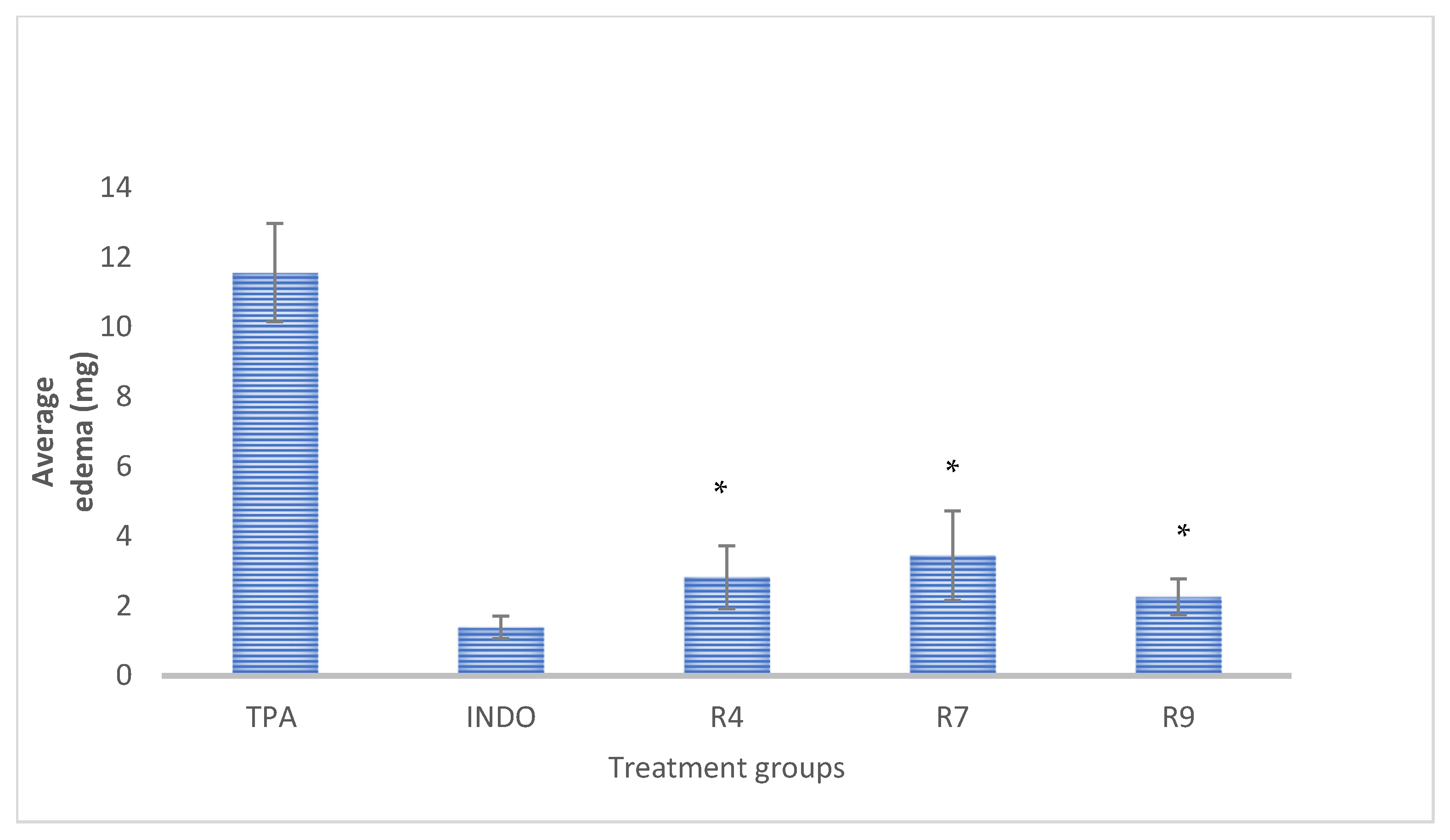
3.3. Anti-Inflammatory Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Organización Mundial de la Salud. Global action plan on antimicrobial resistance. In Plan de Acción Mundial sobre la Resistencia a los Antimicrobianos; OMS: Ginebra, Suiza, 2016; 30p. (In Spanish) [Google Scholar]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Reygaert, C.W. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Kim, M.Y.; Yang, D.C. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Herrera, V.; Wendie, E. Inflamación. 2014. Available online: https://www.academia.edu/38821014/INFLAMACION_I (accessed on 6 September 2025).
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Domínguez, F.; Maldonado-Miranda, J.J.; Castillo-Pérez, L.J.; Carranza-Álvarez, C.; Solano, E.; Isiordia-Espinoza, M.A.; Juárez-Vázquez, M.d.C.; Zapata-Morales, J.R.; Argueta-Fuertes, M.A.; et al. Use of medicinal plants by health professionals in Mexico. J. Ethnopharmacol. 2017, 198, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, V.; González, R.; Sulem, L.; García, B. Is There Scientific Support for the Traditional Use of Medicinal Plants? (El uso tradicional de las plantas medicinales, ¿tiene sustento científico?). Available online: https://www.revistaciencia.amc.edu.mx/images/revista/75_4/PDF/06_75_4_1464.pdf (accessed on 6 September 2025). (In Spanish).
- Prabha, D.S.; Dahms, H.-U.; Malliga, P. Pharmacological potentials of phenolic compounds from Prosopis spp.: A review. J. Coast. Life Med. 2014, 2, 918–924. [Google Scholar]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- González-Cortazar, M.; Salinas-Sánchez, D.O.; Herrera-Ruiz, M.; Román-Ramos, D.C.; Zamilpa, A.; Jiménez-Ferrer, E.; Ble-González, E.A.; Álvarez-Fitz, P.; Castrejón-Salgado, R.; Pérez-García, M.D. Eupatorin and salviandulin-A, with antimicrobial and anti-inflammatory effects from Salvia lavanduloides Kunth leaves. Plants 2022, 11, 1739. [Google Scholar] [CrossRef] [PubMed]
- Paya, M.; Ferrandiz, M.L.; Sanz, M.J.; Bustos, G.; Blasco, R.; Rios, J.L.; Alcaraz, M.J. Study of the antioedema activity of some seaweed and sponge extracts from the Mediterranean coast in mice. Phytother. Res. 1993, 7, 159–162. [Google Scholar] [CrossRef]
- Palacios, R.A. Mexican mesquites: Biodiversity and geographical distribution (Los mezquites mexicanos: Biodiversidad y distribución geográfica). Bol. Soc. Argent. Bot. 2006, 41, 99–121. (In Spanish) [Google Scholar]
- Galindo-Almanza, S.; García-Moya, E. Uses of mesquite (Prosopis L.) in the Potosino Highlands (Usos del mezquite (Prosopis L.) en el Altiplano Potosino). Agrociencia 1986, 63, 7–16. (In Spanish) [Google Scholar]
- Nakamura, A.; Uratsuji, H.; Yamada, Y.; Hashimoto, K.; Nozawa, N.; Matsumoto, T. Anti-inflammatory effect of lanoconazole on 12-O-tetradecanoylphorbol-13-acetate- and 2,4,6-trinitrophenyl chloride–induced skin inflammation in mice. Mycoses 2020, 63, 189–196. [Google Scholar] [CrossRef] [PubMed]
- González-Cortazar, M.; Salinas-Sánchez, D.O.; Herrera-Ruiz, M.; Hernández-Hernández, P.; Zamilpa, A.; Jiménez-Ferrer, E.; Utrera-Hernández, B.E.; Pérez-García, M.D.; Gutiérrez-Roman, A.S.; Ble-González, E.A. Chemical profile analysis of Prosopis laevigata extracts and their topical anti-inflammatory and antibacterial activities. Plants 2025, 14, 1118. [Google Scholar] [CrossRef] [PubMed]
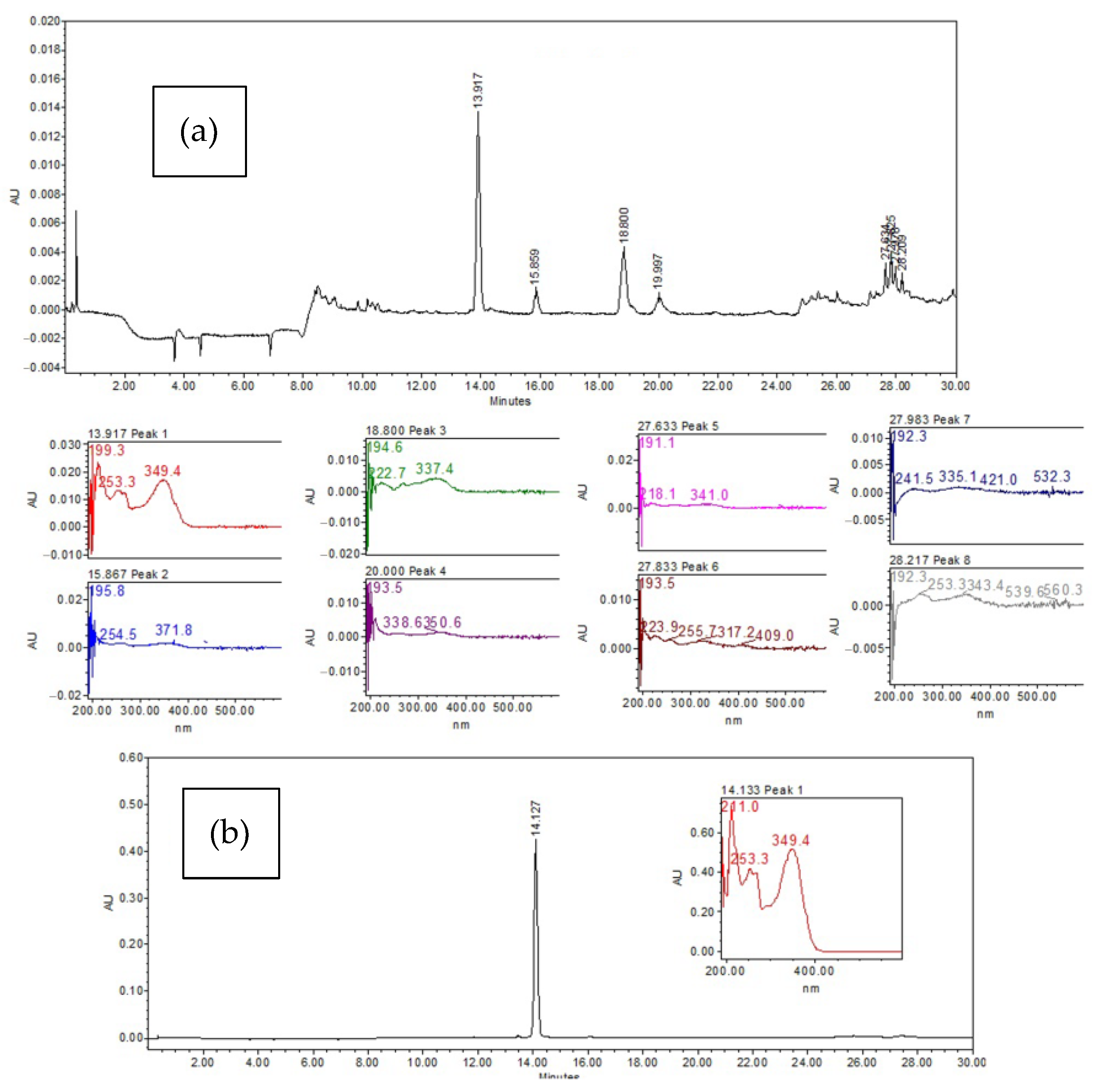
| Retention Time | Absorption Bands λmax |
|---|---|
| 13.917 | 199.3, 253.3, 349.4 |
| 15.867 | 195.8, 254.5, 371.8 |
| 18.800 | 194.6, 222.7, 337.4 |
| 20.000 | 193.5, 338.6, 350.6 |
| 27.633 | 191.1, 218.1, 341.0 |
| 27.833 | 193.5, 223.9, 255.7 |
| 27.983 | 192.3, 241.5, 335.1 |
| 28.217 | 192.3, 253.3, 343.4 |
| Microorganism | R4 | R7 | R9 |
|---|---|---|---|
| Staphylococcus aureus ATCC 29213 | 50 | n/a | 100 |
| Methicillin-resistant S. aureus 43300 | 100 | n/a | 100 |
| Staphylococcus epidermidis ATCC 35984 | <25 | n/a | <25 |
| Staphylococcus epidermidis ATCC 12228 | 200 | n/a | 100 |
| Staphylococcus epidermidis ATCC 1042 | n/a | n/a | <25 |
| Staphylococcus haemolyticus MR isolated | n/a | n/a | <25 |
| Enterococcus faecalis ATCC 29212 | <25 | <25 | <25 |
| Klebsiella pneumoniae ATCC 700603 | <25 | <25 | <25 |
| Pseudomonas aeruginosa ATCC 27853 | <25 | <25 | <25 |
| Escherichia coli ATCC 1042 | n/a | 50 | n/a |
| Escherichia coli ATCC 25922 | n/a | n/a | n/a |
| Salmonella dublin ATCC 9676 | <25 | <25 | <25 |
| Enterobacter cloacae ATCC 700323 | <25 | n/a | n/a |
| Candida albicans ATCC 10231 | <25 | <25 | <25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utrera-Hernández, B.E.; Blé-González, E.A.; González-Cortazar, M.; Pérez-Garcia, M.D.; Zamilpa, A. Bioactive Potential of Ethyl Acetate Extract from Prosopis laevigata: Antimicrobial and Anti-Inflammatory Effects. Chem. Proc. 2025, 18, 66. https://doi.org/10.3390/ecsoc-29-26717
Utrera-Hernández BE, Blé-González EA, González-Cortazar M, Pérez-Garcia MD, Zamilpa A. Bioactive Potential of Ethyl Acetate Extract from Prosopis laevigata: Antimicrobial and Anti-Inflammatory Effects. Chemistry Proceedings. 2025; 18(1):66. https://doi.org/10.3390/ecsoc-29-26717
Chicago/Turabian StyleUtrera-Hernández, Beatriz E., Ever A. Blé-González, Manasés González-Cortazar, Ma Dolores Pérez-Garcia, and Alejandro Zamilpa. 2025. "Bioactive Potential of Ethyl Acetate Extract from Prosopis laevigata: Antimicrobial and Anti-Inflammatory Effects" Chemistry Proceedings 18, no. 1: 66. https://doi.org/10.3390/ecsoc-29-26717
APA StyleUtrera-Hernández, B. E., Blé-González, E. A., González-Cortazar, M., Pérez-Garcia, M. D., & Zamilpa, A. (2025). Bioactive Potential of Ethyl Acetate Extract from Prosopis laevigata: Antimicrobial and Anti-Inflammatory Effects. Chemistry Proceedings, 18(1), 66. https://doi.org/10.3390/ecsoc-29-26717









