Abstract
Chitosan is a biopolymer with excellent properties such as biodegradability, biocompatibility, bioactivity, and non-toxicity, making it an attractive material for various applications. In this study, to enhance these properties particularly for the development of food coatings chitosan derivatives (1,2,3-triazoles) were synthesized via microwave-assisted 1,3-dipolar cycloaddition (CuAAC) using different terminal alkynes. The resulting compounds were obtained in high yields 79.7–88.0% and characterized by vibrational (IR) and electronic (UV–Visible) spectroscopy. Films were formed by combining the derivatives with PVA and characterized using differential scanning calorimetry (DSC), tensile strength testing, and water vapor permeability analysis. The resulting films exhibited improved mechanical properties, homogeneous thicknesses, low-porosity surfaces, and favorable barrier properties, highlighting their potential applicability as food coating materials.
1. Introduction
Chitosan is a natural polymer valued for its aqueous acid solubility, biodegradability, biocompatibility, bioactivity, and non-toxicity [1,2]. Its derivatives exhibit tunable thermoresponsive, photochromic, and pH-sensitive properties, often modified at primary amino groups, which may affect antimicrobial bioadhesion [3]. Click chemistry, particularly copper-catalyzed 1,3-dipolar cycloaddition (Cu-AAC) between alkynes and azides [4,5], efficiently generates 1,2,3-triazoles under mild conditions (Scheme 1), providing a versatile route for new chitosan derivatives.
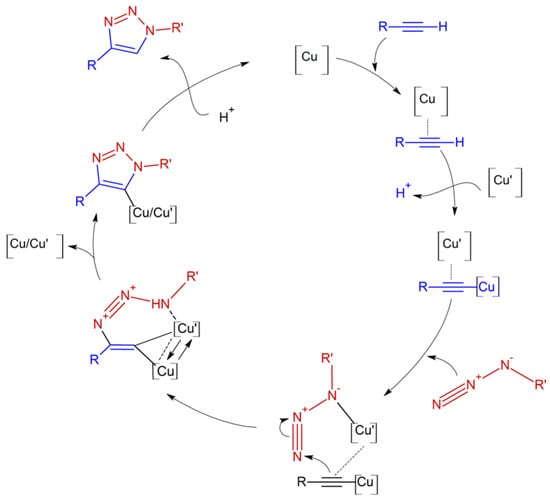
Scheme 1.
Catalytic cycle of the 1,3-dipolar cycloaddition reaction catalyzed by copper CuAAC.
2. Materials and Methods
2.1. Materials and Reagents
Chitosan (CS) from shrimp shells (DD 90.9%, solubility 84.1%) was obtained as previously reported [6], acetic acid (ACS, ≥99.7%), hydrochloric acid (ACS, 37%), diethyl ether, hexadecyltrimethylammonium bromide (CTAB), sodium azide, epichlorohydrin, poly(vinyl alcohol) (PVA). All solvents and reagents were purchased from Sigma-Aldrich (St. Louis, MI, USA) and used without further purification.
2.2. General
The FTIR spectra were recorded using a JASCO FT/IR-4600 (JASCO Corporation, Tokyo, Japan) equipped with attenuated total reflectance (ATR), with a resolution of 2.0 cm−1, over a spectral range of 4000 cm−1 to 400 cm−1. UV-Vis spectra were using a Varian Cary 50 Bio UV-Vis Spectrophotometer (Agilent Technologies, Palo Alto, CA, USA) with a spectral range of 190 nm to 800 nm. Theoretical studies were conducted using the Gaussian 09 (Revision A.02) software package, and the results were visualized with the Gauss View 6.0 (Version 6.0.16) interface.
2.3. Synthesis of 4-Azido-1-chlorobutan-2-ol (1)
CTAB (1.6 mmol) was dissolved in distilled water (40 mL) in an amber vessel, followed by sodium azide (2.5 × 102 mmol). After complete dissolution, epichlorohydrin (2.6 × 102 mmol) was added and stirred for 24 h at room temperature. The product (1) (18.9 g) was washed with diethyl ether, separated by funnel, and dried at room temperature.
2.4. Synthesis of Azido-Modified Chitosan (2)
Chitosan (9.09 mmol) was dissolved in 100 mL of 5% HCl–acetic acid (1:10 v/v), followed by the addition of compound 1 (7.3 × 101 mmol) and sonication for 4 h under light protection. The product (2) with an 89.6% yield (1.3 g) was precipitated with 40% ammonium hydroxide in methanol, filtered, washed (methanol, water, diethyl ether), vacuum-dried (24 h), and stored in the dark.
2.5. Synthesis of Functionalized Chitosan Derivatives (1,2,3-Triazoles)
Compound 2 (1.34 × 10−3 mmol) was reacted with various terminal alkynes under CuAAC conditions (Table 1). Reactions were carried out with CuSO4 (1.1 × 10−2 mmol)/ascorbic acid (2.1 × 10−2 mmol) catalyst in aqueous medium (CH3COOH 1%, 20 mL) at room temperature (Scheme 2). The products were collected by filtration, washed, and dried. Compounds 3, a–f were obtained as solid

Table 1.
Alkynes used in synthesis of functionalized chitosan derivatives.
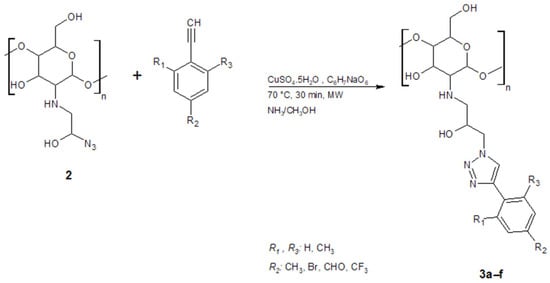
Scheme 2.
Synthesis of functionalized chitosan derivatives by azide−alkyne cycloaddition reaction.
2.6. Theoretical Study
Geometry optimizations and theoretical IR spectra calculations were performed using Gaussian 09 software for compounds 1 and 2, as well as for the monomers of chitosan and compounds 3a–f. The density functional theory (DFT) method with the B3LYP/6-31G level of theory was applied.
2.7. Films Preparation
Films were prepared by solvent casting. Compounds 3a–f (1% acetic acid) and PVA (5%) solutions were sonicated (10 min, 25 °C), mixed 1:1, and stirred for 15 min. The blends were cast into Petri dishes, dried at 36 °C for 24 h, peeled off, and stored in desiccators.
2.8. Characterization of Films
2.8.1. Thermal Stability
Differential scanning calorimetry (DSC) was performed using a TA Instruments Q200, following the procedure reported by Ullah [7] with modifications. A 2 mg film sample was cut and placed into an aluminum pan. The analysis was performed with a continuous purge of nitrogen gas at a flow rate of 40 mL/min with a heating rate of 10 °C/min−1 in the temperature range of 50 to 450 °C. The characteristic transition temperatures values were recorded.
2.8.2. Physical Properties (Thickness)
The thickness of each film type was measured using a HELIOS-PREISSER micrometer (HELIOS-PREISSER, Gammertingen, Germany). Measurements were taken at five different points of each film.
2.8.3. Mechanical Properties
Films (100 × 10 mm) were tested for tensile strength using a universal testing machine (Shimadzu AGS-X, Shimadzu Corporation, Kyoto, Japan) with an initial grip separation of 5 mm and a crosshead speed of 5 mm/min. Tests were performed in triplicate at room temperature.
2.8.4. Topographic Analysis
The roughness and surface topography of the films were analyzed using an atomic force microscope (Park Systems, NX10 model, Park Systems Corp., Suwon, South Korea). The instrument was operated in contact mode with a set point of 180 nm between the silicon nitride cantilever tip and the sample. Portions measuring 1 cm × 1 cm were cut from each film, and their surfaces were mapped.
2.8.5. Water Vapor Permeability (WVP)
The methodology described by Cazón [8] was used with modifications. A 15 mL Falcon tube was filled with 3 mL of water, covered with the film, weighed, and placed in a desiccator. Weight loss was recorded every 30 min for 7 h. WVP was calculated using Equation (1):
where Δw/Δt is the weight loss rate (g/s), T the film thickness (m), A the exposed area (m2), and ΔP the vapor pressure differential (1017 Pa at 15 °C).
3. Results and Discussion
3.1. Synthesis
3.1.1. Synthesis of Azidated Chitosan
The synthesis of compound 2 was a sucessefull with a good yield of 89.55. IR spectrum of azidated chitosan (Figure 1b) exhibited a strong band at 2106 cm−1, which was absent in the IR spectrum of native chitosan (Figure 1a). This band is characteristic of the azide group, confirming the successful functionalization of chitosan.
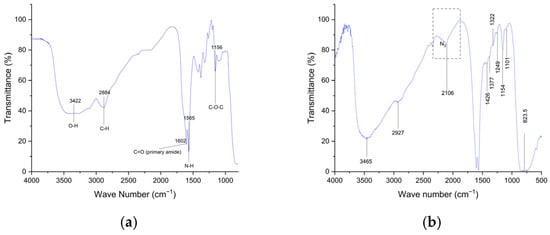
Figure 1.
IR spectrum: (a) Chitosan; (b) Azidated chitosan.
3.1.2. Synthesis of Functionalized Chitosan Derivatives (1,2,3-Triazoles)
Compounds 3a–f were obtained in high yields (Table 1). This is consistent with the copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction, which typically proceeds with high yields and excellent regioselectivity, converting organic azides and terminal alkynes into 1,4-disubstituted 1,2,3-triazoles. The reaction follows a catalytic mechanism involving multiple copper–organic intermediates (Scheme 1).
3.2. Derivatives Characterization
UV–Visible and IR Spectroscopy
The theoretical and experimental IR spectra of the derivatives showed the disappearance of the azide (N3) peak at 2106 cm−1 (Figure 2), confirming the cycloaddition. Significant changes occurred in two regions: 1700–1550 cm−1 and 1500–900 cm−1 (Figure 2). These regions were strongly influenced by the incorporation of the 1,2,3-triazole ring, the benzene ring, and the respective substituents, which altered the peak patterns.
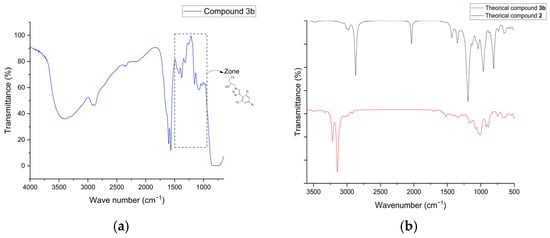
Figure 2.
IR spectrum: (a) Experimental spectrum for Compound 3b (CS-Br); (b) Theoretical spectrum for Compound 3b (CS-Br) and compound 2 (Azidated chitosan).
Chitosan exhibited an absorption band at 203 nm, located in the UV region, whereas the 1,2,3-triazoles showed absorption in the UV–Vis spectrum, with range into [414.6–457] nm. The cycloaddition introduced triazole rings and alkyne substituents, modifying chromophores and increasing auxochromes, resulting in derivatives (3a–f) with colors from yellow to red.
3.3. Characterization of Films
3.3.1. Mechanical Properties
The chitosan film exhibited lower tensile strength and elongation at break than their derivatives (Figure 3). The incorporation of PVA as a plasticizer reduced intermolecular forces and increased polymer chain mobility, thereby enhancing the flexibility and extensibility of the films [9].
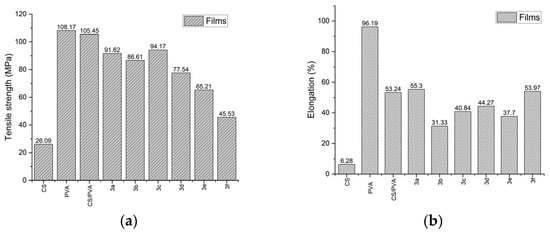
Figure 3.
Comparison of mechanical properties of the different films: (a) Tensile strength (b) Elongation.
3.3.2. Thickness
The thicknesses of the films ranged from 0.0355 to 0.0430 mm. These values indicate that the films can be classified as edible films or coatings. An edible film or coating is defined as any material with a thickness below 0.3 mm, where materials under 0.025 mm are considered coatings, and thicker materials are classified as films [10].
3.3.3. Topographic Analysis
The topographic analysis revealed that films prepared from compounds 3a–f and PVA exhibited lower surface roughness compared to films of chitosan, PVA, or chitosan blended with PVA (Figure 4). These results are consistent with the surface images obtained by AFM (Figure 4).
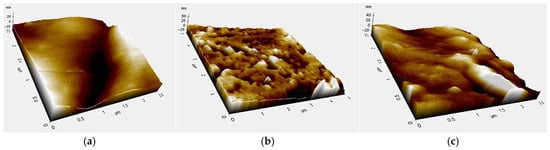
Figure 4.
Topographic analysis of the different films by AFM: (a) Chitosan (b) Chitosan/PVA (c) CS-Br.
The mean roughness (Ra) and root mean square roughness (Rq) of compounds 3b, 3e, and 3f were lower than those of unmodified chitosan or PVA (Table 2), indicating smoother surfaces that can minimize gas and moisture permeability. Such smooth surfaces also facilitate the uniform application of coatings, including antimicrobial or biodegradable layers, thereby enhancing the functionality of the films [1].

Table 2.
Roughness of different films.
3.3.4. Water Vapor Permeability
Chitosan and PVA films exhibited the highest permeability values, indicating relatively low water barrier properties (Figure 5). This behavior is attributed to the abundance of hydroxyl and amino groups in their molecular structure, which readily interact with water molecules and facilitate their permeation through the films [8].
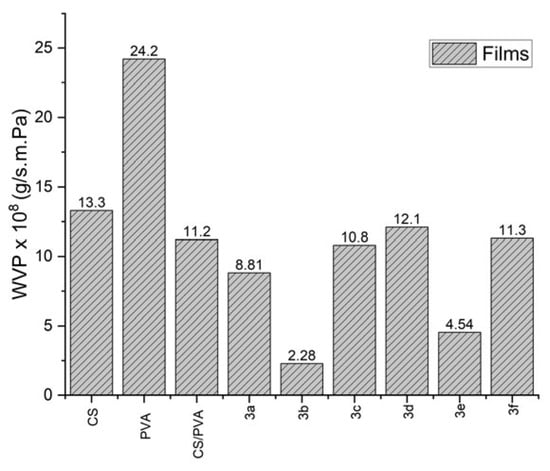
Figure 5.
Comparison of the water vapor permeability of different films.
In contrast, a notable decrease in permeability was observed for the CS-Br and CS-CF3 films (Figure 5). This effect is mainly associated with the presence of bromine and fluorine atoms in their structures. Halogen atoms increase molecular lipophilicity and hydrophobicity, thereby hindering the diffusion of water molecules through the films and enhancing their barrier properties [11].
3.3.5. Thermal Stability
Figure 6 shows the thermograms of the films. Chitosan exhibited an endothermic peak at 112 °C (evaporation of water and acetic acid), a Tg at 187.38 °C, and an exothermic peak at 289.16 °C corresponding to thermal decomposition, consistent with previously reported values [12,13]. PVA films presented peaks at 51.29 °C, 81.16 °C, 219.61 °C, and 311.04 °C, associated with Tg, water loss, melting, and decomposition, in agreement with the literature [14]. Chitosan derivatives showed endothermic peaks related to solvent evaporation and decomposition peaks at 326.12 °C (CS-ACN), 303.49 °C (CS-CH3), 315.13 °C (CS-CF3), and 322.84 °C (CS-Br), with Tg values between 214–219 °C, indicating higher thermal stability than native chitosan and slightly greater stability than PVA. In contrast, CS-TOL and CS-CHO decomposed at lower temperatures (294.27 °C and 288.74 °C), reflecting reduced stability.
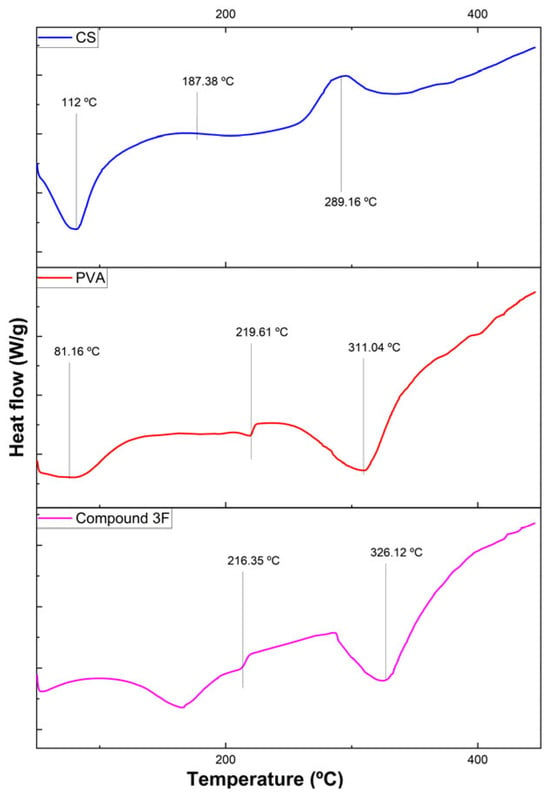
Figure 6.
Comparison of thermograms of different films for Chitosan, PVA and compound 3f.
4. Conclusions
In this study, chitosan–triazole derivatives were efficiently synthesized via CuAAC (79.7–88.0% yield) and characterized spectroscopically. Their PVA-based films showed improved mechanical, structural, and barrier properties, highlighting potential as food coatings.
Author Contributions
Conceptualization, J.G.-G., C.D.A.-L. and P.M.B.-V.; methodology, J.G.-G. and C.D.A.-L.; investigation, J.G.-G.; resources, V.J.T.-T. and R.F.; data curation, V.J.T.-T. and R.F.; writing—review and editing, J.G.-G., C.D.A.-L., V.J.T.-T. and R.F.; supervision, C.D.A.-L.; project administration, C.D.A.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jasrotia, S.; Gupta, S.; Kudipady, M.L.; Puttaiahgowda, Y.M. Development and Characterization of Chitosan–PVA–Tannic Acid Film for Extended Shelf Life and Safety of Food Products. ACS Omega 2025, 10, 19361–19378. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Pranzoni, C.; García, M.G.; Ochoa, N.A. Structural and Conformational Changes on Chitosan after Green Heterogeneous Synthesis of Phenyl Derivatives. Carbohydr. Polym. 2023, 312, 120843. [Google Scholar] [CrossRef] [PubMed]
- Argüelles-Monal, W.M.; Lizardi-Mendoza, J.; Fernández-Quiroz, D.; Recillas-Mota, M.T.; Montiel-Herrera, M. Chitosan Derivatives: Introducing New Functionalities with a Controlled Molecular Architecture for Innovative Materials. Polymers 2018, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, A.K.; Bose, P.; Jaiswal, M.K.; Rajkhowa, S.; Singh, A.S.; Hotha, S.; Mishra, N.; Tiwari, V.K. Cu(I)-Catalyzed Click Chemistry in Glycoscience and Their Diverse Applications. Chem. Rev. 2021, 121, 7638–7956. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Akhtar, M.J.; Gogoi, U.; Meenakshi, D.U.; Das, A. An Overview of 1,2,3-Triazole-Containing Hybrids and Their Potential Anticholinesterase Activities. Pharmaceuticals 2023, 16, 179. [Google Scholar] [CrossRef] [PubMed]
- Pozo Llumiquinga, A.Y. Síntesis de Derivados de 1, 2, 3 Triazol-Quitosano a Partir de Reacciones de Clicloadición Azida-Alquil Benceno Para Aplicaciones en Empaques Alimenticios. Bachelor’s Thesis, Universidad Central del Ecuador, Quito, Ecuador, 2022. [Google Scholar]
- Ullah, A.; Khan, N.R.; Khan, M.H.; Mehmood, S.; Khan, J.; Iftikhar, T.; Gul, I.U.; Basit, H.M.; Qayum, M.; Shah, S.U. Formulation of Microwave-Assisted Natural-Synthetic Polymer Composite Film and Its Physicochemical Characterization. Int. J. Polym. Sci. 2021, 2021, 9961710. [Google Scholar] [CrossRef]
- Cazón, P.; Morales-Sanchez, E.; Velazquez, G.; Vázquez, M. Measurement of the Water Vapor Permeability of Chitosan Films: A Laboratory Experiment on Food Packaging Materials. J. Chem. Educ. 2022, 99, 2403–2408. [Google Scholar] [CrossRef]
- Sánchez, L.A.P.; Ballén, A.B.; Marchand, L.C.S. Preparación y caracterización de una película de quitosano plastificado: Un biomaterial de gran potencial en el campo de la medicina. Reto 2019, 7, 11–24. [Google Scholar] [CrossRef]
- Priya, K.; Thirunavookarasu, N.; Chidanand, D.V. Recent Advances in Edible Coating of Food Products and Its Legislations: A Review. J. Agric. Food Res. 2023, 12, 100623. [Google Scholar] [CrossRef]
- Priimagi, A.; Cavallo, G.; Metrangolo, P.; Resnati, G. The Halogen Bond in the Design of Functional Supramolecular Materials: Recent Advances. Acc. Chem. Res. 2013, 46, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.; Maegawa, T.; Takahashi, T. Glass Transition Temperature of Chitosan and Miscibility of Chitosan/Poly(N-Vinyl Pyrrolidone) Blends. Polymer 2000, 41, 7051–7056. [Google Scholar] [CrossRef]
- Dong, Y.; Ruan, Y.; Wang, H.; Zhao, Y.; Bi, D. Studies on Glass Transition Temperature of Chitosan with Four Techniques. J. Appl. Polym. Sci. 2004, 93, 1553–1558. [Google Scholar] [CrossRef]
- Remiš, T.; Bělský, P.; Kovářík, T.; Kadlec, J.; Ghafouri Azar, M.; Medlín, R.; Vavruňková, V.; Deshmukh, K.; Sadasivuni, K.K. Study on Structure, Thermal Behavior, and Viscoelastic Properties of Nanodiamond-Reinforced Poly (Vinyl Alcohol) Nanocomposites. Polymers 2021, 13, 1426. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).